Abstract
Background/Objectives:
To assess the prevalence of undiagnosed obstructive sleep apnea (OSA) among general medical inpatients and to investigate whether OSA risk is associated with in-hospital sleep quantity and quality.
Design:
Prospective cohort study.
Setting:
General medicine ward in academic medical center
Participants:
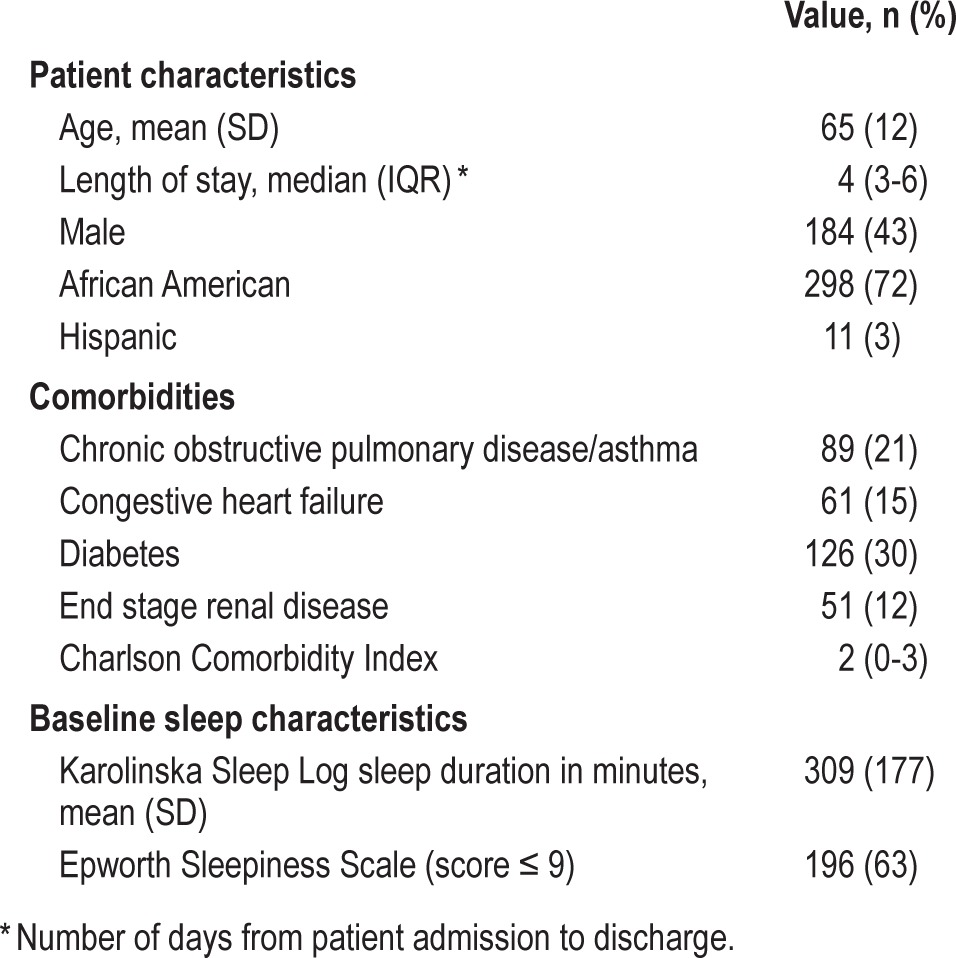
424 hospitalized adult patients ≥ 50 years old without a sleep disorder diagnosis (mean age 65 years, 57% female, 72% African American).
Main Measures:
The Berlin questionnaire, a validated screen for determining risk of OSA, was administered to hospitalized medical patients. Sleep duration and efficiency were measured via wrist actigraphy. Self-reported sleep quality was evaluated using Karolinska Sleep Quality Index (KSQI).
