Abstract
Performing two tasks simultaneously (dual-task) is common in human daily life. The neural correlates of dual-task processing remain unclear. In the current study, we used a dual motor and counting task with functional MRI (fMRI) to determine whether there are any areas additionally activated for dual-task performance. Moreover, we investigated the functional connectivity of these added activated areas, as well as the training effect on brain activity and connectivity. We found that the right cerebellar vermis, left lobule V of the cerebellar anterior lobe and precuneus are additionally activated for this type of dual-tasking. These cerebellar regions had functional connectivity with extensive motor- and cognitive-related regions. Dual-task training induced less activation in several areas, but increased the functional connectivity between these cerebellar regions and numbers of motor- and cognitive-related areas. Our findings demonstrate that some regions within the cerebellum can be additionally activated with dual-task performance. Their role in dual motor and cognitive task processes is likely to integrate motor and cognitive networks, and may be involved in adjusting these networks to be more efficient in order to perform dual-tasking properly. The connectivity of the precuneus differs from the cerebellar regions. A possible role of the precuneus in dual-task may be monitoring the operation of active brain networks.
Keywords: Dual-task Performance, fMRI, Brain Activity, Network Connectivity, Neural Efficient
INTRODUCTION
It is common for people to execute two tasks simultaneously (dual-task). An impairment of performance generally occurs during dual-task performance, which is defined as dual-task interference. However, after extensive practice, dual-task interference becomes less and may even disappear (Van Selst et al., 1999; Levy and Pashler 2001; Ruthruff et al., 2001, 2003; Schumacher et al., 2001) so that some complex dual-tasks can be executed correctly. For example, a skilled typist can type accurately while holding a conversation (Shaffer1975).
The neural basis of dual-task processing is still unclear. One critical issue is whether there is any brain area additionally activated in a dual-task but not in the single component tasks. Previous neuroimaging studies provided mixed results; some studies suggested that there is dual-task performance associated neural activity (D'Esposito et al., 1995; Koechlin et al., 1999; Herath et al., 2001; Dreher and Grafman 2003; Szameitat et al., 2002, 2006; Schubert and Szameitat 2003; Erickson et al., 2005; Dux et al., 2006; Wu and Hallett 2008; Hsieh et al., 2009), while others found no evidence that there are any areas related to dual-task performance (Klingberg, 1998; Adcock et al., 2000; Bunge et al., 2000; Jiang 2004; Smith et al., 2001), or found reduced neural activity associated with dual-task performance (Goldberg et al., 1998; Just et al., 2001, 2008; Jaeggi et al., 2003).
We suppose that one possible reason contributing to these divergent findings is the different paradigms employed. For example, studies with two simple response tasks (Herath et al., 2001), two choice response tasks (Szameitat et al., 2002, 2006; Dreher and Grafman 2003; Schubert and Szameitat 2003; Dux et al., 2006), or a simple motor and a non-motor cognitive task (Wu and Hallett 2008) have frequently detected dual-task related brain activity. In contrast, dual-tasks that combined two working memory tasks yielded controversial findings. For example, D'Esposito et al. (1995) found that the prefrontal cortex was additionally activated for dual-tasking, but some similar subsequent studies did not support this finding (Adcock et al., 2000; Bunge et al., 2000). Another reason may be due to different analysis strategies (for a review, see Szameitat et al., 2011).
A previous study with a dual-task paradigm containing a motor task and a silent counting task found that cerebellar-impaired subjects had difficulty performing the dual-task accurately (Lang and Bastian 2002). Thus, presumably, some regions in the cerebellum should relate to the dual motor and cognitive task. However, our earlier research with a similar paradigm only found that the precuneus, but not the cerebellum, was additionally activated for the dual-task (Wu and Hallett 2008). The motor tasks employed in that study were complex sequential finger movements, which induced extensive activations within the cerebellum; we presume using complex motor task may produce difficulty in distinguishing dual-task related activity from single motor task activations. Therefore, we used a similar dual-task paradigm but with a simpler motor task in the current study and functional MRI (fMRI) method to investigate whether there might be dual-task related areas within the cerebellum. After practice, the simple motor task can be performed automatically (Wu et al., 2004; Poldrack et al, 2005). When one of the two tasks of a dual- task is executed automatically, neural processing capacity will not be exceeded, dual-task specific demands may be absent, and two tasks can be performed simultaneously without costs, then, the dual-task can be executed correctly (Passingham 1996; Meyer and Kieras, 1997a, b).
Moreover, as the role of additionally activated areas in dual-task processing remains unclear, we investigated the neural correlates of the dual-task processing from both local activity and brain network levels, before and after the training of dual-task. We hypothesized that some regions in the cerebellum relate to performance of dual-tasking. These regions should be activated at both before and after training stages, and may be involved in adjusting neural networks to execute the dual-task properly. We hope to provide new insights into understanding the neural correlates of dual-task processing.
METHODS
Subjects
18 healthy volunteers participated in the study (12 males, 6 females; 22–36 years old, mean age 28.2 ± 3.7 years). All subjects were right-handed as measured by the Edinburgh Inventory (Oldfield 1971). The experiments were performed according to the Declaration of Helsinki and were approved by the Institutional Review Board from Xuanwu Hospital. All subjects gave their written informed consent for the study.
Tasks
Subjects were asked to perform two single and one dual-task. Single tasks contained a motor task and a visual letter counting task. The motor task was a self-paced tapping task in which subjects briskly tapped their right index and middle fingers alternatively at 1 Hz frequency, with amplitude of about 2.5 cm. For the visual letter counting task, a random series of the numbers A, G, L, and O were presented on a screen and subjects were asked to identify the number of times they saw a specified target letter. The letters were presented at an irregular interval (average interval 1.5s). In the dual-task, subjects performed the tapping and letter counting tasks simultaneously. The paradigm is similar to that employed in previous studies (Lang and Bastian 2002; Wu et al., 2004; Wu and Hallett 2005a,b; Wu and Hallett, 2008).
This experiment included two fMRI scanning sessions, and was conducted in 2 consecutive days. The time between two fMRI sessions was about 24 hrs. On the first day, before the first scanning session, subjects practiced until they could perform each single task correctly. Because movement frequency has a significant effect on brain activity (Sadato et al., 1997; Deiber et al., 1999), subjects practiced until they could move at the required rate. They briefly learned how to execute the dual-task, but without further practice (“before training” stage). The first scanning session occurred right after. Following the first scanning session, subjects were given enough practice trials until they could perform three dual-task trials in a row without errors (each trial for training lasted 3 min), and without behavioral difference from single tasks (“after training” stage). On the second day, subjects participated in the second fMRI scanning session.
Functional MRI procedure
fMRIs were performed on a 3T Siemens Sonata scanner. A standard head coil was used with foam padding to restrict head motion. High-resolution axial T1- and T2- weighted images were obtained in every participant to detect clinically silent lesions. High-resolution anatomical images were acquired with 3D-MPRAGE sequence (TR = 2530 ms, TE = 3.39 ms, 128 axial slices, 1.33-mm thickness, field of view (FOV) = 256 mm). Blood-oxygen-level dependent (BOLD) data were acquired with gradient-echo echo-planar sequences (TR = 2000 ms, TE = 30 ms, 33 axial slices, 3.5-mm thickness, Flip angle = 900, FOV = 220 mm). An electrical response device that could work inside the MRI scanner was fixed to each subject’s right hand and was used to record finger movements during fMRI sessions. There were three runs in each fMRI scanning session for each subject. Each run was 6 min. All runs were block-designed and contained two conditions, which were defined as the “rest” and “active” condition, respectively. Each condition lasted 30 s and was repeated six times (totally 12 blocks in each run). In the rest condition, subjects were asked to relax and focus on the screen in front of them without moving or thinking while inside the scanner. The active condition in each run contained one of the tapping, counting, or dual-tasks. Visual signals were presented on the screen to inform the subjects to switch from rest to active condition and vice versa. Task order of these three runs was randomized across subjects in each session. No external cue was given to help the subjects move at the specified rate, and no feedback was provided during fMRI scanning to tell subjects whether their finger movements were correct or incorrect. After scanning each run, subjects were asked to report the whole number of target letters in that run.
