Abstract
Evidence shows that artificially lowering body and brain temperature can significantly reduce the deleterious effects of brain injury in both newborns and adults. Although the benefits of therapeutic hypothermia have long been known and applied clinically, the underlying molecular mechanisms have yet to be elucidated. Hypoxic-ischemic brain injury and traumatic brain injury both trigger a series of biochemical and molecular events that cause additional brain insult. Induction of therapeutic hypothermia seems to ameliorate the molecular cascade that culminates in neuronal damage. Hypothermia attenuates the toxicity produced by the initial injury that would normally produce reactive oxygen species, neurotransmitters, inflammatory mediators, and apoptosis. Experiments have been performed on various depths and levels of hypothermia to explore neuroprotection. This review summarizes what is currently known about the beneficial effects of therapeutic hypothermia in experimental models of neonatal hypoxic-ischemic brain injury and traumatic brain injury, and explores the molecular mechanisms that could become the targets of novel therapies. In addition, this review summarizes the clinical implications of therapeutic hypothermia in newborn hypoxic-ischemic encephalopathy and adult traumatic brain injury.
Keywords: Apoptosis, hypothermia, hypoxic-ischemic encephalopathy, neonatal hypoxic-ischemic brain injury, neuroprotection, traumatic brain injury
1. INTRODUCTION
1.1. Therapeutic Hypothermia
The cause of the robust neuroprotective effects of therapeutic hypothermia has been investigated for decades. Hypothermia was first applied therapeutically as a local anesthetic [1]. In the 1930s-1940s hypothermia was developed as a therapeutic modality and utilized to treat head injury, tumors, and other conditions [2–4]. While profound hypothermia (producing body temperatures 20–25°C) was applied in cardiac surgical [5] and neurosurgical procedures [6], in the 1960s, mild hypothermia (body temperatures of 30–34°C) was investigated in attenuating ischemia-induced neurotoxicity. Even a small reduction in temperature provides significant neuroprotection after ischemic neuronal injury [1]. This finding stimulated the use of mild therapeutic hypothermia in various experiments to achieve neuroprotection using various techniques: invasive techniques involving cool saline infusion via femoral or inferior vena cava catheters and non- invasive techniques including use of cooling blankets, ice, etc. over the entire torso or selectively over the head.
In addition to modulating the numerous damaging processes initiated by trauma/ischemia, hypothermia also affects metabolic rate and the cerebral blood flow (CBF) [7], which is altered during different types of injury. CBF is usually reduced after focal or global ischemia, and is further reduced during the reperfusion phase when hyperemia occurs. Some studies show that hypothermia increases CBF during ischemia [8], while others suggest that it has no effect [9]. Brain oxygenation and glucose concentration decrease during hypoxic and ischemic conditions and cause ATP depletion, lactic acid accumulation and acidosis that eventually leads to cell death [10]. Hypothermia reduces cerebral oxygen consumption, tends to increase hemoglobin oxygen-binding affinity, and inhibits the synthesis, release, and/or reuptake of ischemia-induced neurotransmitters and neuromodulators, including glutamate, glycine, GABA, dopamine, norepinephrine, serotonin, and adenosine [11, 12]. Mild to moderate hypothermia inhibits the release of reactive oxygen species including hydrogen peroxide [13], thiobarbituric acid-reactive substances produced after hemorrhage shock [14], and hydroxyl radical formation after traumatic brain injury (TBI) [15].
1.2. Neonatal Hypoxic-Ischemic Brain Injury and Hypoxic-Ischemic Encephalopathy
Perinatal hypoxic ischemic brain injury (HIBI) from a variety of etiologies (including acute perinatal asphyxia, brain hemorrhage, stroke, birth trauma, infections, metabolic disorders, and congenital brain abnormalities) produces hypoxic–ischemic encephalopathy (HIE) in neonates and preterm infants. HIE in newborns is a major cause of death and disability, as up to 60% of infants with moderate or severe HIE die during the neonatal period, and at least 25% of the survivors have significant long-term neurodevelopmental consequences [16] such as epilepsy, mental retardation or cerebral palsy [17]. HIE is a major contributor to global child mortality and morbidity occurring in an estimated 2.5 of every 1000 term births in developed countries [18], with a ten-fold higher incidence (26 per 1000 births) in the developing world [19]. We will focus on the neuroprotection offered by hypothermia in experimental models of neonatal HIBI and discuss the clinical implications of hypothermia in newborn HIE. The search is underway for novel therapeutic regimens targeting pathways that might prevent perinatal injury and/or repair neuronal damage and reduce long-term morbidity [20]. These initiatives have surged along with interest in the molecular mechanisms underlying the neuroprotection induced by hypothermia.
Brain injury in HIE is a complex evolving process initiated at the time of the initial insult extending into the recovery period. HIE results from two recognized phases: primary and secondary energy failure [21, 22]. Primary energy failure occurs during the HI insult and is characterized by decreased cerebral blood flow, which in turn reduces delivery of oxygen and substrates to brain tissue [23]. Following primary energy failure, the cerebral metabolism may recover through reperfusion– reoxygenation, only to deteriorate from secondary energy failure, which is this new phase of neuronal damage, starting at about 6–24 hours after the initial injury mostly characterized by mitochondrial dysfunction, and initiation of the apoptotic cascade. The interval between primary and secondary energy failure represents a latent phase with restoration of cellular energy metabolism, offering an optimal window for neuroprotective interventions such as therapeutic hypothermia.
1.3. Traumatic Brain Injury
TBI, an important cause of morbidity and death, is a major public health problem in the United States and worldwide. Approximately 500,000 new patients suffer from TBI every year in the United States, and as many as 5 million Americans are currently living with the long-term consequences of a previous TBI episode. Moreover, because TBI tends to affect young people disproportionately, the number of productive years lost as a result is staggering compared with other chronic diseases. TBI was originally defined in the 1970s. Similar to the definition of HIE, traumatic brain lesions are defined as primary injuries (inflicted to the brain immediately on impact) and secondary lesions (set in motion by the impact but appearing within minutes, hours, or days after injury) [24, 25]. In 1990, the new term 'tertiary injury' was proposed to define all anomalous cellular events induced by trauma [26]. We and other groups have investigated the pathophysiological mechanisms underlying CNS injury in TBI [27–30]. At the cellular level, traumatic events are mediated by several basic mechanisms: excitotoxicity, free radical–induced alterations, inflammatory events, and calcium-mediated damage [7].
Since hyperthermia is common following TBI, hypothermia has been established as an effective neuroprotective treatment. The administration of artificial hypothermia or 'hibernation' for treatment of TBI cases has been documented since the 1950s. For example, the use of hypothermia “cured” two cases of cerebral concussion [31]. In severe cranium trauma, artificial hypothermia has been used as a supportive measure [32]. In recent years the use of mild therapeutic hypothermia as a means of neuroprotection has become important in the treatment of TBI [33, 34]. Experimental studies over the last decade have reconfirmed that moderate hypothermia confers protection against TBI [7]. Multiple methods of inducing selective cerebral hypothermia are currently in experimental phases, including surface cooling, intranasal selective hypothermia, transarterial or transvenous endovascular cooling, extraluminal vascular cooling, and epidural cerebral cooling [35]. In studies of therapeutic hypothermia for TBI, experimental animal models in vivo [30, 36], organotypic cultures ex vivo [37–39], and cultured cell models in vitro [40] have been used.
2. MOLECULAR TARGETS OF HYPOTHERMIA
2.1. Neuroprotection by Hypothermia in HIBI and HIE
Primary energy failure is associated with acute intracellular derangements, including loss of membrane ionic homeostasis, release and blocked reuptake of excitatory neurotransmitters, defective osmoregulation, and inhibition of protein synthesis [41]. Stimulation of excitatory neurotransmitter receptors causes an increase in intracellular calcium, which in turn sets off a cascade of destructive pathways, ultimately resulting in acute cell death and necrosis [42]. The pathogenesis of secondary energy failure is not as well understood as primary energy failure, but likely involves multiple pathophysiologic processes, including the accumulation of excitatory neurotransmitters such as glutamate, oxidative injury, anaerobic metabolism and free radical production, inflammation, and ultimately initiation of apoptosis. The secondary phase of injury occurs slowly (within hours to days) in the setting of normal intracellular pH and stable cardiorespiratory status [21]. Much of the injury following secondary energy failure is related to apoptosis. In contrast to the cell membrane disruptions that lead to necrosis in primary energy failure, apoptosis is a more nuclear phenomenon, ultimately resulting in DNA fragmentation and condensation [43]. Although acute cellular necrosis and apoptosis are both noted in post-hypoxic–ischemic injury in animal models and human infants, apoptotic cell death appears to be a more significant contributor to damage in the developing brain of the term infant and is the major component of secondary energy failure [44, 45] (Fig. 1 and Table 1).