Key Results:
Two of every 5 inpatients ≥ 50 years old (39.5%, n = 168) were found to be at high risk for OSA. Mean in-hospital sleep duration was ∼ 5 h and mean sleep efficiency was 70%. Using random effects linear regression models, we found that patients who screened at high risk for OSA obtained ∼ 40 min less sleep per night (-39.6 min [-66.5, -12.8], p = 0.004). These findings remained significant after controlling for African American race, sex, and age quartiles. In similar models, those patients who screened at high risk had ∼ 5.5% less sleep efficiency per night (-5.50 [-9.96, -1.05], p = 0.015). In multivariate analysis, patients at high risk for OSA also had lower self-reported sleep quality on KSQI (-0.101 [-0.164, -0.037], p = 0.002).
Conclusion:
Two of every 5 inpatients older than 50 years screened at high risk for OSA. Those screening at high risk have worse in-hospital sleep quantity and quality.
Commentary:
A commentary on this article appears in this issue on page 1067.
Citation:
Shear TC, Balachandran JS, Mokhlesi B, Spampinato LM, Knutson KL, Meltzer DO, Arora VM. Risk of sleep apnea in hospitalized older patients. J Clin Sleep Med 2014;10(10):1061-1066.
Keywords: hospitalized patients, obstructive sleep apnea, sleep
Obstructive sleep apnea (OSA) is a common sleep disorder that affects approximately 1 of every 4 adults in the United States.1,2 Unfortunately, up to 90% of people with OSA are undiagnosed.3 Without treatment, severe OSA is associated with increased risk of cardiovascular and cerebrovascular morbidity and mortality in both men and women.3–5 Early diagnosis and treatment of OSA have been recommended to improve patient health outcomes.
Hospitalized patients are likely at high risk of OSA. One reason is that OSA is especially prevalent among patients with medical conditions that can result in hospitalization, such as congestive heart failure.4–7 Hospitalized patients with OSA also have an increased risk of postoperative complications and exacerbations of chronic conditions.8,9 Because hospitalization has been characterized as a period of acute sleep loss due to environmental factors, inpatients with undiagnosed OSA may experience even more sleep disruptions than their healthier counterparts.10 Despite these associations, in one study of in-patients, there was no documentation of sleep histories or any sleep-associated symptoms in their hospital charts.11 In addition, there is a high frequency of sleep disordered breathing in hospitalized patients referred for polysomnography, especially in patients with underlying cardiopulmonary disease.12
These findings suggest that hospitalization may represent a missed opportunity to screen patients for sleep disorders. Although polysomnography (PSG) is considered the gold standard for diagnosing OSA, it is difficult and resource-intensive to perform among inpatients. For these reasons, screening tools for OSA, such as the Berlin Questionnaire, have been developed with relatively high sensitivity and specificity.13 To date, no study has investigated the prevalence of undiagnosed OSA in medical inpatients and examined how risk of OSA correlates to in-hospital measures of sleep duration and quality.
BRIEF SUMMARY
Current Knowledge/Study Rationale: While the prevalence of OSA in the US is estimated at approximately 25%, it is likely that the prevalence in the in-hospital population is higher. This is because OSA is associated with many medical conditions, such as congestive heart failure, cardiovascular and cerebrovascular disease, postoperative complications, and exacerbations of chronic conditions. Despite this, the prevalence of undiagnosed OSA among hospitalized patients is unknown. Moreover, studies show screening for sleep complaints does not routinely occur in either the clinic or hospital setting.
Study Impact: Understanding the prevalence of OSA as well as the relationship of OSA with inpatient sleep quality is important for recognizing high-risk inpatients as well as diagnosing and treating those who may have OSA. Also, by better understanding where physician knowledge of screening for OSA is lacking, we can hopefully increase awareness of this shortfall and train hospital staff nationwide to recognize and screen patients for sleep disorders.