Behavioral data analysis
Each subject’s performance for each task was recorded. These measurements included the accuracy and frequency for finger-tapping, and number of target letters in each run. Wrong button presses for motor task or incorrect numbers being reported for the counting task were considered errors. We first calculated errors in each single and dual-task separately, then used a repeated-measures ANOVA to calculate the differences in performance between single and dual-task (p < 0.05).
Imaging data analysis
Data preprocessing
Image analysis was performed with SPM8 software (Wellcome Institute of Cognitive Neurology, London, UK). fMRI data were slice-time corrected and aligned to the first image of each run for motion correction. Functional images were co-registered to high-resolution anatomical images. After spatial normalization, all images were resampled into voxels that were 3×3×3 mm in size, and smoothed with a 4-mm Gaussian smoothing kernel to suppress noise and effects due to residual differences in functional and gyral anatomy.
Brain activity analysis
Data were analyzed for each single participant separately on a voxel-by-voxel basis using the general linear model approach for the time series. We defined a model using a fixed effect boxcar design convolved with a hemodynamic response function for analysis of task-dependent activation. We added the 6 head motion parameters as regressors to optimally control for the motion effects. A contrast representing the effect of the active condition compared with the rest condition was calculated in each participant. A one-sample t-test model was used to identify the brain activity for each task (p < 0.05, family-wise error (FWE) corrected). Then, we used one-way ANOVA to explore the brain regions that were additionally activated in the dual-task compared to single tasks (dual-task - tapping - counting; Wu and Hallett, 2008; Szameitat et al., 2011). In addition, we used the contrast of the letter counting task as the inclusive mask (p < 0.001, uncorrected) to explore areas that are commonly activated by the motor and letter counting task (p < 0.05, FWE corrected). The activations specific to the cerebellum were overlaid on the high-resolution, Spatially Unbiased Infraorbital Template (SUIT Version 2.5) atlas of the human cerebellum (Diedrichsen 2006; Diedrichsen et al., 2009).
Network connectivity analysis
We defined brain regions that showed additional activation in the dual-task rather than in the single component tasks as the regions of interest (ROIs). The ROIs were centered at the voxels showing the maximum magnitude of activation within each additionally activated area, with a radius of 5 mm, and were applied as the seeds for functional connectivity analysis. We only chose the data at active conditions for connectivity analysis. The functional connectivity analysis procedure was done by a toolkit REST (www.restfmri.net) and SPM8. Eight nuisance covariates were regressed, including: the white matter signal, cerebrospinal fluid signal, and six head motion parameters. A seed reference time course was obtained by averaging the time courses within each ROI. Correlation analysis was carried out between the seed reference and the whole brain in a voxel-wise manner. A random effect one-sample t-test was used to determine brain regions showing significant connectivity with each ROI while performing each task within each condition. Finally, we applied one-way ANOVA to investigate the training effect on brain activity and connectivity ((dual-task - tapping - counting) from the after training session-(dual-task - tapping - counting) from the before training session).
RESULTS
Task Performance
Behavioral data are shown in Table 1. In the before training session, the subjects had no error in performing the single tasks. But they committed some errors of finger tapping and letter counting while performing the dual-task. The difference of performance between the dual-task and the single tasks was statistically significant (repeated-measures ANOVA, F-value = 67.1, p < 0.001). After practice (average 14.06 ± 2.46 training trials, 42.18±7.38 min), subjects had no errors in performing the single and dual-tasks. There was no difference in tapping interval between single and dual-task during either the first or second fMRI sessions. Thus, movement rate had no effect on our imaging findings.
Table 1.
Performance (number of errors) of finger tapping, letter-counting, and dual-task during the two fMRI sessions.
| Task | First fMRI Session | Second fMRI Session | ||
|---|---|---|---|---|
| Errors | Tapping Interval | Errors | Tapping Interval | |
| Tapping Task | 0 | 0.97±0.09 | 0 | 0.98±0.08 |
| Counting Task | 0 | 0 | ||
| Dual-Task | 1.44±1.29/2.94±1.35 | 1.03±0.11 | 0 | 0.96±0.07 |
Values are given as mean ±SD for number of errors. The results of the dual-task are given as errors of finger movements/errors of letter-counting.
FMRI Results
Dual-task related brain activity
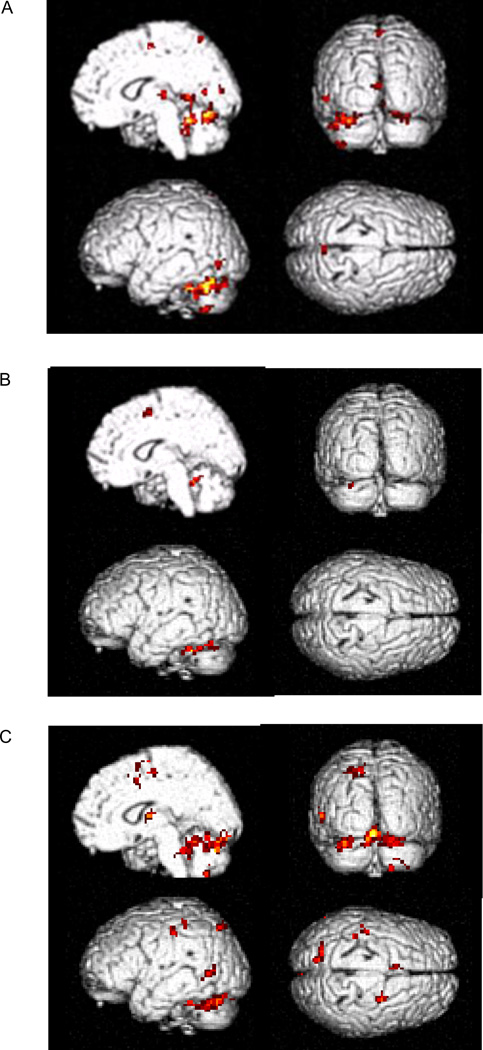
In both fMRI sessions, performance of the single tapping task was associated with activation in the left primary sensorimotor cortex (M1), caudal supplementary motor area (SMA-proper), left dorsal premotor cortex (PMd), and right lobule VI of cerebellum (Fig. 1A, Tables 2 and 3). The letter counting task activated the rostral SMA (pre-SMA), bilateral PMd, and left lobule VI of cerebellum (Fig. 1B, Tables 2 and 3). The dual-task produced activation in the left M1, SMA, bilateral PMd, bilateral parietal cortex, precuneus, and bilateral cerebellum (Fig. 1C, Tables 2 and 3).
Figure 1. Brain activations in dual and single tasks.
Brain areas activated while performing single tapping (A), counting (B), and dual-task (C) at the after training stage (p < 0.05, FWE corrected).
Table 2.