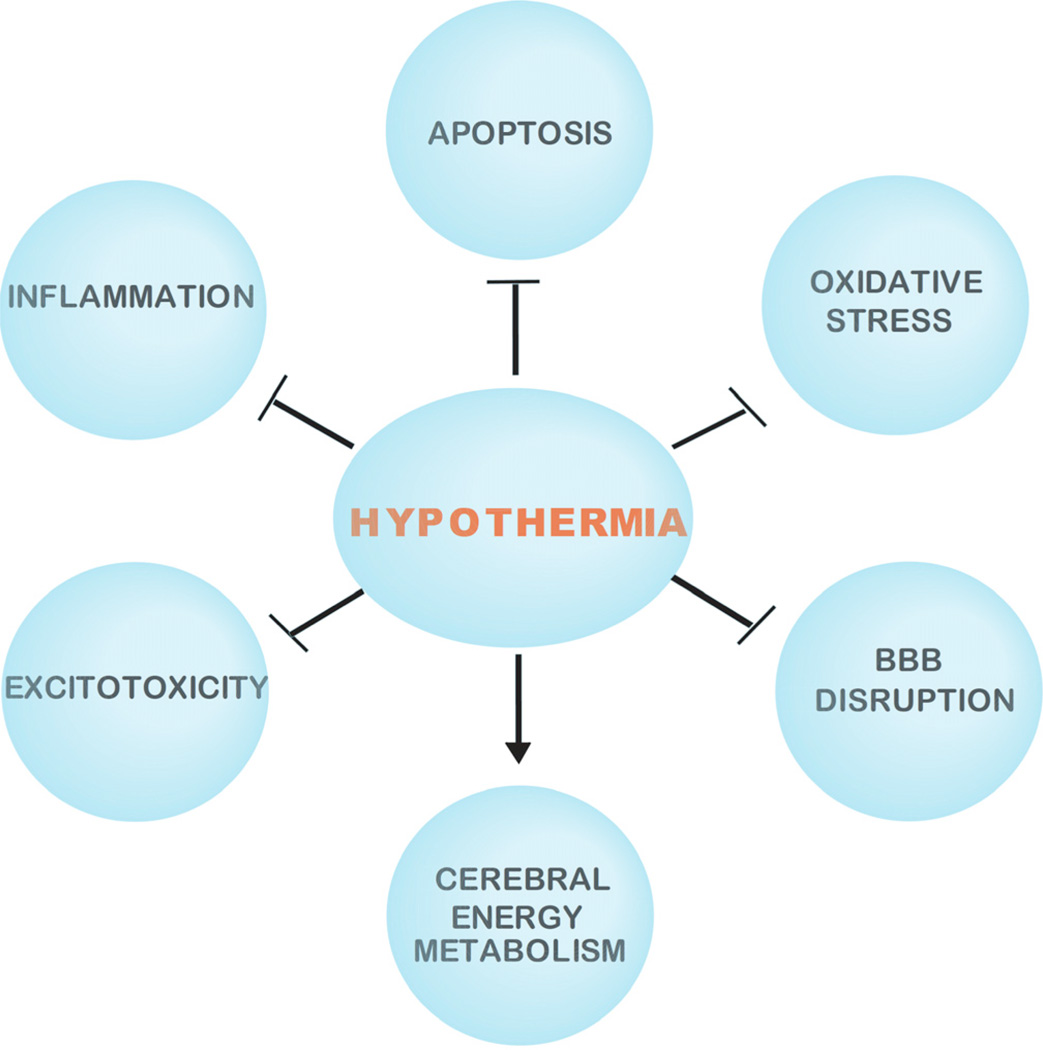
Fig. 1. Protective mechanisms of hypothermia in HIBI and TBI.
The neuroprotection of hypothermia in HIBI and TBI is mediated by various mechanisms including the inhibition (⊥) of oxidative stress, apoptosis, inflammation, and excitotoxicity, as well as the enhancement (→) of cerebral energy metabolism. In addition hypothermia reduces BBB disruption in TBI (⊥).
Table 1.
Molecular Effects of Therapeutic Hypothermia on HIBI and TBI
| Injury Types |
Protective Mechanism |
Hypothermia (°C) |
Hypothermia Length (Hour) /Condition |
Molecular Effect | Model | Refs. |
|---|---|---|---|---|---|---|
| HIBI | Anti-apoptotic | 34.9 | 12 post-ischemic |
Reduce apoptotic cell death | Piglets | [44] |
| HIBI | Anti-apoptotic | 30 | 72 post-ischemic |
Suppress cytochrome c release and activation of caspase-3 and calpain |
Wistar rat pups |
[46] |
| HIBI | Anti-apoptotic | 30 | 10 post-ischemic |
Reduce levels of cytochrome c and activation of caspase-3 and −2 |
Wistar rat pups |
[47] |
| HIBI | Anti-apoptotic | 30 | 24 post-ischemic |
Decrease phosphor-Akt and caspase-3 levels, total Akt levels remain unchanged |
Sprague- dawley rat pups |
[50] |
| HIBI | Anti-apoptotic | 30–32 | >24 | Reduce caspase-8 and −9 activity, delay caspase-3 activation, inhibit cytochrome c and AIF release, and attenuate markers of oxidation |
PC12 cells | [53] |
| TBI | Anti-apoptotic | 32 | 72 /combination with propofol |
Decrease tissue trauma | Organotypic hippocampal brain slices |
[39] |
| TBI | Anti-apoptotic | 30.8 | 24 post-injury |
Suppress JNK activation and prevent apoptosis |
Sprague- Dawley rats |
[63] |
| TBI | Anti-apoptotic | 32 ± 0.5 | 2 | Decrease calpain activity | Cultured rat neurons |
[65] |
| TBI | Anti-apoptotic | 32 | 24, 72 | Decrease the levels of caspase-3 mRNA and immunoreactivity and attenuate TIMP-3 activation |
Sprague- Dawley rats |
[66] |
| TBI | Anti-apoptotic | 30 | 0.75/pre-injury 1/post-injury |
Attenuate MAP-2 degradation | Rats | [67] |
| HIBI | Anti- inflammatory |
33 | 72 | Reduce levels of IL-6, IL-10, and NO | Neonatal wistar rats Microglia culture |
[54] |
| TBI | Anti- inflammatory |
30 | 1.5 | Cold pre-conditioning evoke neuroprotection with the involvement of TNF-α, and IL-11 |
Rat hippocampal slices |
[69] |
| TBI | Anti- inflammatory |
35 | 4 post-injury |
The enhancing capability of heat acclimation mice to maintain hypothermia after injury may contribute to the functional benefit of the acclimation process |
Mice | [68] |
| TBI | Anti- inflammatory |
33 | 3 | Downregulate inflammatory response genes effects on synapse organization, Biogenesis (upregulated) and regulate the mRNA expression of Ank3, Cmbp, Nrxn3, Tgm2, and Fcgr3 |
Rat hippocampus |
[36] |
| TBI | Anti- inflammatory |
32–35 | <24 | Attenuate MMP-9 and IL-6 levels | Human patients |
[70] |
| HIBI | Anti-oxidative | 33–34 | 72 | Decrease rise in CRP and slower rise in hydroperoxides |
Term infants | [56] |
| TBI | Anti-oxidative | 32–33 | 24 post-ischemia |
Reduce astrocytes and inflammatory cytokine levels |
Sprague dawley |
[55] |
| HIBI | Anti-excitation | 34 | 3 | Suppress NMDA receptor phosphorylation and protein oxidation |
Piglets | [57] |
| TBI | Anti-excitation | 23, 32 | 0.5, 1 | Attenuate the uptake of extracellular glutamate via hGLT-1 |
Chinese hamster ovary cells |
[40] |
| TBI | Anti-excitation | 30 | 24 /after 0.5–1.67 hour of oxygen glucose deprivation |
Reduce the release of glutamate to the bathing medium |
Murine cortical cultures |
[72] |
| TBI | Anti-excitation | 35 | 1–3 | Attenuate excitatory damage to hippocampal neurons |
Rats | [73] |
| HIBI | Enhance energy metabolism |
33, 35 | 24 | Prolong nucleotide triphosphate/ phosphate pool and reduce the secondary fall in phosphocreatine/inorganic phosphate ratios |
Piglets | [59] |
| HIBI | Enhance energy metabolism |
31 | 31 intra-ischemic |
Increase high energy phosphates and glucose levels, decrease lactate levels |
Rat pups | [58] |
| TBI | Reduce BBB disruption |
30 or 33 | 1 /alone or combination with FK506 |
Reduce BBB disruption following TBI, significantly enhance vascular and axonal protection, and prevent BBB dysfunction |
Rats | [76] [81] |
| TBI | Protect Cerebral Circulation |
32 | 1.5 | Protect cerebral microcirculation | Rats | [80] |
| TBI | Anti- brain edema |
23.17 + 0.95 25.19 + 0.76 30.53 + 0.8 30.37 + 0.67 27.33 + 1.13 27.43 + 1.18 |
5 / combination with decompressive craniectomy |
Reduce posttraumatic brain edema | Male CD-1 mice |
[78] |
Explanation of abbreviations can be found in the list of abbreviations.
2.1.1. Anti-Apoptotic Effects of Hypothermia on HIBI
Various studies have examined the beneficial effects of hypothermia at the molecular level in different animal models. It has been determined that post-insult mild therapeutic hypothermia (34.9°C for 12 hours beginning soon after resuscitation) reduces apoptotic cell death in piglets [44]. The effects of prolonged hypothermia (30°C for 72 hours) begun immediately after HI insult have been evaluated in P7 Wistar rats [46]. In this setting, not only were the number of apoptotic cells in the parietal cortex and hippocampus reduced, but also was cytochrome c release at 24–72 hours post HI (by western blot) and also the number of cytochrome c-immunopositive cells at 24–160 hours (by immunohistochemistry staining) [46]. Caspase-3 levels in the induced hypothermia groups were lower than in the normothermic group 24–48 hours post HI [46]. A study of systemic induced hypothermia (30°C for 10 hours immediately after HI insult) in P7 Wistar rat pups also showed anti-apoptotic neuroprotective effects [47]. The brain infarct volumes as evaluated by loss of MAP-2 staining were reduced in cortex, thalamus, and hippocampus in the hypothermia group animals. The number of cytochrome c-positive cells was significantly reduced in cortex and dentate gyrus. The activation of caspase-3 and −2 was also significantly reduced at 24 hours post HI injury in the hypothermic group [47]. In addition, the expression of IL-18 was reduced 24, 48, and 72 hours after treatment of neonates with moderate or severe HIE by selective head cooling [48]. These results indicate that hypothermia may act at least partially through inhibition of the intrinsic pathway of caspase activation in the neonatal brain, thereby preventing apoptotic cell death. Mild hypothermia (34°C) decreases expression of caspase-3 mRNA and lowers caspase-3 enzyme activity [49]. Protein kinases like Akt have been implicated in inhibition of apoptosis. Although active Akt plays a role in the immature brain by supporting neuron survival in the absence of trophic factors, it has not been implicated in the neuroprotective effects of hypothermia. This was shown in Sprague-Dawley rat pups treated with 30°C hypothermia for 24 hours post ischemia; the levels of caspase −3 were reduced, but the phosphorylation of Akt post HI was also found to be decreased, while total Akt remained unchanged [50]. This indicated that hypothermia may have some depressant effects on both cell death and cell survival signal pathways, and that Akt may not play a major role in the neuroprotective effect of hypothermia in the immature brain [50]. In infants with HIE, elevated levels of FeSO4 and ascorbic acid are seen in the cerebrospinal fluid compared with normoxic infants [51]. The synergistic effect of FeSO4 and ascorbic acid induces apoptosis in PC12 cells through formation of hydroxyl radicals, inducing lipid peroxidation [52]. Differentiated PC12 cells were more susceptible than undifferentiated cells. Moderate hypothermia (30–32°C) improved the viability of differentiated PC12 cells subjected to toxicity mediated via 1 mmol/L FeSO4 and ascorbic acid as compared with normothermia. The exposure was continued for 48 hours [52]. Hypothermia was induced within 6 hours after the insult and when continued until 24 hours or longer, improved cell viability at 48 hours [52]. Several important steps in the apoptotic cascade activated by Fe and ascorbic acid were inhibited by hypothermia [53]. Caspase-3, −8, and −9 activities measured at different times showed that hypothermia reduced total caspase activity as compared with untreated cells. Caspase-3 activation was found to be significantly reduced at 24 hours [53]. Cytochrome c and apoptosis inducing factor (AIF) levels were also reduced. Akt phosphorylation was minimally affected by hypothermia [53]. Hypothermia also reduced the oxidative damage induced by Fe and ascorbic acid, as shown by the significantly lower level of oxidation products and products of lipid peroxidation at 32°C compared with 37°C [53].