We were specifically interested in studying sleep in older inpatients. Because of their susceptibility to sleep disruptions as they age, existing studies in hospitalized older patients are largely descriptive and based on observations or self-report. The few hospital interventions to date, such as the well-known Yale Hospital Elder Life Program, target sleep for older patients as part of a multicomponent intervention to reduce delirium.19 Unfortunately, prior studies of interventions to improve sleep for older adults do not objectively measure sleep duration or quality and often have very low adherence for the sleep portion of the protocol.20 Before effective interventions can be designed and implemented, it is essential to fully characterize sleep duration and quality in hospitalized older patients using both subjective and objective assessments.
The aim of this study is to better understand the prevalence of undiagnosed OSA in the hospital setting. Our secondary aims are to investigate whether inpatients at high risk for OSA have lower inpatient sleep duration and quality, and to assess physician knowledge of and behaviors surrounding screening for OSA.
METHODS
Study Design
We enrolled patients from an ongoing prospective study of general medicine inpatients at the University of Chicago Medical Center.14 Eligible patients were ambulatory, community-dwelling adults aged 50 years or older who were cognitively intact, as defined by the Short Portable Mental Status Questionnaire (SPMSQ).15 Patients were excluded if they were transferred from an intensive care unit, had a known sleep disorder, were on bed rest, in respiratory isolation, had already been hospitalized for the 3 days prior to eligibility screening, or were readmitted within 2 weeks. The study was approved by the University of Chicago Institutional Review Board and appropriate informed consent was obtained from the human subjects.
Data Collection
OSA Risk
We screened patients for OSA risk using the Berlin questionnaire,13 an 11-item survey on snoring, sleepiness, and hyper-tension, which has been validated against polysomnography in a variety of populations and is considered the most accurate screening questionnaire to predict a diagnosis of OSA.16–18 Using a scoring guide adapted from a prior study, an individual was identified at high risk of OSA if they scored positive in ≥ 2 categories.13 A category was positive if scores of questions within each category summed to ≥ 2.
Subjective Sleep Quality
Self-reported sleep quality in the hospital at night was assessed using the Karolinska Sleep Log, which includes questions regarding an individual's sleep the previous night, such as how many awakenings he or she had.21 The Karolinska Sleep Quality Index (KSQI) was calculated from the average of 4 items, each on a 5-point scale (sleep quality, sleep restlessness, ease of falling asleep, ability to sleep throughout night). A KSQI score ≤ 3 placed someone in an “insomniac” range.22,23 The Epworth Sleepiness Scale, a standard validated survey examining sleepiness in common situations, was administered on admission.24 A score ≥ 9 indicated excessive sleepiness. Using the Pittsburgh Sleep Quality Index (PSQI), patients reported their sleep quality for the month prior to hospitalization.25 A PSQI score ≥ 5 indicated poor sleep quality.
Objective Sleep Quality
Objective sleep data were obtained with wrist-watch like activity monitors (Actiwatch 2; Respironics Inc, Murrysville, Pennsylvania), which have been validated to calculate sleep duration and efficiency.26 Subjects who consented wore the watch continuously from the day of consent until discharge or until electing to no longer wear the watch. The data were downloaded and analyzed using Actiware 5 (Respironics, Inc., Murrysville, Pennsylvania). To ensure sleep time was calculated accurately, the rest interval for each patient was based on self-reported sleep onset at night and awakening from the Karolinska Sleep Log. Sleep duration was defined as total time spent asleep during the rest interval, while sleep efficiency was calculated as the percentage of time during the rest-interval spent asleep.
Demographic Information, Disease Severity, and Patient Outcomes
Demographic information, including age, race, ethnicity, gender, comorbidities, use of sleep medications, and number of hospitalizations prior to discharge, was collected from thorough chart reviews and patient surveys as part of an ongoing study of admitted general medicine patients at the University of Chicago Medical Center.14 We also obtained patient outcome data such as length of stay from this chart review. Lastly, all patients were called after discharge to assess whether they had been readmitted or presented to the emergency room after discharge (acute care visits).