Brain activity in each single and dual-tasks at the beforer training stage.
| Brain region | Brodmann area |
MNI Coordinates | t value | Cluster size |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Tapping Task | ||||||
| L M1 | 4 | −39 | −26 | 55 | 13.79 | 98 |
| L PMd | 6 | −26 | −14 | 52 | 11.89 | 53 |
| L SMA-Proper | 6 | −4 | −8 | 56 | 12.75 | 41 |
| R Cerebellum, Anterior Lobe, Culmen, Lobule VI | 21 | −50 | −28 | 15.16 | 103 | |
| Counting Task | ||||||
| L PMd | 6 | −32 | −10 | 51 | 9.75 | 16 |
| R PMd | 6 | 38 | −7 | 56 | 10.85 | 29 |
| R Pre-SMA | 6 | 3 | 10 | 52 | 9.98 | 62 |
| L Cerebellum, Posterior Lobe, Declive, Lobule VI | −27 | −63 | −27 | 12.08 | 59 | |
| Dual-Task | ||||||
| R Postcentral Gyrus | 3 | 39 | −30 | 57 | 11.75 | 44 |
| L M1 | 4 | −33 | −29 | 58 | 14.79 | 198 |
| L PMd | 6 | −28 | −10 | 52 | 15.69 | 127 |
| R PMd | 6 | 34 | −12 | 49 | 14.11 | 65 |
| L Pre-SMA | 6 | −6 | 8 | 52 | 14.56 | 89 |
| L Precuneus | 7 | −4 | −57 | 58 | 11.46 | 108 |
| L Postcentral Gyrus | 40 | −27 | −39 | 57 | 10.25 | 34 |
| R Cerebellum, Vermis | 3 | −51 | −18 | 11.84 | 77 | |
| R Cerebellum, Anterior Lobe, Culmen, Lobule VI | 27 | −54 | −27 | 11.98 | 179 | |
| L Cerebellum, Posterior Lobe, Declive, Lobule VI | −24 | −63 | −30 | 10.71 | 52 | |
| L Cerebellum, Anterior Lobe, Culmen, Lobule V | −24 | −47 | −24 | 11.46 | 60 | |
| L Cerebellum, Anterior Lobe, Dentate | −15 | −54 | −33 | 9.09 | 21 | |
Brain regions activated during performance of tapping, counting, or dual-task at the before training stage (one-sample t-test, p < 0.05, FWE corrected). Abbreviations: L, left; R, right; M1, primary sensorimotor cortex; PMd, dorsal premotor cortex; Pre-SMA, rostral supplementary motor area; SMAproper, caudal supplementary motor area.
Table 3.
Brain activity in each single and dual-tasks at the after training stage.
| Brain region | Brodmann area |
MNI Coordinates | t value | Cluster size |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Tapping Task | ||||||
| L M1 | 4 | −45 | −27 | 51 | 14.63 | 107 |
| L PMd | 6 | −30 | −12 | 54 | 13.15 | 41 |
| SMA-Proper | 6 | 0 | −6 | 54 | 11.97 | 28 |
| R Cerebellum, Anterior Lobe, Culmen, Lobule VI | 24 | −48 | −30 | 13.97 | 85 | |
| Counting Task | ||||||
| L PMd | 6 | −39 | −6 | 51 | 10.06 | 18 |
| R PMd | 6 | 45 | −3 | 54 | 10.41 | 35 |
| R Pre-SMA | 6 | 9 | 15 | 54 | 10.57 | 51 |
| L Cerebellum, Posterior Lobe, Declive, Lobule VI | −30 | −60 | −30 | 10.11 | 42 | |
| Dual-Task | ||||||
| R Postcentral Gyrus | 2 | 45 | −30 | 39 | 10.83 | 10 |
| L M1 | 4 | −39 | −27 | 57 | 15.35 | 257 |
| L PMd | 6 | −36 | −15 | 54 | 13.53 | 87 |
| L SMA | 6 | −3 | 0 | 51 | 13.46 | 34 |
| R PMd | 6 | 39 | −6 | 45 | 11.25 | 19 |
| L Superior Parietal Lobule | 7 | −24 | −69 | 57 | 11.92 | 37 |
| R Precuneus | 7 | 5 | −58 | 54 | 10.36 | 81 |
| L Thalamus, Pulvinar | −15 | −24 | 9 | 12.38 | 14 | |
| R Cerebellum, Vermis | 5 | −54 | −15 | 13.04 | 72 | |
| R Cerebellum, Anterior Lobe, Culmen, Lobule VI | 24 | −51 | −30 | 12.96 | 142 | |
| L Cerebellum, Posterior Lobe, Declive, Lobule VI | −18 | −66 | −27 | 9.14 | 36 | |
| L Cerebellum, Anterior Lobe, Culmen, Lobule V | −21 | −48 | −28 | 10.21 | 42 | |
| L Cerebellum, Anterior Lobe, Dentate | −12 | −57 | −30 | 8.23 | 10 | |
Brain regions activated during performance of tapping, counting, or dual-task at the after training stage (one-sample t-test, p < 0.05, FWE corrected). Abbreviations: L, left; R, right; M1, primary sensorimotor cortex; PMd, dorsal premotor cortex; Pre-SMA, rostral supplementary motor area; SMAproper, caudal supplementary motor area.
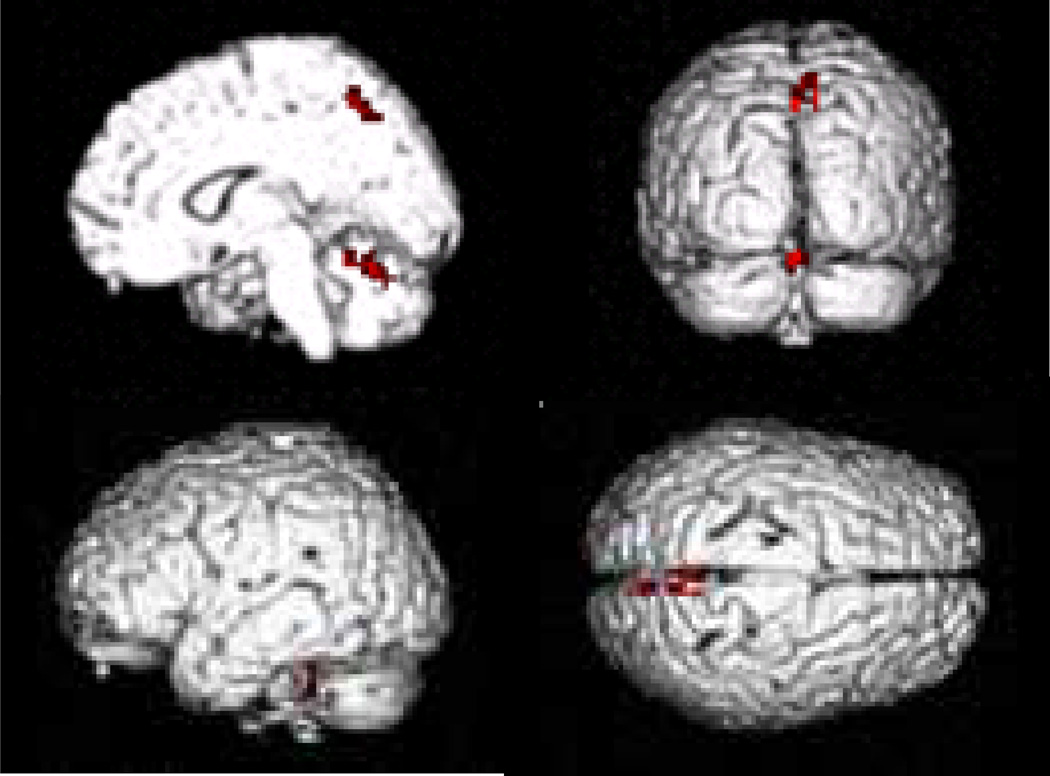
Dual-task performance additionally activated the vermis of the right cerebellum (RVM), lobule V of left cerebellum (LCV), and precuneus compared with single component tasks at both before and after training stages (one-way ANOVA, p < 0.05, FWE corrected; Fig. 2, Table 4). These three additionally activated areas were further used for the functional connectivity analysis. The activity within the cerebellum for each task, including those areas additionally activated for the dual-task compared with the component tasks are shown in the Fig. 3. In both the before and after-training states, the single motor and letter-counting task commonly activated the left PMd.