2.1.2. Anti-Inflammatory Role of Hypothermia in HIBI
Activated microglia produce potentially cytotoxic factors including IL-6, IL-10, and nitric oxide, which exacerbate neuronal injury. In microglia isolated from neonatal Wistar rats treated with induced hypothermia at 33°C for 72 hours, levels of pro-inflammatory cytokines and nitric oxide were reduced [54]. In neonatal Sprague-Dawley rats similarly treated with induced hypothermia at 32–33°C for 24 hours after ischemia, the expected increase in GFAP-mRNA transcription was significantly reduced at all time points. The number of astrocytes was reduced by hypothermia treatment at 3 and 7 days. The levels of inflammatory cytokines TNF- α mRNA and IL-6 mRNA were also lower in the hypothermic group compared to the normothermic group; in addition, the infarct volume in CA1 hippocampus after HI was reduced [55]. Finally, the inhibition of calcium-calmodulin-dependent protein kinase II triggered by normothermic ischemia is prevented by hypothermia [1].
2.1.3. Anti-Oxidative Role of Hypothermia in HIBI
Oxidative stress plays a key role in the morbidity associated with HIBI. Though oxidative stress occurs in all neonates irrespective of hypoxia, its effect is magnified by hypoxic conditions. Free radicals produced by the Fenton reaction exert damaging effects even at 7 days after birth. It has been determined that therapeutic hypothermia can play an anti-oxidant neuroprotective role. The levels of hydroperoxides and kinetics of C-reactive protein were increased during the first 72 hours irrespective of treatment [56]. However, with application of therapeutic hypothermia, a tendency towards a slow increase in hydroperoxides was observed in the first postnatal days. Among a group of term infants randomly selected for hypothermia or normal care (normothermia), the increase in C-reactive protein was also lower in the hypothermic than in the normothermic infants. Thus therapeutic hypothermia has been suggested to have an antioxidant role post-asphyxia [56].
2.1.4. Effect of Hypothermia on Excitotoxicity
Brain damage caused by perinatal HI is partially mediated by excitotoxic mechanism while the N-methyl-D-aspartate (NMDA) receptor and intracellular signaling pathways have important roles in perinatal brain injury. Rapid NMDA receptor phosphorylation is elevated after HI in the newborn piglets model of HI [57]. Postasphyxic, mild whole body hypothermia by applying water circulating blankets, provides neuroprotection after HI in newborn piglets by suppressing NMDA receptor phosphorylation and protein oxidation [57].
2.1.5. Effect of Hypothermia on Energy Metabolism
ATP is crucial for maintaining the energy-demanding processes of brain cells, including cell membrane integrity and the active transport of various ions. The loss of high-energy phosphates has been implicated as one of the mechanisms initiating cellular dysfunction in HIBI. In 7-day-old rat pups, HI was induced and intraischemic mild hypothermia at 31°C or 34°C was administered [58]. At 31°C the blood glucose levels were considerably higher than in the 34°C or 37°C groups, and lactate levels mirrored the glucose levels in all groups. Higher phosphocreatine and ATP levels were also seen in 31°C group animals, indicating the neuroprotective role of hypothermia on cerebral metabolism [58]. High-energy phosphate depletion is also attenuated by hypothermia, improving post-ischemic metabolic recovery. Delayed hypothermia (either 35 or 33°C) induced in piglets starting 2 hours after resuscitation and lasting for 24 hours, preserved the nucleotide triphosphate/exchangeable phosphate pool and reduced the secondary fall in phosphocreatine/inorganic phosphate ratios [59].
2.2. Neuroprotection by Hypothermia in Traumatic Brain Injury
Many posttraumatic adverse events at the cellular and molecular level in TBI are highly temperature-sensitive and are therefore a good target for treatment with hypothermia [60]. The basic mechanisms through which hypothermia protects the traumatically injured brain are multifactorial including a reduction in brain metabolic rate and brain thermo pooling, and effects on cerebral blood flow and the white matter [7]. However, in this review, we will focus on the common neuroprotective mechanisms of hypothermia in TBI and HIBI (Fig. 1 and Table 1):
2.2.1. Modulation of Apoptotic Cell Death
Because apoptotic cell death is the most important mechanism contributing to cell loss following head injury [61], modulation of apoptotic cell death after TBI through hypothermia has been investigated. The MAPK family includes c-Jun NH2-terminal kinase (JNK), extracellular signal regulated kinase (ERK), and p38 mitogen-activated protein kinase (p38 MAPK); another kinase is MAP kinase kinase (MEK). The activation of JNK, ERK, and MEK has been implicated in TBI [62, 63]. Hypothermia protects against TBI via early suppression of JNK activation and subsequent prevention of apoptosis in astrocytes. Manipulation of the JNK pathway in astrocytes may become a therapeutic strategy for ameliorating the devastating progression of tissue damage after TBI [63]. Moreover, hypothermia has been found to stimulate early activation of signaling intermediates in the JNK, but not the IKK (IκB kinase)-NF- κB (nuclear factor κB) signaling pathways. TNFR1 (tumor necrosis factor receptor-1) signaling after TBI was associated with increased TNFR1 expression, and hypothermia treatment significantly reduced TNFR1 levels 15 and 30 mins after TBI [64]. Hypothermia promoted a rapid activation of caspase-3 acutely after injury but suppressed caspase-3 activation at later time points. These data suggest that hypothermia may regulate both the JNK signaling cascade via X-linked inhibitor of apoptosis protein (XIAP) and the preconditioning pathways that activate caspases. Current interventional therapies have been linked with targeting caspase-mediated apoptosis [20]. Hypothermia mediates TNFR1 responses via early activation of the JNK signaling pathway and caspase-3, leading to endogenous neuroprotective events [64], providing more evidence that therapeutic hypothermia targeting caspase-mediated apoptosis is a viable neuroprotective strategy in TBI.
Calpains are calcium-activated cytoplasmic proteases that disrupt the cytoskeleton and nucleus; thus, increases in intracellular calcium can cause cells to undergo apoptosis. Hypothermia is a calcium antagonist, and it has been suggested that reduced calcium influx is one of the key mechanisms underlying hypothermia-induced neuroprotection [7]. Calpain activity and levels of apoptotic protein TIMP-3 increase following fluid percussion injury, resulting in cellular injury and death. Induction of hypothermia at 32 +/− 0.5°C regulates the calpain changes and provides protection against TBI as shown in in vitro cultured rat neurons [65]. Further, 32°C hypothermia decrease the levels of caspase-3 mRNA and immunoreactivity and attenuated the activation of TIMP-3 after fluid percussion injury in Sprague-Dawley rats [66]. Hypothermia at 30°C attenuates calpain-mediated degradation of microtublule-associated protein 2, thus reducing cytoskeletal damage in the hippocampus following TBI [67].
2.2.2. Modulation of the Inflammatory Response
As mild hypothermia has been linked to the attenuation of inflammatory-associated entities, long-term exposure to moderate ambient heat (heat acclimation, HA, 30 days at 34+/−1°C) provides protection against TBI. It has been reported that HA mice display sustained post-injury hypothermia and demonstrate enhanced pre-injury IL-10 expression of anti-inflammatory mediators or a reduction in postinjury TNF α expression [68]. Moreover, given that slice cultures ex vivo closely resemble their in vivo counterpart, it was not surprising that in rat hippocampal slice cultures ex vivo, cold preconditioning coupled with blockade of IL-11 signaling enhanced neuroprotection compared to that seen with cold pre-conditioning alone [69].
Elevation of matrix metalloproteinase-9 (MMP-9) produces inflammatory changes following TBI. Also, the elevation of MMP-9 is correlated with interleukin-6 (IL-6) in arterial and internal jugular venous blood following TBI. Hypothermia attenuated the elevated levels of MMP-9 and hence provided neuroprotection by an anti-inflammatory mechanism [70].
2.2.3. Anti-Oxidative Stress
The pathological sequelae of TBI include increased oxidative stress due to the production of reactive oxygen species. Free radical-induced oxidative damage reactions and lipid peroxidation are among the best-validated injury mechanisms in preclinical TBI models. Patients treated with hypothermia were cooled to 32–33°C (within approximately 6 h of injury) for either 24 or 48 h and then re-warmed. Levels of F(2)-isoprostane (a marker of lipid peroxidation) were assessed in ventricular cerebrospinal fluid (CSF) samples. Hypothermia tended to decrease F(2)-isoprostane levels only in male adult patients after TBI [71]. The results suggest that gender should be an important consideration in clinical hypothermia trial design, particularly in the case of antioxidant strategies.