Staff Surveys
We surveyed a convenience sample of internal medicine resident physicians who attended a lunch conference at the University of Chicago Medical Center on their knowledge of and practice in screening inpatients for OSA. The survey consisted of 5-point Likert type questions, and included original questions as well as questions adapted from prior studies.27 We specifically examined 4 OSA screening-related questions from this survey. The survey was approved by the University of Chicago Institutional Review Board.
Data Analysis
Data were entered directly into REDCap, a secure web application to facilitate access and storage of data.28 Demographics and disease severity variables from the ongoing study were merged into this database. Descriptive statistics were used to calculate sleep duration and efficiency and the prevalence of patients at high risk for OSA, as well as to evaluate the physician sleep survey responses. Chi-square tests were used to examine associations between patient demographics (age, race, sex, Charlson comorbidity index) and OSA risk.33 Because our data were clustered with multiple observations per subject, random effects linear regression models were used to test the association between risk of OSA and sleep duration, sleep efficiency, and KSQI score. This enabled use of all sleep data over multiple nights per subject. Models were adjusted for patient demographics (African American race, age category, sex). Statistical significance was defined as p < 0.05, and statistical analysis was performed using Stata 12.0 (Stata Corp., College Station, TX).
RESULTS
From November 2009 to July 2013, of the 800 patients eligible to participate, 424 (53%) consented to participate in the sleep study and completed the Berlin questionnaire (Figure 1). The majority of patients were African American (72%) and female (57%) (Table 1). The mean age was 65 years (SD = 12), the median length of hospital stay was 4 days (IQR = 3-6), and the median length of enrollment in our study was 2 days (IQR = 1-2), while the mean was 1.8 days. The majority of subjects (63%) did not report significant hypersomnolence as determined by an Epworth Sleepiness Scale. Using wrist actigraphy, average sleep duration was approximately 5 h (313 min, SD = 146), and mean sleep efficiency was 70% (95% CI 67.6%-71.6%), which is below the normal range of 85% to 100%.21,29,30 For 268 (63%) of the patients enrolled, 30-day follow-up interview regarding acute care visits was completed, and approximately 26% had acute care visits.
Figure 1. Patient flow through the study.
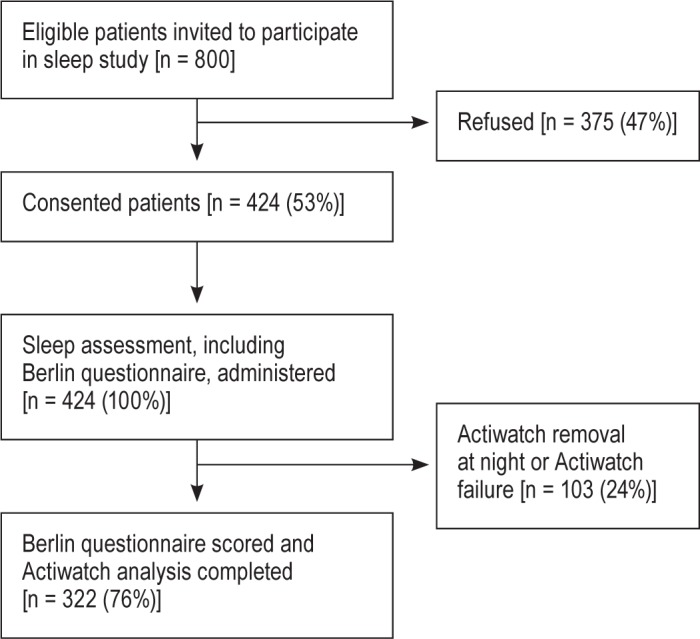
Table 1.
Patient demographics and baseline sleep characteristics (total n = 424).