Figure 2. Brain regions added activated in the dual-task.
Brain areas more activated in the dual-task compared with single component tasks at the after training stage (one-way ANOVA, p < 0.05, FWE corrected).
Table 4.
Brain regions additionally activated in dual-task at the after training stage.
| Brain region | Brodmann area |
MNI Coordinates | t value | Cluster size |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| R Precuneus | 7 | 3 | −54 | 54 | 7.16 | 22 |
| L Cerebellum, Anterior Lobe, Culmen, Lobule V | −24 | −45 | −24 | 8.18 | 32 | |
| R Cerebellum, Anterior Lobe, Vermis | 3 | −54 | −15 | 7.57 | 28 | |
Brain regions additionally activated during performance of dual-task compared with tapping and counting tasks at the after training stage (one-way ANOVA, dual-task - tapping - counting, p < 0.05, FWE corrected). Abbreviations: L, left; R, right.
Figure 3. Activations within the cerebellum.
Activations within the cerebellum during performing tapping (A, left column), counting (A, right column), and dual-task (B), and more activated in the dual-task compared with single component tasks at the after training stage (C).
Dual-task related network connectivity
In both sessions, the LCV had functional connectivity with the posterior lobe of the ipsilateral cerebellum, anterior lobe of the contralateral cerebellum, SMA-proper, parietal cortex, thalamus, and midbrain during execution of the single tapping task. In performing the single counting task, the LCV had connectivity with the posterior lobe of the ipsilateral cerebellum, anterior lobe of the contralateral cerebellum, and pre-SMA. During performance of the dual-task, the LCV showed connectivity with the posterior lobe of the ipsilateral cerebellum, anterior lobe of the contralateral cerebellum, M1, pre-SMA, SMA-proper, PMd, parietal cortex, and thalamus (Fig. 4, Table 5).
Figure 4. Functional connectivity in the left cerebellum.
Functional connectivity in the left lobule V of the cerebellum during performing tapping (A), counting (B), and dual-task (C) at the after training stage (p < 0.05, FWE corrected).
Table 5.
Network connectivity in the left cerebellum at the after training stage.
| Brain region | Brodmann area |
MNI Coordinates | t value | Cluster size |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Tapping Task | ||||||
| R SMA-proper | 6 | 2 | −3 | 51 | 10.16 | 12 |
| R Occipital Lobe, Cuneus | 18 | 3 | −84 | 12 | 9.61 | 14 |
| L Inferior Temporal Gyrus | 19 | −55 | −72 | −6 | 11.31 | 16 |
| L Inferior Parietal Lobule | 40 | −32 | −57 | 39 | 11.60 | 20 |
| R Thalamus, Medial Dorsal Nucleus | 6 | −21 | 6 | 11.55 | 22 | |
| L Cerebellum, Posterior Lobe, Declive, Lobule VI | −27 | −63 | −24 | 11.84 | 353 | |
| R Cerebellum, Anterior Lobe, Culmen, Lobule V | 24 | −48 | −21 | 13.31 | 356 | |
| L Midbrain | −3 | −36 | −3 | 10.78 | 11 | |
| Counting Task | ||||||
| R Pre-SMA | 6 | 6 | 2 | 51 | 10.95 | 14 |
| L Cerebellum, Posterior Lobe, Declive, Lobule VI | −24 | −63 | −24 | 12.35 | 133 | |
| R Cerebellum, Anterior Lobe, Culmen, Lobule V | 9 | −42 | −24 | 12.38 | 15 | |
| Dual-Task | ||||||
| L M1 | 4 | −37 | −24 | 50 | 10.25 | 17 |
| R PMd | 6 | 27 | −6 | 63 | 12.90 | 30 |
| L Pre-SMA | 6 | −6 | 6 | 54 | 15.25 | 26 |
| R Pre-SMA | 6 | 6 | 9 | 48 | 9.88 | 10 |
| R SMA-proper | 6 | 9 | −6 | 54 | 9.64 | 14 |
| L Precuneus | 7 | −18 | −69 | 48 | 10.17 | 35 |
| L Middle Temporal Gyrus | 37 | −52 | −66 | 6 | 13.22 | 21 |
| L Postcentral Gyrus | 40 | −39 | −36 | 57 | 10.12 | 10 |
| L Thalamus, Medial Dorsal Nucleus | −3 | −9 | 8 | 11.29 | 30 | |
| L Cerebellum, Posterior Lobe, Declive, Lobule VI | −18 | −63 | −18 | 16.20 | 217 | |
| R Cerebellum, Posterior Lobe, Declive, Lobule VI | 3 | −79 | −18 | 15.22 | 99 | |
| R Cerebellum, Anterior Lobe, Culmen, Lobule VI | 24 | −54 | −21 | 1.18 | 114 | |
Brain regions functionally connected with the left cerebellum (anterior lobe, culmen, lobule V) during performance of tapping, counting, or dual-task at the after training stage (one-sample t-test, p < 0.05, FWE corrected). Abbreviations: L, left; R, right; M1, primary sensorimotor cortex; PMd, dorsal premotor cortex; Pre-SMA, rostral supplementary motor area; SMA-proper, caudal supplementary motor area.
The RVM had functional connectivity with the posterior lobe of the ipsilateral cerebellum, M1, PMd, SMA-proper, parietal cortex, and thalamus while performing the tapping task. When performing the counting task, the RVM had connectivity with the ipsilateral cerebellar lobule VI, pre-SMA, medial frontal gyrus, and anterior and posterior cingulate cortices. During performance of the dual-task, the RVM functionally connected with lobule VI of the ipsilateral cerebellum, anterior and posterior of the contralateral cerebellum, M1, PMd, pre-SMA, SMA-proper, parietal cortex, and thalamus (Fig. 5, Table 6).
Figure 5. Functional connectivity in the right cerebellum.
Functional connectivity in the right vermisof the cerebellum during performing tapping (A), counting (B), and dual-task (C) at the after training stage (p < 0.05, FWE corrected).
Table 6.
Network connectivity in the right cerebellumatthe after training stage.