2.2.4. Blockade of Excitotoxic Mechanisms
Inhibition of glutamate release and modulation of excitotoxic brain damage in TBI is an important mechanism underlying hypothermic neuroprotection [7, 15]. To elucidate molecular mechanisms of hypothermia acting on excitotoxicity, a human glial glutamate transporter (hGLT-1) cDNA was cloned and stably transfected into Chinese hamster ovary cells. Experiments demonstrate that hypothermia attenuated the uptake of extracellular glutamate via hGLT-1 in a temperature- and time-dependent manner [40]. Moreover, the neuroprotective effects of hypothermia are observed in murine cortical cultures exposed to insults such as excitatory amino acids or oxygen glucose deprivation (OGD). Hypothermia during and after a period of OGD reduced the release of glutamate [72]. In addition, fluid percussion injury (FPI) induces hyperactivity of the excitatory synapses in CA1 hippocampal pyramidal neurons, and mild hypothermia at 35°C maintained for 1–3 hours after 15 mins of FPI attenuates the excitatory damage to hippocampal neurons [73]. Taken together, these studies confim the neuroprotective effects of hypothermia via the blockade of excitotoxicity.
2.2.5. Attenuate BBB Permeability and Decrease Edema Formation
Blood brain barrier (BBB) leakage facilitates abnormal passage of blood-borne substances that might result in secondary brain injury [74]. In addition, increased posttraumatic vascular permeability results in edema and activates several inflammatory cascades culminating in neuronal cell death. Disruption of BBB has been implicated in posttraumatic epilepsy and posttraumatic concussion [75]. Increased vascular permeability has been noted early (after 4 hours and after 3 days) and late (7 days) after FPI in Sprague-Dawley rats. In the early posttraumatic period, both large- and small-molecular-weight tracer substances leak into the brain parenchyma as evidence of BBB leakage. However, in the longer posttraumatic period only small-molecular-weight tracer substances leaked. Temperature modifications can prevent BBB leakage and influence both neuronal viability and vascular permeability. The protective effects of moderate hypothermia on BBB permeability have been confirmed in TBI rats [76]. Moreover, moderate posttraumatic hypothermia at 33°C for 4 hours reduces the permeability of the BBB as evidenced by reduction in the extravasation of BDA-3K in the ipsilateral rat cortex and hippocampus after moderate TBI. A temporal and spatial relationship was found between BBB and CD-68 positive macrophage infiltration; stereological analyses of cortex and hippocampus stained by anti-CD 68 antibodies show fewer macrophages in the hypothermia group than in the normothermia group [77].
Hypothermia acts not only on the modulation of BBB permeability, but also on the consequent decreases in the edema formation [60]. A recent investigation of the effects of selective brain hypothermia and decompressive craniectomy on brain edema after closed head injury in mice furthermore demonstrated that focal hypothermia may be effective in the treatment of posttraumatic brain edema [78].
Specifically, on the basis of our current understanding of its action, hypothermia should be introduced relatively early in the treatment of TBI, with special attention paid to gradual rewarming in conjunction with the introduction of other treatment strategies such as the drug FK-506, which has already proven its potential to inhibit diffuse brain injury associated with post-hypothermic rewarming [70].
In posttraumatic hypothermia, the duration of initiation, duration, as well as the speed of posttraumatic rewarming have been recognized as key factors determining the degree of neuroprotection in both animal models and clinical applications [79, 80]. The neuroprotective benefits of posttraumatic hypothermia followed by slow rewarming have been demonstrated to extend the protection of cerebral microcirculation in rats with TBI [80].
Another promising approach is to combine pharmacological neuroprotective agents with mild hypothermia [62]. Either delayed hypothermia or administration of the immunophilin ligand FK506 after TBI provided limited protection, while combinational therapy using both posttraumatic hypothermia and FK506 in rats attenuated the most dangerous features of TBI by significantly enhancing vascular and axonal protection, but lent no protection against changes in the BBB. Thus hypothermia is not only protective but also extends the therapeutic window for improved FK506 efficacy against TBI [81]. In addition, the anesthetic agent propofol (2,6-diisopropylphenol) has been shown to be an effective neuroprotective agent in different models of brain injury. In an organotypic hippocampal brain-slice cultures ex vivo model of TBI, slices were subjected to focal mechanical trauma and subsequently treated with propofol under both normo-and hypothermic conditions. The dose-dependent neuroprotective effect of propofol is additive to the neuroprotective effect of hypothermia [39].
2.2.6. Effect of Hypothermia on Energy Metabolism and Gene Expression
The energy crisis and membrane phospholipid degradation in perilensional tissue are easier to happen after TBI, and mild hypothermia shows protective effect on glucose metabolism and glycerol of brain tissue in patients with severe traumatic brain injury [33]. To evaluate the role of hypothermia on gene expression, the effect of mild hypothermic therapy on the genomics of the hippocampus after moderate TBI in rats was investigated. Posttraumatic mild hypothermia had a significant effect on the gene expression profiles of the hippocampus, especially those genes belonging to the nine gene ontology categories most affected: synapse organization, biogenesis (upregulated), and inflammatory response genes (downregulated). In addition, hypothermia regulates the mRNA expression of Ank3, Cmbp, Nrxn3, Tgm2, and Fcgr3 [36].
3. CLINICAL IMPLICATIONS OF HYPOTHERMIA
3.1. Clinical Implications of Therapeutic Hypothermia in HIBI and HIE
Evidence from multicenter randomized trials indicates that therapeutic hypothermia can significantly improve clinical and neurologic outcomes in term infants with HIE [82]. It has been documented that post hypoxic-ischemic hypothermia improves the outcome of HIBI and HIE [82].
Neonatal encephalopathy may present as altered consciousness, abnormal muscle tone or reflexes, altered respiration, and sometimes seizures. HIE is brain injury resulting from inadequate blood flow and oxygen delivery to the brain. Until recently, management of a newborn with HIE has consisted largely of supportive care to restore and maintain cerebral perfusion, providing adequate gas exchange, and treatment of seizure activity as no specific neuroprotective treatments were available. Although animal models of HI injury using fetal sheep, neonatal pigs, and neonatal rats have shown the benefits of hypothermia, including reduced neuronal loss and improved survival and functional outcome [83–85], it was only recently that this therapy was brought to clinical use in humans [86]. The knowledge that brain injury after HI is an evolving process has provided a “window of opportunity” for therapeutic interventions that may arrest or reduce secondary brain injury. Hypothermia has now emerged as the single most promising intervention for infants with neonatal encephalopathy and HIE. For every 1°C reduction in temperature from normothermic values, brain energy utilization was reduced by approximately 5% [87]. Therapeutic hypothermia aims to lower the temperature of the vulnerable deep brain structures, the basal ganglia, to 32–34°C. Hypothermia in newborns can be accomplished through whole-body cooling (placing the infant on a cooling blanket or mattress circulated with coolant fluid to maintain esophageal/rectal temperature of 33°C-34°C) and selective head cooling with mild systemic hypothermia (circulating cold water in a cap fitted around the head, reducing rectal temperature to 34°C-35°C) [88]. These two methods of therapeutic hypothermia have generally been found to be equally safe and effective, although a small study by Rutherford et al. reported a lower incidence of severe cortical lesions on MRI in infants with encephalopathy treated with selective head cooling compared with infants treated with whole-body cooling [88]. These results may be due to differential temperature gradients demonstrated by selective head cooling with associated colder temperature in the cortex [89, 90]. Nevertheless, more research is needed to determine whether one of these cooling methods is superior. Studies in fetal sheep subjected to HI suggest that the potential window for treatment in animal models may be up to 6 hours post injury; this finding has led to the incorporation of a 6-hour therapeutic window into protocols of clinical trials to maximize the neuroprotective effect of hypothermia therapy.
Shah et al. [91] reported a meta-analysis of the large randomized controlled cooling trials, with the primary outcome of death or impairment in childhood [83, 92–94] (Table 2). This meta-analysis of 249 cooled infants and 248 control infants showed significant reduction in the risk of death or moderate to severe neurodevelopmental disability in infants who received hypothermia compared with control infants, with a relative risk (RR) of 0.76, and a 95% confidence interval (CI) of 0.65–0.88. The number to treat to avoid one encephalopathic infant from dying or surviving with moderate-to-severe disability was estimated to be six. In the more recently published TOBY trial, mortality was the same for cooled and regular care, but among survivors, disability was significantly decreased among hypothermia-treated infants [95]. In both the NICHD trial [96] and the CoolCap trial [97], adverse outcomes were more likely in those infants with HIE who subsequently had higher body temperatures. The recent Infant Cooling Evaluation (ICE) trial, which adopted whole-body hypothermia, is the only individual trial to demonstrate reduced mortality in cooled infants, consistent with the pooled analyses in the published systematic reviews described above [43].
Table 2.