In our study sample of patients ≥ 50 years old, which by design did not include anyone with a prior diagnosis of a sleep disorder, 2 of every 5 patients (39.5%, n = 168) were screened as high risk for OSA based on the Berlin questionnaire (Figure 2). Patients who were at high risk of OSA were less likely to be in an older age category, 74-93 years old, (χ2 = 7.52, p = 0.006), and more likely to have a higher BMI (30.9 kg/m2 high risk vs. 26.1 kg/m2 low risk, χ2 = 7.43, p = 0.006). Risk of OSA was not associated with male sex (43.5% high risk vs. 43.5% low risk, p = 0.988) or prior hospitalization in the past year (16.7% high risk vs. 13.7% low risk, p = 0.397). There was a trend towards fewer African American patients screening at high risk for OSA (40.3% high risk vs. 59.7% low risk, p = 0.06). Risk of OSA was not associated with a higher Charlson comorbidity score (t = -1.88, p = 0.178).
Figure 2. Percentage of hospitalized patients screened at high risk for obstructive sleep apnea (total n = 424).
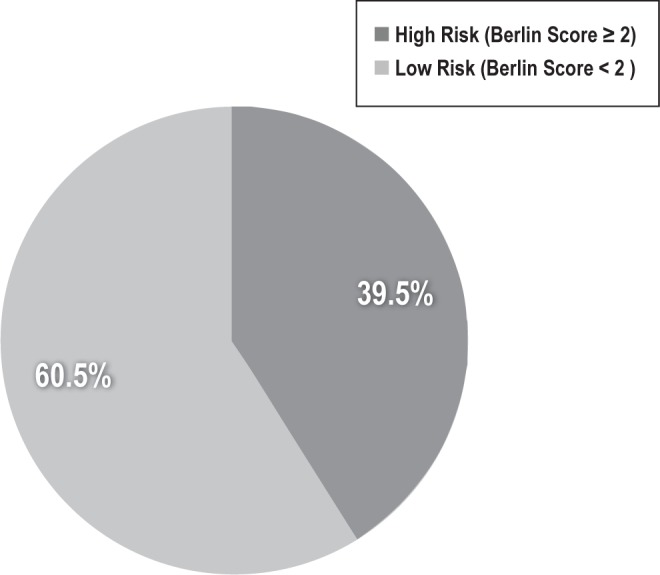
Percentage of hospitalized patients on the general medicine service at the University of Chicago screened as at high or low risk for obstructive sleep apnea (OSA) using the Berlin questionnaire. High risk for OSA is defined as a score on the Berlin questionnaire of ≥ 2.
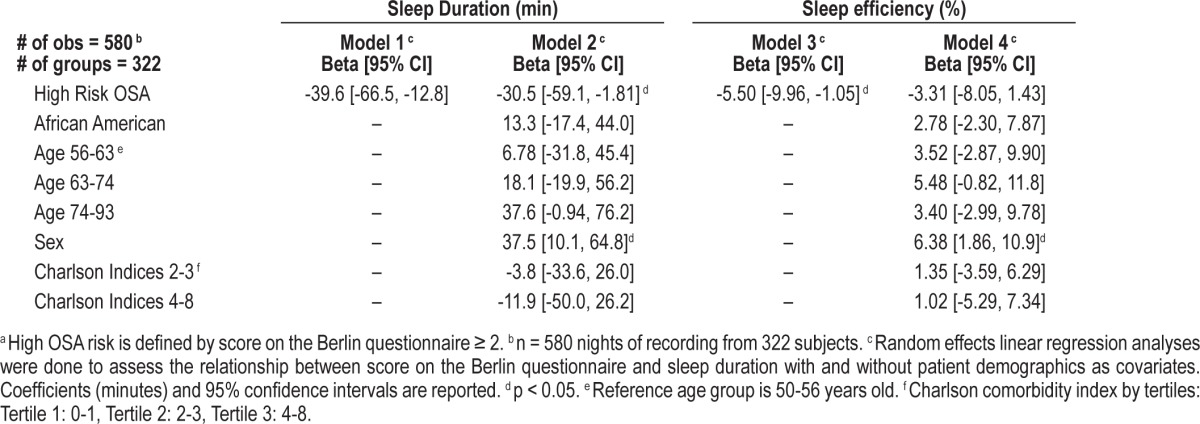
For 322 (76%) of the patients who completed the Berlin questionnaire, actigraphy data were available for regression analyses. Using random effects linear regression models, clustered by subject, patients at high risk for OSA obtained approximately 40 min less sleep per night per actigraphy during hospitalization (-39.6 min [-66.5, -12.8], p = 0.004, Table 2). These findings remained significant after controlling for African American race, Charlson comorbidity index, sex, and age (Table 2). Patients at high risk of OSA also had lower inpatient sleep efficiency (-5.50% [-9.96, -1.05], p = 0.015). This finding also remained significant in multivariate analysis.