| Brain region | Brodmann area |
MNI Coordinates | t value | Cluster size |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Tapping Task | ||||||
| L M1 | 4 | −43 | −24 | 51 | 11.54 | 27 |
| L PMd | 6 | −24 | −15 | 52 | 11.32 | 32 |
| SMA-Proper | 6 | 0 | −3 | 51 | 10.08 | 29 |
| R Inferior Parietal Lobule | 40 | 36 | −54 | 48 | 9.81 | 16 |
| L Thalamus, Medial Dorsal Nucleus | −9 | −18 | 9 | 9.21 | 12 | |
| R Cerebellum, Posterior Lobe, Declive, Lobule VI | 30 | −66 | −24 | 10.48 | 220 | |
| Counting Task | ||||||
| L Pre-SMA | 6 | −4 | 5 | 69 | 15.18 | 12 |
| R Medial Frontal Gyrus | 9 | 24 | 36 | 21 | 12.85 | 15 |
| R Posterior Cingulate Cortex | 30 | 3 | −69 | 9 | 10.14 | 18 |
| R Anterior Cingulate Cortex | 32 | 9 | 42 | 6 | 10.40 | 17 |
| L Anterior Cingulate Cortex | 32 | −3 | 39 | 18 | 9.10 | 26 |
| R Cerebellum, Anterior Lobe, Culmen, Lobule VI | 33 | −48 | −24 | 9.85 | 86 | |
| Dual-Task | ||||||
| L Postcentral Gyrus | 2 | −54 | −24 | 42 | 9.13 | 13 |
| R Postcentral Gyrus | 3 | 57 | −21 | 36 | 10.16 | 13 |
| L M1 | 4 | −35 | −30 | 51 | 15.75 | 212 |
| L PMd | 6 | −42 | −9 | 57 | 15.75 | 109 |
| R PMd | 6 | 27 | −9 | 57 | 12.06 | 48 |
| R Pre-SMA | 6 | 6 | 6 | 51 | 12.47 | 31 |
| SMA-Proper | 6 | 0 | −3 | 51 | 13.19 | 71 |
| L Superior Parietal Lobule | 7 | −27 | −66 | 57 | 13.60 | 44 |
| R Superior Parietal Lobule | 7 | 21 | −66 | 60 | 13.62 | 16 |
| L Precuneus | 7 | −18 | −72 | 45 | 11.08 | 16 |
| R Middle Frontal Gyrus | 9 | 30 | 39 | 27 | 12.12 | 15 |
| R Inferior Parietal Lobule | 40 | 45 | −42 | 48 | 10.11 | 15 |
| L Thalamus, Ventral Lateral Nucleus | −12 | −16 | 3 | 12.57 | 34 | |
| L Cerebellum, Posterior Lobe, Declive, Crus I | −33 | −66 | −27 | 14.38 | 123 | |
| L Cerebellum, Anterior Lobe, Culmen, Lobule VI | −30 | −54 | −21 | 11.2 | 36 | |
| R Cerebellum, Anterior Lobe, Culmen, Lobule VI | 24 | −45 | −30 | 16.75 | 578 | |
Brain regions functionally connected with the right cerebellar vermisduring performance of tapping, counting, or dual-task at the after training stage (one-sample t-test, p < 0.05, FWE corrected). Abbreviations: L, left; R, right; M1, primary sensorimotor cortex; PMd, dorsal premotor cortex; Pre-SMA, rostral supplementary motor area; SMA-proper, caudal supplementary motor area.
The precuneus only had significant connectivity with the superior temporal gyrus while performing the counting task and had connectivity with the occipital lobe, pre-SMA, and medial frontal gyrus while performing the tapping task. During performance of the dual-task, the precuneus showed connectivity with the PMd, pre-SMA, SMA-proper, parietal cortex, temporal lobe, posterior cingulate cortex, occipital lobe, thalamus, and putamen (Fig. 6, Table 7).
Figure 6. Functional connectivity in the precuneus.
Functional connectivity in the precuneus during performing tapping (A), counting (B), and dual-task (C) at the after training stage (p < 0.05, FWE corrected).
Table 7.
Network connectivity in the precuneus at the after training stage.
| Brain region | Brodmann area |
MNI Coordinates | t value | Cluster size |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Tapping Task | ||||||
| R SMA-proper | 6 | 6 | −8 | 51 | 10.06 | 19 |
| R Medial Frontal Gyrus | 9 | 6 | 30 | 33 | 9.34 | 10 |
| L Occipital Lobe, Cuneus | 19 | −6 | −78 | 30 | 12.04 | 18 |
| R Occipital Lobe, Cuneus | 19 | 6 | −78 | 30 | 10.91 | 16 |
| L Middle Occipital Gyrus | 19 | −48 | −78 | 6 | 10.22 | 10 |
| Counting Task | ||||||
| R Superior Temporal Gyrus | 19 | 48 | −60 | 15 | 11.73 | 15 |
| Dual-Task | ||||||
| R PMd | 6 | 33 | −1 | 54 | 10.40 | 11 |
| R SMA-proper | 6 | 6 | −11 | 54 | 13.69 | 15 |
| R Pre-SMA | 6 | 3 | 13 | 47 | 9.59 | 18 |
| R Superior Parietal Lobule | 7 | 26 | −63 | 51 | 11.19 | 26 |
| R Occipital Lobe, Cuneus | 17 | 12 | −87 | 3 | 11.27 | 10 |
| L Superior Occipital Gyrus | 19 | −33 | −78 | 24 | 12.87 | 36 |
| R Occipital Lobe, Cuneus | 30 | 9 | −60 | 6 | 14.70 | 18 |
| R Posterior Cingulate Cortex | 31 | 3 | −39 | 42 | 13.96 | 53 |
| R Temporal Lobe, Fusiform Gyrus | 37 | 48 | −57 | −15 | 12.44 | 20 |
| L Inferior Parietal Lobule | 39 | −32 | −66 | 42 | 9.41 | 11 |
| L Thalamus, Medial Dorsal Nucleus | −6 | −12 | 6 | 12.81 | 15 | |
| R Putamen | 27 | −6 | 6 | 10.83 | 22 | |
Brain regions functionally connected with the precuneus during performance of tapping, counting, or dual-task at the after training stage (one-sample t-test, p < 0.05, FWE corrected). Abbreviations: L, left; R, right; M1, primary sensorimotor cortex; PMd, dorsal premotor cortex; Pre-SMA, rostral supplementary motor area; SMA-proper, caudal supplementary motor area.
Comparison between the before and after training stage
During performance of the dual-task, there was less activation in the pre-SMA, bilateral PMd, and right postcentral gyrus in the after training stage compared with the before training condition. Comparing network connectivity showed that the LCV had increased connectivity with the pre-SMA, right PMd, and right lobule VI of the cerebellar anterior lobe, while the RVM showed increased connectivity with the pre-SMA, bilateral PMd, bilateral postcentral gyrus, left superior parietal lobule, and bilateral lobule VI of the cerebellar anterior lobe in the after training stage rather than in the before training stage while performing the dual-task (paired t-test, p < 0.05, FWE corrected, Table 8). There was no significant difference in functional connectivity in the precuneus between the two scanning sessions in performing the dual-task.
Table 8.
Brain areas less activated or more functionally connected with the ROIs during performing dual-task at aftertrainingcondition than that at beforetraining stage.
| Brain region | Brodmann area |
MNI Coordinates | t value | Cluster size |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Brain regions less activated | ||||||
| LPre-SMA | 6 | −6 | 8 | 52 | 8.10 | 22 |
| L PMd | 6 | −34 | −17 | 54 | 8.32 | 20 |
| R PMd | 6 | 32 | −8 | 57 | 7.81 | 16 |
| R PostcentralGyrus | 40 | 39 | −33 | 54 | 6.82 | 15 |
| Brain regions more connected with the LCV | ||||||
| L Pre-SMA | 6 | −3 | 9 | 51 | 7.45 | 21 |
| R PMd | 6 | 28 | −12 | 54 | 7.28 | 16 |
| R Cerebellum, Anterior Lobe, Culmen, Lobule VI | 21 | −51 | −27 | 8.36 | 45 | |
| Brain regions more connected with the RVM | ||||||
| L Postcentral Gyrus | 3 | −34 | −33 | 58 | 7.89 | 36 |
| R Postcentral Gyrus | 3 | 29 | −34 | 55 | 6.35 | 11 |
| R Pre-SMA | 6 | 6 | 9 | 52 | 7.77 | 19 |
| L PMd | 6 | −29 | −12 | 54 | 8.14 | 26 |
| R PMd | 6 | 36 | −9 | 57 | 7.68 | 27 |
| L Cerebellum, Anterior Lobe, Culmen, Lobule VI | −26 | −42 | −30 | 8.68 | 69 | |
| R Cerebellum, Anterior Lobe, Culmen, Lobule VI | 30 | −45 | −32 | 8.51 | 68 | |
Brain regions less activated or more connected with the LCV or RVM at after training than that at before training stage during performing dual-task (paired t-test, p < 0.05, FWE corrected). Abbreviations: L, left; R, right; LCV, lobule V of left cerebellum;PMd, dorsal premotor cortex; Pre-SMA, rostral supplementary motor area; RVM, vermis of right cerebellum.