Randomized Controlled Clinical Trials on Hypothermia for the Management of Neonatal HIE
| Injury Types |
No. of Patients |
Inclusion Criteria | Hypother mia (°C) |
Cooling Method |
Cooling Length (Hour) |
Follow- Up (Month) |
Neurological Outcome |
Refs. |
|---|---|---|---|---|---|---|---|---|
| HIE | 22 | Gestational age (GA) > 37 weeks, defined criteria for low Apgar scores, low arterial cord pH and clinical signs of encephalopathy |
36.5-36 and 35.9-35.5 |
Selective head |
72 | 3, 6, 12 | Established the safety of mild hypothermia |
[78] |
| HIE | 208 | GA > 36 weeks, seizures or moderate encephalopathy with defined criteria for low cord or arterial pH, increased base deficit and low Apgar scores |
33.5 | Whole body |
72 | 18–22 | Significant reduction in the risk of death or moderate to severe neurodevelopment al disability in hypothermia group |
[92] |
| HIE | 65 | GA > 35 weeks, Birth weight > 2000g with defined criteria for low cord or infant gas pH, low Apgar scores, increased need for resuscitation and signs of neonatal encephalopathy |
33 ± 0.5 | Whole body |
48 | 12 | Significant reduction in severely abnormal motor scores and combined outcome of death or severe motor scores in the hypothermia group |
[93] |
| HIE | 218 | GA > 36 weeks with defined criteria for low Apgar scores, increased need for resuscitation, low cord blood or infant blood pH and amplitude integrated EEG (aEEG) suggestive of moderate to severe encephalopathy |
34–35 | Selective head |
72 | 18 | Reduction in death or severe disability at 18 months in hypothermia group |
[91] |
| HIE | 325 | GA > 36 weeks with defined criteria for low Apgar scores, increased need for resuscitation, low cord or infant blood pH, moderate to severe encephalopathy and an abnormal aEEG |
33–34 | Whole body |
72 | 18 | Significant increase in rate of survival without neurologic abnormality and reduced risks of cerebral palsy among survivors in cooled group |
[94] |
Explanation of abbreviations can be found in the list of abbreviations.
Serious adverse effects are rare with hypothermia and have not been reported in neonates with HIE treated with mild-moderate hypothermia. However, hypothermia can affect other organs and may lower heart rate, necessitate longer use of vasopressors, increase the duration of prothrombin use, lower platelet levels, and increase rates of seizures [94]. These side effects should be closely monitored during administration of hypothermia.
3.2. Clinical Implications of Therapeutic Hypothermia in Traumatic Brain Injury
While prevention has been the main focus of reducing the incidence of primary brain injury, multiple medical modalities have been employed to manage the secondary injury caused by TBI. In 1993, small-scale investigations suggested a trend toward improved outcomes among patients treated with hypothermia after suffering TBI [98, 99]. A number of large-scale, randomized controlled clinical trials (RCTs) testing systemic mild-to-moderate hypothermia after TBI were then carried out over the last two decades; their results are summarized in Table 3.
Table 3.
Recent Randomized Controlled Clinical Trials on Systemic Hypothermia for the Management of TBI
| Injury Types |
No. of Patients |
Admission GCS |
Hypothermia (°C) |
Cooling Length (Hour) |
Follow-Up (Month) |
Neurological Outcome | Refs. |
|---|---|---|---|---|---|---|---|
| TBI | 232 | 3–8 | 33 | 48 | 6 | Poor outcome (severe disability, vegetative state, or death) in 31 of 52 patients in HT group and 25 of 56 in NT group (RR = 1.08, 95% CI 0.76-1.53; p = 0.67). 12 patients in HT group died compared with eight in NT group (RR 1.30, 95% CI 0.58-2.52; p = 0.52) |
[106] |
| TBI | 86 | 3–8 | 33–35 | 103.2 | 24 | Recovery rates were 65.1% in HT group and 37. 2% in NT group (p < 0.05). The mortality rates were 25.6% in HT group and 51.2% in NT group (p < 0.05) |
[124] |
| TBI | 392 | 3–8 | 33 | 48 | 6 | 57% had poor outcome in both groups. Mortality was 28% in HT group and 27% in NT group (p = 0.79). Patients in HT group had more hospital days with complications |
[102] |
| TBI | 91 | 3–8 | 34 | 48 | 3 | No difference in clinical outcome. Significantly greater use of neuromuscular blocking agents (p = 0.011) and higher rates of medical complications (p < 0.05) in HT group |
*[125] |
| TBI | 87 | 3–8 | 33–35 | 72–336 | 12 | Favorable outcome (good recovery or moderate disability) in 20 out of 43 patients (46.5%) in HT group and 27.3% in NT group. Mortality rates were 25.6% in HT group and 45.5% (p < 0.05) |
[126] |
| TBI | 82 | 3–7 | 32–33 | 24 | 12 | 62% in hypothermia group and 38% in normothermia group had good outcomes (OR = 0.5, 95% CI = 0.2–1.2, p = 0.18) |
[100] |
Explanation of abbreviations can be found in the list of abbreviations.
includes patients with low ICP.
Marion et al. at the University of Pittsburgh Medical Center conducted the first well-publicized RCT on 82 patients with severe closed head injuries and published the results in 1997 [100]. The patients in the hypothermia group were cooled to 32–33°C for 24 hours and were followed for 12 months after discharge. The authors reported good clinical outcomes (Glasgow Outcome Scale (GOS) 4 and 5) in 62% of in the hypothermia (HT) group versus 38% of the normothermia (NT) group. There was a statistically significant advantage in patients with admission Glasgow Coma Scale (GCS) scores of 5 to 7: HT was associated with improved outcome at 3 and 6 months, although not at 12 months. These encouraging results formed the basis for subsequent multi-center investigations using systemic moderate hypothermia in TBI in both adult [101, 102] and pediatric [103] populations.
The National Acute Brain Injury Study of hypothermia was the largest prospective, randomized controlled trial to date and enrolled a total of 392 patients [102]. Hypothermia was initiated within 6 hours after injury, and patients in the HT group were maintained at 33°C for 48 hours, with functional status (as measured by GOS) assessed 6 months after injury. The authors reported poor outcomes in 57% of patients in both HT (108/190) and NT (102/178) groups, with 28% mortality in the HT group (53/190) versus 27% in the NT group (48/178). Contrary to previous studies, these results suggest that moderate systemic hypothermia was ineffective in reducing mortality or improving the neurologic outcome after severe TBI. One key difference between this study and the single-center trial published in 1997, as the authors stated, was the fact that patients with hypothermia on admission who were randomized to the NT group were allowed to spontaneously rewarm in the 2001 study, but were actively rewarmed in the 1997 study [100–102]. Other possible explanations of the difference in outcome included the timing of initiating the hypothermia treatment and intercenter variation between high-enrollment and low-enrollment centers.
Subsequently, another large multi-center RCT focused specifically on initiating early hypothermia after severe brain trauma [104, 105]. Patients were enrolled within 2.5 hours of injury and randomized to HT and NT group. Those randomized to the HT group were cooled to 33°C in approximately 4 hours. However, hypothermia was again shown ineffective in improving the poor outcome after severe TBI, with a trend toward higher mortality in the HT group (23%) compared with the NT group (18%). The trial was
However, hypothermia was again shown ineffective in improving the poor outcome after severe TBI, with a trend toward higher mortality in the HT group (23%) compared with the NT group (18%) [106]. The trial was terminated in 2009 as futile [106]. Another multi-center international trial involving pediatric patients obtained largely similar results [103]. These findings collectively suggest that systemic, mild-to-moderate hypothermia does not improve neurologic outcome after severe TBI, despite the positive results of animal studies and earlier trials. The timing of hypothermia initiation, length of treatment, and rewarming strategies are all matters of debate that can be optimized in future investigations. General improvements in ICU care over the last two decades have significantly decreased the overall mortality rate among patients with severe TBI, making it more difficult to detect any possible benefits of therapeutic hypothermia. Furthermore, systemic hypothermia carries an appreciable risk of adverse events in other systems, including hypotension, thrombocytopenia, and infections [107–109]. Given the lack of evidence for a clearly beneficial effect, the Brain Trauma Foundation, the American Association of Neurological Surgeons, and the Congress of Neurological Surgeons issued a level III recommendation for optional and cautious use of hypothermia in TBI patients [110, 111].
Recently, a number of investigators have focused on a different strategy for using selective hypothermia to manage TBI [112–115] (Table 4). In these studies, hypothermia was administered preferentially to the brain via a cranial cooling device while a significant gradient between the systemic and intracranial temperatures was maintained during the treatment period. The results of a number of small-scale studies have been inconsistent: while two groups reported significant benefits of using selective hypothermia in patients with severe TBI [113, 114], a recent randomized trial using a commercially available cooling cap showed no difference in clinical outcomes between the selective hypothermia group and the normothermia group [112]. Additional studies are needed to further evaluate the effectiveness and safety of such an approach in TBI patients.
Table 4.
Major Randomized Controlled Clinical Trials on the Effectiveness of Selective Hypothermia for TBI
| Injury Types |
No. of Patients |
Admission GCS |
Hypothermia (°C) |
Cooling Method |
Cooling Length (Hour) |
Follow Up (Month) |
Neurological Outcome | Refs. |
|---|---|---|---|---|---|---|---|---|
| TBI | 14 | N/A | 34 | Cooling Helmet |
48–72 | N/A | N/A | [115] |
| TBI | 90 | 3–8 | 33–35 | Cooling cap and neck band |
72 | 6 | Intracranial pressure (ICP) was significantly lower in SHT group than in NT group (p <0.01). Good neurological outcome (GOS of 4 to 5) rates were 68.9% for the SBC group and 46.7% for the NT group (p <0.05). No complications resulted in severe sequelae. |
[113] |
| TBI | 66 | 3–8 | 33–35 | Cooling Head and neck |
72 | 24 | The percentage of patients with a good neurological outcome was 72.7%, 57.1%, and 34.8% in the SHT, systemic HT, and NT groups, respectively. Complications were managed without severe sequelae. |
[114] |
| TBI | 25 | 3–8 | 33 | Cooling cap |
24 | 1 | Mean intracranial temperature of the SHT group was significantly lower than that of the NT. Target intracranial temperature of 33 degrees C was achieved in only 2 patients. Six (50.0%) of 12 patients in the SHT group and 4 (30.8%) of 13 in the NT group died (p = 0.43). There was no significant difference in change in GOS or complication rates between the groups (p value range 0.20–1.0). |
[112] |
Explanation of abbreviations can be found in the list of abbreviations.
4. PERSPECTIVE AND FUTURE DIRECTIONS
The evidence strongly suggests that hypothermia can effectively improve outcomes of neonate HIE [92, 116] and adult HIBI [117, 118], but the results for patients with TBI have largely been inconsistent. To date, hypothermia has been incorporated into clinical practice as the standard of care for infants with HIE and adults who suffer cardiac arrest and subsequent coma. However, hypothermia can not be routinely recommended for patients with TBI.