Table 2.
Association between OSA riska and in-hospital sleep.
Patients with high risk of OSA reported lower sleep quality on the KSQI (-0.101 [-0.164, -0.037], p = 0.002). This relationship did not remain statistically significant in multivariate analysis, adjusted for Charlson comorbidity index, age, sex, and race (-0.035 [-0.094, -0.024], p = 0.240). Compared to those at low risk of OSA, more patients who were at high risk for OSA reported excessive daytime sleepiness (50.8% > 9 Epworth Sleepiness scale high risk vs. 27.4% low risk, p < 0.001) and worse sleep quality over the last month (83.3% ≥ 5 PSQI high risk vs. 69.1% low risk, p = 0.001). Interestingly, patients who were high risk for OSA were 3 times more likely to receive pharmacologic sleep aids while in the hospital (15.5% of high risk vs. 5.1% of low risk, p < 0.001). Risk of OSA was not associated with hospital outcomes, such as length of stay (high risk median = 4, IQR: 3-7 days, vs. low risk median = 4, IQR: 2.5-6 days) or acute care visits (32% high risk vs. 25% low risk, p = 0.536).
In our convenience sample of 26 resident physicians, 50% reported feeling responsibility to screen inpatients for sleep disorders, but 0% reported routinely doing so. Only 19% reported knowing how to screen patients for sleep disorders, and no residents (0%) were satisfied with their training on sleep disorders.
DISCUSSION
This is the first study to assess the prevalence of OSA risk in adult general medicine inpatients and its relationship to inhospital sleep duration and quality. Our study demonstrated that two of five hospitalized general medicine patients older than 50 years of age were at high risk for OSA. These patients had shorter in-hospital sleep duration, lower sleep efficiency, and worse self-reported sleep quality.
Our finding that 40% of inpatients screened at high risk for OSA is approximately 1.5 times greater than the estimated prevalence in the general population.2 However, because we did not use polysomnography, we were only able to assess risk for, and not actually diagnose, OSA. However, given the positive predictive value of the Berlin questionnaire, it is likely that at least 70% of the inpatients in this study would actually be formally diagnosed with OSA by polysomnography.16 This higher in-hospital prevalence may be explained by several studies that have reported an association between OSA and medical conditions that may result in hospitalization.6 Because untreated OSA is also associated with an increased risk of cardiovascular and cerebrovascular disease, it is theoretically possible that some of our patients were, at least in part, admitted due to consequences related to untreated OSA.
While the difference in sleep time of forty minutes between those who were high risk for OSA and those who were not seems small, it is clinically significant, especially given that sleep duration in both groups was already low. This difference in sleep duration may adversely impact cortisol levels, glucose tolerance, and sympathetic nervous system activity in patients.31 While OSA has been associated with worse postoperative outcome, we did not find a significant association between risk of OSA and various health outcomes such as length of hospital stay, number of hospitalizations after discharge, and number of ER visits after discharge. This may be because our group includes patients who are at risk for OSA but excludes patients with known severe OSA. Nevertheless, undiagnosed OSA patients in the hospital are at risk of preventable adverse events, such as respiratory failure due to inappropriate use of sedatives or narcotics.32 This is especially important because of the higher rate of pharmacologic sleep aid use among inpatients with high risk of OSA, which could lead to patient harm.