DISCUSSION
In the current study, we investigated brain activity and network interactions during dual-task performance. The novel finding is that some regions within the cerebellum additionally relate to executing a dual motor and counting task. Moreover, these cerebellar regions functionally connect to extensive brain networks in the dual-task condition.
A concern about dual-tasks is whether the critical mental operations of the two component tasks are executed simultaneously; that is, whether subjects actually perform them simultaneously or instead smoothly switch between the two tasks (Pashler 1994, 2001). Because our tasks were self-paced and no reaction time could be measured, we used intervals between finger movements of the sequences to examine if the subjects were switching between the two tasks. The intervals were stable. Performing the secondary (letter-counting) task did not influence movement intervals. Moreover, the rates of the two component tasks overlapped, which made switching between the two tasks unlikely. In addition, these two single tasks commonly activated the left PMd. It was suggested that dual-task interference is associated with overlapping cortical activation (Klingberg and Roland, 1997). A similar dual-task paradigm was used to study automaticity (Lang and Bastian, 2002; Wu et al., 2004; Wu and Hallett 2005a,b) or dual-task performance (Wu and Hallett 2008) in healthy subjects and Parkinson’s disease patients.
Our results provide further evidence concerning the existence of dual-task related areas. In addition, comparing results from the current study and our earlier study (Wu and Hallett 2008), in which the motor task was more difficult, demonstrates that the complexity of the component tasks is also an important reason as to whether dual-task related regions can be explored. A complex motor task activates extensive cerebellar areas, including the LCV and RVM (Wu et al., 2004; Wu and Hallett 2008). Therefore, we have difficulty in difficulty in distinguishing dual-task related activity from single task activations. Presumably, with further improved data analyze method, we can find out additionally activated areas while performing complex dual motor and cognitive tasks.
Previous studies identified certain added brain areas, such as the lateral prefrontal cortex (D’Esposito et al., 1995; Goldberg et al., 1998; Dove et al., 2000; Herath et al., 2001; Szameitat et al., 2002), left inferior frontal sulcus (Schubert and Szameitat, 2003), anterior cingulate cortex (Dreher and Grafman, 2003), or precuneus (Wenderoth et al., 2005; Mochizuki et al., 2007; Wu and Hallett 2008) that related to dual-task processing. This study, for the first time, identified that two subregions of the cerebellum, the left lobule V and right vermis are additionally activated for dual-task execution compared with single tasks. The dentate was also activated in the dual-task, but not in any component tasks (Fig. 3b, Table 2). However, as direct comparison between the dual and single tasks did not find activation in the dentate (Fig. 3C, Table 3), we cannot consider this region as a dual-task related area (Szameitat et al., 2011). Finding these different dual-task related areas suggests that the neural correlates of dual-task execution depend on the component tasks being combined. The prefrontal area is critical for dual cognitive tasks (D’Esposito et al., 1995; Goldberg et al., 1998; Dove et al., 2000; Herath et al., 2001; Szameitat et al., 2002), whereas the cerebellum is important in dual motor and cognitive tasks. The prefrontal area may be additionally activated when dual-tasks contain two response tasks, or two working memory tasks. Whether the cerebellum is also involved in dual-task including two motor tasks need further investigation.
According to our local activity and network connectivity results, we identified some distinct characteristics of the LCV and RVM in dual-task processing. Both areas functionally connect to extensive brain networks in the dual-task condition; in contrast, they only connect with limited areas for either the single-tapping or counting task alone (Figs. 4 and 5). The LCV and RVM functionally connect with both pre-SMA and SMA-proper in executing dual-task, but only connect with the SMA-proper while performing the tapping task, and connect with the pre-SMA while performing the counting task. Extensive studies found that the SMA-proper is primarily activated with regard to aspects of movement behavior, while the pre-SMA is usually associated with the cognitive aspects of a variety of tasks (Shima et al., 1996; Deiber et al., 1999; Jenkins et al, 2000; Kurata et al., 2000; Sakai et al., 2000). Although a common perception is that the pre-SMA is also a motor area, anatomical and physiological evidence suggests that the pre-SMA is more like a prefrontal area than a motor area, providing cognitive, sensory or motivational inputs for motor behavior (Picard and Strick 2001). Our findings that a tapping task activated SMA-proper and a counting task activated pre-SMA also support this classification (Table 2).
The LCV and RVM also functionally connect with other cerebellar regions, including both the anterior and posterior lobes. The cerebellum is involved in a wide range of neural processes, including motor and cognitive functions. For the single component tasks, the tapping task activated the ipsilateral cerebellar anterior lobe while the counting task activated the left cerebellar posterior lobe. These findings are consistent with previous reports that motor activation is located in the anterior lobe and the posterior lobe is involved in higher-order cognitive tasks (Stoodley and Schmahmann 2009). Functional connectivity reflects the integration within functionally specialized areas in a given task (Friston 1994). Thus, the phenomenon that the LCV and RVM functionally connect with almost all motor-related (i.e., M1, SMA-proper, PMd, and contralateral cerebellar anterior lobe) and cognitive-related (i.e., pre-SMA and cerebellar posterior lobe) areas activated in our dual-task, suggests that the LCV and RVM are parts of the executive networks for the dual-task; their role is likely to integrate motor and cognitive networks in order to perform motor and cognitive tasks simultaneously.
In addition, the LCV and RVM showed distinct patterns of training-associated changes. The poor performance of the dual-task compared to single tasks during the first fMRI session indicates that dual-task interference occurred at the before training stage. After training, dual-tasking was performed with high accuracy, proving that practice can diminish dual-task interference (Van Selst et al., 1999; Levy and Pashler 2001; Ruthruff et al., 2001, 2003; Schumacher et al., 2001; Erickson et al., 2007; Dux et al., 2009). The training process resulted in decreased activation of the pre-SMA, PMd, and superior parietal lobule, but activity in the LCV and RVM were unchanged. The decreased activity in a number of regions accompanied the diminishing of dual-task interference agrees with previous reports (Erickson et al., 2007) and may represent a training-induced increase in neural efficiency such that fewer neural demands are needed to perform the task (Poldrack and Gabrieli 2001; Wu et al., 2004; Erickson et al., 2007).
Performing a dual-task with high accuracy indicates that the component tasks are performed automatically (Passingham 1996; Wu et al., 2004). It was shown that when a new motor task is practiced until subjects can perform it without attention being clearly directed toward the details of the movement (automatic stage), major changes include not only reduced neural activity in a number of areas (Wu et al., 2004), but also a group of motor areas become more tightly connected (Wu et al., 2008). The combination of reduced activation and strengthening of functional connectivity suggests a more efficient neural code for controlling a given task. In the current study, in addition to local activity changes, we found that the LCV and RVM had strengthened connectivity with several regions, including both motor (i.e., lobule VI of cerebellar anterior lobe), and cognitive (i.e., pre-SMA) networks after training. The unchanged local activity and strengthened functional connectivity in the cerebellum suggests that, the neural activity of LCV and RVM themselves may not become more efficient; indeed, their role is likely to adjust both brain motor and cognitive networks to be more efficient in order to perform the dual-task properly.