Although hypothermia has been a revolutionary therapy in HIE, clinical trials have shown that more than 40% of the cooled infants either died or survived with impairments. Therefore, the search for more effective therapeutic interventions in addition to hypothermia continues. Several drugs including magnesium, xenon, erythropoietin, and free radical inhibitors, have proved to be neuroprotective in animal studies [119–123] but have not reached clinical use. The molecular mechanisms underlying the effects of therapeutic hypothermia are multiple. An effective therapy with hypothermia or combination of multiple modalities of treatment with hypothermia and drugs such as anti-apoptotic drugs in HIE and TBI should block multiple action mechanisms. It may be that a safe neuroprotective drug will potentiate the beneficial effect of hypothermia and further improve outcomes. Gaining a more comprehensive understanding of multiple molecular mechanisms of hypothermia in HIE and TBI may be one future direction.
However, problems in translating results from experimental studies to clinical practice may arise from choosing the wrong targets of injury and poor experimental design. Experimental studies should be robust, preferably showing benefit in more than one model, and in more than one species, with effects on long term behavioral and functional endpoints rather than just short term histological changes. Also, it is essential to design strategies that block brain damage without disrupting normal development.
In spite of the established role of hypothermia as a neuroprotective strategy for HIE, there are gaps in our knowledge. The longer-term effects of hypothermia treatment for HIE are unknown. We await data from long-term follow-up as the subjects of the current large clinical trials reach school age. Exact cooling strategies including depth, duration, timing, and re-warming approaches are also areas requiring further study and refinement. The optimal mode of cooling, i.e., head versus whole body, also needs to be clarified further.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (to X.W.) and the Bill & Melinda Gates Foundation (to X.W.).
ABBREVIATIONS
- aEEG
Amplitude integrated EEG
- AIF
Apoptosis inducing factor
- BBB
Blood brain barrier
- CBF
Cerebral blood flow
- CI
Confidence interval
- ERK
Extracellular signal regulated kinase
- FPI
Fluid percussion injury
- GA
Gestational age
- GOS
Glasgow outcome score
- HA
Heat acclimation
- hGLT-1
Human brain glutamate transporter-1
- HI
Hypoxic-ischemic
- HIBI
Hypoxic ischemic brain injury
- HIE
Hypoxic ischemic encephalopathy
- HT
Hypothermia group
- ICE
Infant Cooling Evaluation
- ICU
Intensive care unit
- IKK
IκB kinase
- NF-κB
Nuclear factor-κB
- JNK
c-Jun NH2-terminal kinase
- MAPK
Mitogen-activated protein kinase
- NMDA
N-Methyl-D-aspartate
- NT
Normothermia group
- OGD
Oxygen glucose deprivation
- RCT
Randomized control trial
- RR
Relative risk
- SHT
Selective hypothermia
- TBI
Traumatic brain injury
- TNFR1
Tumor necrosis factor receptor-1
- XIAP
X-linked inhibitor of apoptosis protein
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Ginsberg MD, Sternau LL, Globus MY, Dietrich WD, Busto R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev. 1992;4:189–225. [PubMed] [Google Scholar]
- 2.Smith LW, Fay T. Observations on human beings with cancer, maintained at reduced temperatures of 75–90 °F. Am J Clin Pathol. 1940;10:1–11. [Google Scholar]
- 3.Fay T. Observations on prolonged human refrigeration. N Y State J Med. 1940;40:1351–1354. [Google Scholar]
- 4.Fay T. Observations on generalized refrigeration in cases of severe cerebral trauma. Assoc Res Nerv Ment Dis Proc. 1943;24:611–619. [Google Scholar]
- 5.Spencer FC, Bahnson HT. The present role of hypothermia in cardiac surgery. Circulation. 1962;26:292–300. doi: 10.1161/01.cir.26.2.292. [DOI] [PubMed] [Google Scholar]
- 6.Botterell EH, Lougheed WM, Morley TP, Vandewater SL. Hypothermia in the surgical treatment of ruptured intracranial aneurysms. J Neurosurg. 1958;15:4–18. doi: 10.3171/jns.1958.15.1.0004. [DOI] [PubMed] [Google Scholar]
- 7.Sahuquillo J, Vilalta A. Cooling the injured brain: how does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des. 2007;13:2310–2322. doi: 10.2174/138161207781368756. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins LW, DeWitt DS, Johnston WE, Davis KL, Prough DS. Intraischemic mild hypothermia increases hippocampal CA1 blood flow during forebrain ischemia. Brain Res. 2001;890:1–10. doi: 10.1016/s0006-8993(00)03011-0. [DOI] [PubMed] [Google Scholar]
- 9.Sick TJ, Tang R, Perez-Pinzon MA. Cerebral blood flow does not mediate the effect of brain temperature on recovery of extracellular potassium ion activity after transient focal ischemia in the rat. Brain Res. 1999;821:400–406. doi: 10.1016/s0006-8993(99)01119-1. [DOI] [PubMed] [Google Scholar]
- 10.Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513–530. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- 11.Globus MY, Busto R, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of ischemia on the in vivo release of striatal dopamine, glutamate, and gamma-aminobutyric acid studied by intracerebral microdialysis. J Neurochem. 1988;51:1455–1464. doi: 10.1111/j.1471-4159.1988.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 12.Okuda C, Saito A, Miyazaki M, Kuriyama K. Alteration of the turnover of dopamine and 5-hydroxytryptamine in rat brain associated with hypothermia. Pharmacol Biochem Behav. 1986;24:79–83. doi: 10.1016/0091-3057(86)90048-1. [DOI] [PubMed] [Google Scholar]
- 13.Lei B, Adachi N, Arai T. The effect of hypothermia on H2O2 production during ischemia and reperfusion: a microdialysis study in the gerbil hippocampus. Neurosci Lett. 1997;222:91–94. doi: 10.1016/s0304-3940(97)13349-3. [DOI] [PubMed] [Google Scholar]
- 14.Guven H, Amanvermez R, Malazgirt Z, et al. Moderate hypothermia prevents brain stem oxidative stress injury after hemorrhagic shock. J Trauma. 2002;53:66–72. doi: 10.1097/00005373-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- 16.Vannucci RC. Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res. 1990;27:317–326. doi: 10.1203/00006450-199004000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Dilenge ME, Majnemer A, Shevell MI. Long-term developmental outcome of asphyxiated term neonates. J Child Neurol. 2001;16:781–792. doi: 10.1177/08830738010160110201. [DOI] [PubMed] [Google Scholar]
- 18.Graham WJ, Ahmed S, Stanton C, Abou-Zahr C, Campbell OM. Measuring maternal mortality: an overview of opportunities and options for developing countries. BMC Med. 2008;6:12. doi: 10.1186/1741-7015-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Airede AI. Birth asphyxia and hypoxic-ischaemic encephalopathy: incidence and severity. Ann Trop Paediatr. 1991;11:331–335. doi: 10.1080/02724936.1991.11747524. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Panyda R, Yao J, Ma H, Li J. Targeting caspases in neonatal hypoxia-ischemic brain injury and traumatic brain injury. In: McNeill AS, editor. Neurodegeneration: Theory, Disorders and Treatments. New York: NOVA Science Publishers Inc; 2010. pp. 125–134. [Google Scholar]
- 21.Lorek A, Takei Y, Cady EB, et al. Delayed ("secondary") cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994;36:699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28:1353–1365. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Laptook AR, Corbett RJ, Ruley J, Olivares E. Blood flow and metabolism during and after repeated partial brain ischemia in neonatal piglets. Stroke. 1992;23:380–387. doi: 10.1161/01.str.23.3.380. [DOI] [PubMed] [Google Scholar]
- 24.Adams H, Mitchell DE, Graham DI, Doyle D. Diffuse brain damage of immediate impact type. Its relationship to 'primary brain-stem damage' in head injury. Brain. 1977;100:489–502. doi: 10.1093/brain/100.3.489. [DOI] [PubMed] [Google Scholar]
- 25.Graham DI, Adams JH, Gennarelli TA. Pathology of brain damage in head injury. In: Cooper PR, editor. Head injury. Baltimore: Williams and Wilkins; 1987. pp. 72–88. [Google Scholar]
- 26.Pitts LH, Mcintosh TK. Dynamic changes after brain trauma. In: Braakman R, editor. Handbook of Clinical Neurology. Head Injury. 57. Vol. 13. Amsterdam: Elsevier Science Publishers BV; 1990. pp. 65–100. [Google Scholar]
- 27.Petrov T, Page AB, Owen CR, Rafols JA. Expression of the inducible nitric oxide synthase in distinct cellular types after traumatic brain injury: an in situ hybridization and immunocytochemical study Acta. Neuropathol. 2000;100:196–204. doi: 10.1007/s004019900167. [DOI] [PubMed] [Google Scholar]
- 28.Yao J, Zeng X, Zhang J. Research change and anlysis of endothelins in the plasma of patients after severe head injury. Guangxi Medical Journal. 1999;21:63–65. [Google Scholar]
- 29.Teasdale GM, Graham DI. Craniocerebral trauma: protection and retrieval of the neuronal population after injury. Neurosurgery. 1998;43:723–737. doi: 10.1097/00006123-199810000-00001. discussion 737–8. [DOI] [PubMed] [Google Scholar]
- 30.Bramlett HM, Dietrich WD, Green EJ, Busto R. Chronic histopathological consequences of fluid-percussion brain injury in rats: effects of post-traumatic hypothermia. Acta Neuropathol. 1997;93:190–199. doi: 10.1007/s004010050602. [DOI] [PubMed] [Google Scholar]
- 31.Vialard PL. Navarranne. [Two cases of severe cerebral concussion treated and cured by artificial hibernation] Anesth Anal. 1954;11:431–436. [PubMed] [Google Scholar]
- 32.Mayrhofer O, Kuhlmayer R. [Artificial hypothermia (hibernation) as supporting measure in the treatment of severe cranium trauma] Wien Klin Wochenschr. 1954;66:495–497. [PubMed] [Google Scholar]