The strengthened connectivity in the LCV and RVM after training supports the suggestion that the cerebellum may be important for shifting performance from an attentionally demanding state to a more automatic state (Lang and Bastian 2002). Presumably, damage to the cerebellum may impede the integration and efficiency of neural networks, which in turn will decrease the ability to shift the component tasks to be automatic, and will impair dual-task performance in cerebellar damaged patients (Lang and Bastian 2002). Most patients in that study (Lang and Bastian 2002) had damage in the vermis (e.g., vermal split, vermal tumor, olivopontocerebellar atrophy, or spinocerebellar atrophy type 6), which gave particular support to the idea that the vermis is critical in dual-task networks.
Agreeing with previous reports (Wenderoth et al., 2005; Mochizuki et al., 2007; Wu and Hallett 2008), we also found that the precuneus is related to dual-task performance. Additionally, this study found that the precuneus had functional connectivity with several brain regions and also included both motor- and cognitive- related areas (Fig. 6). However, unlike the LCV and RVM, the precuneus had no connectivity with the M1 or cerebellum. The precuneus had connectivity with a number of areas that did not activate with the dual-tasks, i.e., the occipital lobe, posterior cingulate cortex, and temporal lobe. Therefore, the involvement of the precuneus in the execution network and its role in integrating motor and cognitive networks is not as obvious as the LCV and RVM. In addition, as neither the local activity nor the interactions with brain networks were changed during the training process, the precuneus had no significant effect in adjusting brain networks to be more efficient in dual-task performance. A possible role of the precuneus in dual-task may be monitoring the operation of networks or switching attention between the two tasks (Wenderoth et al., 2005). These effects are constant in dual-task processing regardless of the state of automaticity.
Our findings suggest that when two different tasks are being performed at the same time, the neural networks for each task may not operate separately or in parallel. It is very likely these networks are being integrated into a single network by linkage of some distinct brain areas. Instead of only serving as the points of joining different networks, some regions may also be involved in modifying the networks to be more efficient. The ability to integrate and adjust various brain networks is possibly the neural basis that allows performance of multiple tasks at the same time in daily life.
An argument is that counting task may have motor components, the additionally activated cerebellum might to coordinate motor demands in the two component tasks. However, our counting task is simple, and it is unlikely the subjects need to make finger movements to help with counting. Children typically learn counting using finger-based representations, but counting becomes a more abstract process in adults (Moeller et al., 2011). Additionally, we did not There was no activity in the M1, which does not support the possibility that there were finger movements during counting. Recent studies (Kansaku et al., 2006, 2007; Zago et al., 2010) found that the left ventral premotor cortex was more activated during the enumeration of large numerosity compared to the enumeration of smaller numerosity, possibly because large numerosity is related to more subvocalizing of counting (Zago et al., 2010). In contrast, in our study, counting only activated the dorsal premotor cortex. Moreover, if these additionally activated cerebellar regions are due to coordination of motor demands, the activations of these regions should be different between the before and after training stages, as there must be less motor demand, and much easier coordination of motor demands after training. However, there was no difference of activity in these additionally activated cerebellar regions between before and after training stages. Thus, although we can not totally rule out motor involvement in our counting task, the additionally activated cerebellar regions are really likely dual-task related, but not due to coordination of motor demands.
The single motor and counting task commonly activated the left PMd. Although was traditionally regarded as a motor area, increasing evidence show that the PMd also has cognitive functions (Abe et al., 2007; Dennis et al., 2011). As both tasks may contain more or less motor and cognitive components, the overlapped activation in this area can be explained as either shared common cognitive, or motor, or both demands of the two single tasks, which needs further clarification.
A recent study found that heart rate has influence on activation in the cerebellum (Schlerf et al., 2012). Using heart rate as a regressor can reduce the noise in BOLD signal. As we did not monitor heart rate during fMRI scanning, the potential influence of heart rate was not regressed out in the current study. Although fluctuations in heart rate unlikely have significant influence on our findings, we will measure and regress heart rate in future studies to improve the quality of data analysis.
In conclusion, the current study found that the right cerebellar vermis, left lobule V of cerebellar anterior lobe and precuneus were additionally activated for dual-task performance (combining a right hand task and cognitive counting task). The cerebellar regions functionally connect with motor and cognitive networks, and these connections were strengthened after training. These cerebellar regions are parts of the executive networks (Buckner et al., 2011) and their role in dual motor and cognitive task-processing is likely to integrate motor and cognitive networks, and may modify these networks to be more efficient to perform the dual-task properly. The role of the precuneus in dual-task processes seems different and may well be to monitor the operation of active networks regardless of the state of automaticity.
Acknowledgments
This work was supported by grants from the National Science Foundation of China (grant numbers 30570530, 30870693 and 81071012). We wish to thank D. G. Schoenberg for skillful editing.
REFERENCES
- Abe M, Hanakawa T, Takayama Y, Kuroki C, Ogawa S, Fukuyama H. Functional coupling of human prefrontal and premotor areas during cognitive manipulation. J. Neurosci. 2007;27:3429–3438. doi: 10.1523/JNEUROSCI.4273-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock RA, Constable RT, Gore JC, Goldman-Rakic SG. Functional neuroanatomy of executive processes involved in dual task performance. Proc. Natl. Acad. Sci. USA. 2000;97:3567–3572. doi: 10.1073/pnas.060588897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Klingberg T, Jacobsen RB, Gabrieli JD. A resource model of the neural basis of executive working memory. Proc. Natl. Acad. Sci. USA. 2000;97:3573–3578. doi: 10.1073/pnas.050583797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibañez V, Sadato N, Hallett M. Medial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J. Neurophysiol. 1999;81:3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- Dennis A, Bosnell R, Dawes H, Howells K, Cockburn J, Kischka U, Matthews P, Johansen-Berg H. Cognitive context determines dorsal premotor cortical activity during hand movement in patients after stroke. Stroke. 2011;42:1056–1061. doi: 10.1161/STROKEAHA.110.597880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res. Cogn. Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Dreher J, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb. Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- Dux PE, Ivanoff J, Asplund CL, Marois R. Isolation of a central bottleneck of information processing with time-resolved FMRI. Neuron. 2006;52:1109–1120. doi: 10.1016/j.neuron.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron. 2009;63:127–138. doi: 10.1016/j.neuron.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kramer AF. Neural correlates of dual-task performance after minimizing task-preparation. Neuroimage. 2005;28:967–979. doi: 10.1016/j.neuroimage.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvarado M, Kramer AF. Training-induced functional activation changes in dual-task processing: an FMRI study. Cereb. Cortex. 2007;17:192–204. doi: 10.1093/cercor/bhj137. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and Effective Connectivity in Neuroimaging: A Synthesis. Hum. Brain Mapp. 1994;2:56–78. [Google Scholar]
- Goldberg TE, Berman KF, Fleming K, Ostrem J, Horn JDV, Esposito G, Mattay VS, Gold JM, Weinberger DR. Uncoupling cognitive workload and prefrontal cortical physiology: a PET rCBF study. Neuroimage. 1998;7:296–303. doi: 10.1006/nimg.1998.0338. [DOI] [PubMed] [Google Scholar]