- 33.Wang Q, Li AL, Zhi DS, Huang HL. Effect of mild hypothermia on glucose metabolism and glycerol of brain tissue in patients with severe traumatic brain injury. Chin J Traumatol. 2007;10:246–249. [PubMed] [Google Scholar]
- 34.Kimberger O, Kurz A. Thermoregulatory management for mild therapeutic hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22:729–744. doi: 10.1016/j.bpa.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Christian E, Zada G, Sung G, Giannotta SL. A review of selective hypothermia in the management of traumatic brain injury. Neurosurg Focus. 2008;25:E9. doi: 10.3171/FOC.2008.25.10.E9. [DOI] [PubMed] [Google Scholar]
- 36.Feng JF, Zhang KM, Jiang JY, Gao GY, Fu X, Liang YM. Effect of therapeutic mild hypothermia on the genomics of the hippocampus after moderate traumatic brain injury in rats. Neurosurgery. 2010;67(3):730–742. doi: 10.1227/01.NEU.0000378023.81727.6E. [DOI] [PubMed] [Google Scholar]
- 37.Adamchik Y, Frantseva MV, Weisspapir M, Carlen PL, Perez Velazquez JL. Methods to induce primary and secondary traumatic damage in organotypic hippocampal slice cultures. Brain Res Brain Res Protoc. 2000;5:153–158. doi: 10.1016/s1385-299x(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 38.Newman GC, Qi H, Hospod FE, Grundmann K. Preservation of hippocampal brain slices with in vivo or in vitro hypothermia. Brain Res. 1992;575:159–163. doi: 10.1016/0006-8993(92)90438-f. [DOI] [PubMed] [Google Scholar]
- 39.Rossaint J, Rossaint R, Weis J, Fries M, Rex S, Coburn M. Propofol: neuroprotection in an in vitro model of traumatic brain injury. Crit Care. 2009;13:R61. doi: 10.1186/cc7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakai F, Amaha K. The effects of hypothermia on a cloned human brain glutamate transporter (hGLT-1) expressed in Chinese hamster ovary cells: -[3H]L-glutamate uptake study. Anesth Analg. 1999;89:1546–1550. doi: 10.1097/00000539-199912000-00044. [DOI] [PubMed] [Google Scholar]
- 41.Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–741. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Siesjo BK, Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab. 1989;9:127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson MD. Programmed cell death: a missing link is found. Trends Cell Biol. 1997;7:467–469. doi: 10.1016/S0962-8924(97)01182-3. [DOI] [PubMed] [Google Scholar]
- 44.Edwards AD, Yue X, Squier MV, et al. Specific inhibition of apoptosis after cerebral hypoxia-ischaemia by moderate post-insult hypothermia. Biochem Biophys Res Commun. 1995;217:1193–1199. doi: 10.1006/bbrc.1995.2895. [DOI] [PubMed] [Google Scholar]
- 45.Rothstein RP, Levison SW. Gray matter oligodendrocyte progenitors and neurons die caspase-3 mediated deaths subsequent to mild perinatal hypoxic/ischemic insults. Dev Neurosci. 2005;27:149–159. doi: 10.1159/000085987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohmura A, Nakajima W, Ishida A, et al. Prolonged hypothermia protects neonatal rat brain against hypoxic-ischemia by reducing both apoptosis and necrosis. Brain Dev. 2005;27:517–526. doi: 10.1016/j.braindev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Zhu C, Wang X, Cheng X, et al. Post-ischemic hypothermia-induced tissue protection and diminished apoptosis after neonatal cerebral hypoxia-ischemia. Brain Res. 2004;996:67–75. doi: 10.1016/j.brainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Liu CQ, Xia YF, Yuan YX, Li L, Qiu XL. [Effects of selective head cooling with mild hypothermia on serum levels of caspase-3 and IL-18 in neonates with hypoxic-ischemic encephalopathy] Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:690–692. [PubMed] [Google Scholar]
- 49.Wang LS, Yu LJ, Shao XM. [Mild hypothermia attenuates neuronal apoptosis after cerebral hypoxia-ischemia in neonatal rats] Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:37–41. [PubMed] [Google Scholar]
- 50.Tomimatsu T, Fukuda H, Endo M, et al. Effects of hypothermia on neonatal hypoxic-ischemic brain injury in the rat: phosphorylation of Akt, activation of caspase-3-like protease. Neurosci Lett. 2001;312:21–24. doi: 10.1016/s0304-3940(01)02178-4. [DOI] [PubMed] [Google Scholar]
- 51.Ogihara T, Hirano K, Ogihara H, et al. Non-protein-bound transition metals and hydroxyl radical generation in cerebrospinal fluid of newborn infants with hypoxic ischemic encephalopathy. Pediatr Res. 2003;53:594–599. doi: 10.1203/01.PDR.0000054685.87405.59. [DOI] [PubMed] [Google Scholar]
- 52.Hiroi M, Ogihara T, Hirano K, et al. Regulation of apoptosis by glutathione redox state in PC12 cells exposed simultaneously to iron and ascorbic acid. Free Radic Biol Med. 2005;38:1057–1072. doi: 10.1016/j.freeradbiomed.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Hasegawa M, Ogihara T, Tamai H, Hiroi M. Hypothermic inhibition of apoptotic pathways for combined neurotoxicity of iron and ascorbic acid in differentiated PC12 cells: reduction of oxidative stress and maintenance of the glutathione redox state. Brain Res. 2009;1283:1–13. doi: 10.1016/j.brainres.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Matsui T, Kakeda T. IL-10 production is reduced by hypothermia but augmented by hyperthermia in rat microglia. J Neurotrauma. 2008;25:709–715. doi: 10.1089/neu.2007.0482. [DOI] [PubMed] [Google Scholar]
- 55.Xiong M, Yang Y, Chen GQ, Zhou WH. Post-ischemic hypothermia for 24h in P7 rats rescues hippocampal neuron: association with decreased astrocyte activation and inflammatory cytokine expression. Brain Res Bull. 2009;79:351–357. doi: 10.1016/j.brainresbull.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 56.Perrone S, Szabo M, Bellieni CV, et al. Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury. Pediatr Neurol. 2010;43:236–240. doi: 10.1016/j.pediatrneurol.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Mueller-Burke D, Koehler RC, Martin LJ. Rapid NMDA receptor phosphorylation and oxidative stress precede striatal neurodegeneration after hypoxic ischemia in newborn piglets and are attenuated with hypothermia. Int J Dev Neurosci. 2008;26:67–76. doi: 10.1016/j.ijdevneu.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yager JY, Asselin J. Effect of mild hypothermia on cerebral energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. Stroke. 1996;27:919–925. doi: 10.1161/01.str.27.5.919. discussion 926. [DOI] [PubMed] [Google Scholar]
- 59.O’Brien FE, Iwata O, Thornton JS, et al. Delayed whole-body cooling to 33 or 35 degrees C and the development of impaired energy generation consequential to transient cerebral hypoxia-ischemia in the newborn piglet. Pediatrics. 2006;117:1549–1559. doi: 10.1542/peds.2005-1649. [DOI] [PubMed] [Google Scholar]
- 60.Dietrich WD. Therapeutic hypothermia in experimental models of traumatic brain injury. In: Hayashi N, editor. Brain Hypothermia. Tokyo: Springer-Verlag; 2000. pp. 39–46. Hayashi N Ed. Brain Hypothermia. [Google Scholar]
- 61.McCarthy MM. Stretching the truth. Why hippocampal neurons are so vulnerable following traumatic brain injury. Exp Neurol. 2003;184:40–43. doi: 10.1016/j.expneurol.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Clausen F, Lundqvist H, Ekmark S, Lewen A, Ebendal T, Hillered L. Oxygen free radical-dependent activation of extracellular signal-regulated kinase mediates apoptosis-like cell death after traumatic brain injury. J Neurotrauma. 2004;21:1168–1182. doi: 10.1089/neu.2004.21.1168. [DOI] [PubMed] [Google Scholar]
- 63.Huang T, Solano J, He D, Loutfi M, Dietrich WD, Kuluz JW. Traumatic injury activates MAP kinases in astrocytes: mechanisms of hypothermia and hyperthermia. J Neurotrauma. 2009;26:1535–1545. doi: 10.1089/neu.2008.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lotocki G, de Rivero Vaccari JP, Perez ER, et al. Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage. Eur J Neurosci. 2006;24:2283–2290. doi: 10.1111/j.1460-9568.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- 65.Sui LS, Han F, Guo YW, et al. [Time course of calpain activity changes in rat neurons following fluid percussion injury and the interventional effect of mild hypothermia] Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1149–1151. [PubMed] [Google Scholar]
- 66.Jia F, Jiang JY, Liang YM, Mao Q. The effect of hypothermia on the expression of TIMP-3 after traumatic brain injury in rats. J Neurotrauma. 2009 doi: 10.1089/neu.2008-0814. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 67.Haranishi Y, Kawata R, Fukuda S, et al. Moderate hypothermia, but not calpain inhibitor 2, attenuates the proteolysis of microtubule-associated protein 2 in the hippocampus following traumatic brain injury in rats. Eur J Anaesthesiol. 2005;22:140–147. doi: 10.1017/s0265021505000268. [DOI] [PubMed] [Google Scholar]
- 68.Shein NA, Doron H, Horowitz M, et al. Altered cytokine expression and sustained hypothermia following traumatic brain injury in heat acclimated mice. Brain Res. 2007;1185:313–320. doi: 10.1016/j.brainres.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell HM, White DM, Domowicz MS, Kraig RP. Cold preconditioning neuroprotection depends on TNF-alpha and is enhanced by blockade of interleukin-11. J Neurochem. 2011;117:187–196. doi: 10.1111/j.1471-4159.2010.07103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suehiro E, Fujisawa H, Akimura T, et al. Increased matrix metalloproteinase-9 in blood in association with activation of interleukin-6 after traumatic brain injury: influence of hypothermic therapy. J Neurotrauma. 2004;21:1706–1711. doi: 10.1089/neu.2004.21.1706. [DOI] [PubMed] [Google Scholar]