- Herath P, Klingberg T, Young J, Amunts K, Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb. Cortex. 2001;11:796–805. doi: 10.1093/cercor/11.9.796. [DOI] [PubMed] [Google Scholar]
- Hsieh L, Young RA, Bowyer SM, Moran JE, Genik RJ, II, Green CC, Chiang YR, Yu YJ, Liao CC, Seaman S. Conversation effects on neural mechanisms underlying reaction time to visual events while viewing a driving scene: fMRI analysis and asynchrony model. Brain Res. 2009;1251:162–175. doi: 10.1016/j.brainres.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Seewer R, Nirkko AC, Eckstein D, Schroth G, Groner R, Gutbrodb K. Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. Neuroimage. 2003;19:210–225. doi: 10.1016/s1053-8119(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Resolving dual-task interference: an fMRI study. Neuroimage. 2004;22:748–754. doi: 10.1016/j.neuroimage.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Emery L, Zajac H, Thulborn KR. Interdependence of nonoverlapping cortical systems in dual cognitive tasks. Neuroimage. 2001;14:417–426. doi: 10.1006/nimg.2001.0826. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Cynkar J. A decrease in brain activation associated with driving when listening to someone speak. Brain Res. 2008;1205:70–80. doi: 10.1016/j.brainres.2007.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansaku K, Johnson A, Grillon ML, Garraux G, Sadato N, Hallett M. Neural correlates of counting of sequential sensory and motor events in the human brain. Neuroimage. 2006;31:649–660. doi: 10.1016/j.neuroimage.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Kansaku K, Carver B, Johnson A, Matsuda K, Sadato N, Hallett M. The role of the human ventral premotor cortex in counting successive stimuli. Exp. Brain. Res. 2007;178:339–350. doi: 10.1007/s00221-006-0736-8. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Concurrent performance of two working memory tasks: potential mechanisms of interference. Cere. Cortex. 1998;8:593–601. doi: 10.1093/cercor/8.7.593. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Roland PE. Interference between two concurrent tasks is associated with activation of overlapping fields in the cortex. Brain Res. Cogn. Brain Res. 1997;6:1–8. doi: 10.1016/s0926-6410(97)00010-4. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Kurata K, Tsuji T, Naraki S, Seino M, Abe Y. Activation of the dorsal premotor cortex and pre-supplementary motor area of humans during an auditory conditional motor task. J. Neurophysiol. 2000;84:1667–1672. doi: 10.1152/jn.2000.84.3.1667. [DOI] [PubMed] [Google Scholar]
- Lang CE, Bastian AJ. Cerebellar damage impairs automaticity of a recently practiced movement. J. Neurophysiol. 2002;87:1336–1347. doi: 10.1152/jn.00368.2001. [DOI] [PubMed] [Google Scholar]
- Levy J, Pashler H. Is dual task slowing instruction dependent? J. Exp. Psychol. Hum. Percept. Perform. 2001;27:862–869. [PubMed] [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part 1. Basic mechanisms. Psychological Review. 1997a;104:3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part 2. Accounts of psychological refractory-period phenomena. Psychological Review. 1997b;104:749–791. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Tashiro M, Gyoba J, Suzuki M, Okamura N, Itoh M, Yanai K. Brain activity associated with dual-task management differs depending on the combinations of response modalities. Brain Res. 2007;1172:82–92. doi: 10.1016/j.brainres.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Moeller K, Martignon L, Wessolowski S, Engel J, Nuerk HC. Effects of finger counting on numerical development - the opposing views of neurocognition and mathematics education. Front. Psychol. 2011;2:328. doi: 10.3389/fpsyg.2011.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual task in simple tasks: data and theory. Paychol. Bull. 1994;2:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Pashler H. Perception and production of brief durations: beat-based versus interval-based timing. J. Exp. Psychol. Hum. Percept. Perform. 2001;27:485–493. [PubMed] [Google Scholar]
- Passingham RE. Attention to action. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996;351:1473–1479. doi: 10.1098/rstb.1996.0132. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Medial wall motor areas: a review of their location and functional activation. Cereb. Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick P. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JD. Characterizing the neural mechanisms of skill learning and repetition priming: evidence from mirror reading. Brain. 2001;124:67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. J. Neurosci. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthruff E, Johnston JC, Van Selst M, Whitsell S, Remington R. Vanishing dual task interference after practice: has the bottleneck been eliminated or is it merely latent? J. Exp. Psychol. Hum. Percept. Perform. 2003;29:280–289. doi: 10.1037/0096-1523.29.2.280. [DOI] [PubMed] [Google Scholar]
- Ruthruff E, Johnston JC, VanSelst MA. Why practice reduces dual task interference. J. Exp. Psychol. Hum. Percept. Perform. 2001;27:3–21. [PubMed] [Google Scholar]
- Sadato N, Ibanez V, Campbell G, Deiber MP, Le Bihan D, Hallett M. Frequency-dependent changes of regional cerebral blood flow during finger movements: functional MRI compared to PET. J. Cereb. Blood Flow Metab. 1997;17:670–679. doi: 10.1097/00004647-199706000-00008. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Takino R, Miyachi S, Nielsen M, Tamada T. What and when: parallel and convergent processing in motor control. J. Neurosci. 2000;20:2691–2700. doi: 10.1523/JNEUROSCI.20-07-02691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Szameitat AJ. Functional neuroanatomy of interference in overlapping dual tasks: an fMRI study. Brain Res. Cogn. Brain Res. 2003;17:733–746. doi: 10.1016/s0926-6410(03)00198-8. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Seymour TL, Glass JM, Kieras DE, Meyer DE. Virtually perfect time sharing in dual task performance: Uncorking the central attentional bottleneck. Psychological Science. 2001;121:101–108. doi: 10.1111/1467-9280.00318. [DOI] [PubMed] [Google Scholar]
- Shaffer LH. Multiple attention in continuous verbal tasks.In: Attention and Performance, edited by Rabbitt PMA and Dornic S. New York: Academic Press; 1975. pp. 205–213. [Google Scholar]
- Shima K, Mushiake H, Saito N, Tanji J. Role for cells in the presupplementary motor area in updating motor plans. Proc. Natl. Acad. Sci. USA. 1996;93:8694–8698. doi: 10.1073/pnas.93.16.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: effects of performance and aging. Proc. Natl. Acad. Sci. USA. 2001;98:2095–2100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Lepsien J, Cramon DY, Sterr A, Schubert T. Task-order coordination in dual-task performance and the lateral prefrontal cortex: an event-related fMRI study. Psychol. Res. 2006;70:541–552. doi: 10.1007/s00426-005-0015-5. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Schubert T, Müller K, von Cramon DY. Localization of executive functions in dual-task performance with fMRI. J. Cogn. Neurosci. 2002;14:1184–1199. doi: 10.1162/089892902760807195. [DOI] [PubMed] [Google Scholar]
- Szameitat A, Schuberta T, Müller H. How to test for dual-task-specific effects in brain imaging studies - An evaluation of potential analysis methods. NeuroImage. 2011;54:1765–1773. doi: 10.1016/j.neuroimage.2010.07.069. [DOI] [PubMed] [Google Scholar]
- Van Selst MA, Ruthruff E, Johnston JC. Can practice eliminate the Psychological Refractory Period effect? J. Exp. Psychol. Hum. Percept. Perform. 1999;25:1268–1283. doi: 10.1037//0096-1523.25.5.1268. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur. J. Neurosci. 2005;22:235–246. doi: 10.1111/j.1460-9568.2005.04176.x. [DOI] [PubMed] [Google Scholar]
- Wu T, Chan P, Hallett M. Modifications of the interactions in the motor network when a movement becomes automatic. J. Physiol. 2008;586:4295–4304. doi: 10.1113/jphysiol.2008.153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005a;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. The influence of normal human ageing on automatic movements. J. Physiol. 2005b;562:605–615. doi: 10.1113/jphysiol.2004.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2008;79:760–766. doi: 10.1136/jnnp.2007.126599. [DOI] [PubMed] [Google Scholar]
- Wu T, Kansaku K, Hallett M. How self-initiated memorized movements become automatic: a fMRI study. J. Neurophysiol. 2004;91:1690–1698. doi: 10.1152/jn.01052.2003. [DOI] [PubMed] [Google Scholar]