- 71.Bayir H, Marion DW, Puccio AM, et al. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- 72.Bruno VM, Goldberg MP, Dugan LL, Giffard RG, Choi DW. Neuroprotective effect of hypothermia in cortical cultures exposed to oxygen-glucose deprivation or excitatory amino acids. J Neurochem. 1994;63:1398–1406. doi: 10.1046/j.1471-4159.1994.63041398.x. [DOI] [PubMed] [Google Scholar]
- 73.Ooba S, Hasuo H, Shigemori M, Yamashita S, Akasu T. Mild hypothermia prevents post-traumatic hyperactivity of excitatory synapses in rat hippocampal CA1 pyramidal neurons. Kurume Med J. 2009;56:49–59. doi: 10.2739/kurumemedj.56.49. [DOI] [PubMed] [Google Scholar]
- 74.Stahel PF, Shohami E, Younis FM, et al. Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J Cereb Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- 75.Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A. Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. J Clin Neurophysiol. 2005;22:1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- 76.Jiang JY, Lyeth BG, Kapasi MZ, Jenkins LW, Povlishock JT. Moderate hypothermia reduces blood-brain barrier disruption following traumatic brain injury in the rat. Acta Neuropathol. 1992;84:495–500. doi: 10.1007/BF00304468. [DOI] [PubMed] [Google Scholar]
- 77.Lotocki G, Vaccari JP, Perez ER, et al. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J Neurotrauma. 2009;26:1123–1134. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szczygielski J, Mautes AE, Schwerdtfeger K, Steudel WI. The effects of selective brain hypothermia and decompressive craniectomy on brain edema after closed head injury in mice. Acta Neurochir Suppl. 2010;106:225–229. doi: 10.1007/978-3-211-98811-4_42. [DOI] [PubMed] [Google Scholar]
- 79.Polderman KH. Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: Indications and evidence Posttraumatic hypothermia followed by slow rewarming protects the cerebral microcirculation. Intensive Care Med. 2004;30:556–575. doi: 10.1007/s00134-003-2152-x. [DOI] [PubMed] [Google Scholar]
- 80.Suehiro E, Ueda Y, Wei EP, Kontos HA, Povlishock JT. Posttraumatic hypothermia followed by slow rewarming protects the cerebral microcirculation. J Neurotrauma. 2003;20:381–390. doi: 10.1089/089771503765172336. [DOI] [PubMed] [Google Scholar]
- 81.Oda Y, Gao G, Wei EP, Povlishock JT. Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood-brain barrier dysfunction after traumatic brain injury in rat. J Cereb Blood Flow Metab. 2011;4:1143–1154. doi: 10.1038/jcbfm.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Azzopardi D, Brocklehurst P, Edwards D, et al. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics. 1998;102:885–892. doi: 10.1542/peds.102.4.885. [DOI] [PubMed] [Google Scholar]
- 84.Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport. 1997;8:3359–3362. doi: 10.1097/00001756-199710200-00033. [DOI] [PubMed] [Google Scholar]
- 85.Fukuda H, Tomimatsu T, Watanabe N, et al. Post-ischemic hypothermia blocks caspase-3 activation in the newborn rat brain after hypoxia-ischemia. Brain Res. 2001;910:187–191. doi: 10.1016/s0006-8993(01)02659-2. [DOI] [PubMed] [Google Scholar]
- 86.Zanelli SA, Naylor M, Dobbins N, et al. Implementation of a 'Hypothermia for HIE' program: 2-year experience in a single NICU. J Perinatol. 2008;28:171–175. doi: 10.1038/sj.jp.7211896. [DOI] [PubMed] [Google Scholar]
- 87.Laptook AR, Corbett RJ, Burns D, Sterett R. Neonatal ischemic neuroprotection by modest hypothermia is associated with attenuated brain acidosis. Stroke. 1995;26:1240–1246. doi: 10.1161/01.str.26.7.1240. [DOI] [PubMed] [Google Scholar]
- 88.Rutherford MA, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116:1001–1006. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 89.Laptook AR, Shalak L, Corbett RJ. Differences in brain temperature and cerebral blood flow during selective head versus whole-body cooling. Pediatrics. 2001;108:1103–1110. doi: 10.1542/peds.108.5.1103. [DOI] [PubMed] [Google Scholar]
- 90.Thoresen M, Simmonds M, Satas S, Tooley J, Silver IA. Effective selective head cooling during posthypoxic hypothermia in newborn piglets. Pediatr Res. 2001;49:594–599. doi: 10.1203/00006450-200104000-00024. [DOI] [PubMed] [Google Scholar]
- 91.Shah PS, Ohlsson A, Perlman M. Hypothermia to treat neonatal hypoxic ischemic encephalopathy: systematic review. Arch Pediatr Adolesc Med. 2007;161:951–958. doi: 10.1001/archpedi.161.10.951. [DOI] [PubMed] [Google Scholar]
- 92.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 93.Shankaran S, Laptook A, Wright LL, et al. Whole-body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics. 2002;110:377–385. doi: 10.1542/peds.110.2.377. [DOI] [PubMed] [Google Scholar]
- 94.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 95.Azzopardi D, Strohm B, Edwards AD, et al. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94:F260–F264. doi: 10.1136/adc.2008.146977. [DOI] [PubMed] [Google Scholar]
- 96.Laptook A, Tyson J, Shankaran S, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008;122:491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 98.Shiozaki T, Sugimoto H, Taneda M, et al. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg. 1993;79:363–368. doi: 10.3171/jns.1993.79.3.0363. [DOI] [PubMed] [Google Scholar]
- 99.Clifton GL, Allen S, Barrodale P, et al. A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma. 1993;10:263–271. doi: 10.1089/neu.1993.10.263. discussion 273. [DOI] [PubMed] [Google Scholar]
- 100.Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 101.Narayan RK. Hypothermia for traumatic brain injury--a good idea proved ineffective. N Engl J Med. 2001;344:602–603. doi: 10.1056/NEJM200102223440810. [DOI] [PubMed] [Google Scholar]
- 102.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 103.Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 104.Clifton GL, Drever P, Valadka A, Zygun D, Okonkwo D. Multicenter trial of early hypothermia in severe brain injury. J Neurotrauma. 2009;26:393–397. doi: 10.1089/neu.2008.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clifton GL. Is keeping cool still hot? An update on hypothermia in brain injury. Curr Opin Crit Care. 2004;10:116–119. doi: 10.1097/00075198-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 106.Clifton GL, Valadka A, Zygun D, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kochanek PM, Safar PJ. Therapeutic hypothermia for severe traumatic brain injury. JAMA. 2003;289:3007–3009. doi: 10.1001/jama.289.22.3007. [DOI] [PubMed] [Google Scholar]
- 108.Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62–71. doi: 10.1089/neu.2007.0424. [DOI] [PubMed] [Google Scholar]
- 109.Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD001048.pub3. CD001048. [DOI] [PubMed] [Google Scholar]
- 110.Bullock MR, Povlishock JT. Guidelines for the management of severe traumatic brain injury. Editor’s Commentary. J Neurotrauma. 2007;24(Suppl 1) doi: 10.1089/neu.2007.9998. 2 p preceding S1. [DOI] [PubMed] [Google Scholar]
- 111.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. III. Prophylactic hypothermia. J Neurotrauma. 2007;24(Suppl 1):S21–S25. doi: 10.1089/neu.2007.9993. [DOI] [PubMed] [Google Scholar]
- 112.Harris OA, Muh CR, Surles MC, et al. Discrete cerebral hypothermia in the management of traumatic brain injury: a randomized controlled trial. J Neurosurg. 2009;110:1256–1264. doi: 10.3171/2009.1.JNS081320. [DOI] [PubMed] [Google Scholar]
- 113.Qiu W, Shen H, Zhang Y, et al. Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci. 2006;13:995–1000. doi: 10.1016/j.jocn.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 114.Liu WG, Qiu WS, Zhang Y, Wang WM, Lu F, Yang XF. Effects of selective brain cooling in patients with severe traumatic brain injury: a preliminary study. J Int Med Res. 2006;34:58–64. doi: 10.1177/147323000603400107. [DOI] [PubMed] [Google Scholar]
- 115.Wang H, Olivero W, Lanzino G, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg. 2004;100:272–277. doi: 10.3171/jns.2004.100.2.0272. [DOI] [PubMed] [Google Scholar]
- 116.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 117.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 118.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 119.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dingley J, Tooley J, Porter H, Thoresen M. Xenon provides short-term neuroprotection in neonatal rats when administered after hypoxia-ischemia. Stroke. 2006;37:501–506. doi: 10.1161/01.STR.0000198867.31134.ac. [DOI] [PubMed] [Google Scholar]
- 121.Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–e226. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- 122.Van Bel F, Shadid M, Moison RM, et al. Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, and electrical brain activity. Pediatrics. 1998;101:185–193. doi: 10.1542/peds.101.2.185. [DOI] [PubMed] [Google Scholar]
- 123.Jatana M, Singh I, Singh AK, Jenkins D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2006;59:684–689. doi: 10.1203/01.pdr.0000215045.91122.44. [DOI] [PubMed] [Google Scholar]
- 124.Qiu WS, Liu WG, Shen H, et al. Therapeutic effect of mild hypothermia on severe traumatic head injury. Chin J Traumatol. 2005;8:27–32. [PubMed] [Google Scholar]
- 125.Shiozaki T, Hayakata T, Taneda M, et al. A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. Mild Hypothermia Study Group in Japan. J Neurosurg. 2001;94:50–54. doi: 10.3171/jns.2001.94.1.0050. [DOI] [PubMed] [Google Scholar]
- 126.Jiang J, Yu M, Zhu C. Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg. 2000;93:546–549. doi: 10.3171/jns.2000.93.4.0546. [DOI] [PubMed] [Google Scholar]