While half of residents responded that it was their responsibility to screen inpatients for sleep disorders, none reported routinely doing so. This is likely due to the low level on knowledge on how to screen for sleep disorders and marked dissatisfaction with the training they have received. Although patients were wearing Actiwatches, this did not seem to increase residents' interest in screening for sleep disorders; this may be due to the residents being unaware of what an Actiwatch looks like or its purpose. Furthermore, the Actiwatch model is discrete; in any given week, approximately one of the residents' patients may have been enrolled in the study.
These findings have implications for future interventions to improve screening for OSA in hospitalized older adults. While this may be the first such study done on older medical inpatients, large studies on OSA have been conducted in other patient populations. Interestingly, compared to one of these studies of surgical patients by Lockhart et al., our study found twice as many general medicine inpatients screening at high risk as surgical patients.34 Given the potentially high rates of undiagnosed OSA on medical wards, hospital staff should be trained to routinely screen patients who are at high risk for OSA and refer them for diagnosis and treatment.
This study has several limitations. First, generalizability is limited since it was conducted with general medicine patients at a single institution, and excluded non-English speaking or patients with cognitive impairments. We did not perform polysomnography on the patients in the study and therefore cannot report the actual prevalence of OSA. Moreover, even though we tried to use set inclusion and exclusion criteria to select patients appropriate for actigraphy, it is possible that actigraphy does not accurately measure sleep in hospitalized patients. While studies have assessed the accuracy of actigraphy in ICU patients, as well as in controlled sleep conditions, no known study of the accuracy of actigraphy in general medicine patients has been conducted.35,36 Another study limitation was the rate of Actiwatch removal or failure (24%); the most common reason was removal of the Actiwatch for a procedure, such as MRI, as clinical activities took precedence over our research. It is possible that the patients who consented to the sleep study differ from those that did not consent, raising the concern for selection bias. We also used a convenience sample of internal medicine resident physicians which may not represent all physicians.
In conclusion, a substantial portion of hospitalized adults age 50 or older are at high risk for OSA. These high-risk patients have lower in-hospital sleep duration and efficiency, as well as lower subjective sleep quality, and are more likely to receive pharmacologic sleep aids in the hospital. Screening hospitalized patients for OSA could be a way to identify and treat un-diagnosed OSA patients, as well as potentially improve their long-term health.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was funded by the National Institutes on Aging through a Short-Term Aging-Related Research Program (1 T35 AG029795), National Institute on Aging career development award (K23AG033763), a midcareer career development award (1K24AG031326), a Program Project (P01AG-11412), an Agency for Healthcare Research and Quality Centers for Education and Research on Therapeutics (1U18HS016967) a National Institutes on Aging Clinical Translational Sciences Award (UL1 RR024999), and the American Sleep Medicine Foundation (99-EP-13). The funding agencies had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Dr. Arora had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the statistical analysis.
REFERENCES
- 1.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US Population: Results from the National Sleep Foundation Sleep in America 2005 Poll. Chest. 2006;130:780–86. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 2.Peppard P, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Ballard RD. Sleep and medical disorders. Prim Care. 2005;32:511–33. doi: 10.1016/j.pop.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without treatment with continuous positive airway pressure: a cohort study. Ann Intern Med. 2012;156:115–22. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 6.Correia L, Souza AC, Garcia G, et al. Obstructive sleep apnea affects hospital outcomes of patients with non-ST-elevation acute coronary syndromes. Sleep. 2012;35:1241–5A. doi: 10.5665/sleep.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009;15:739–46. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after elective surgery: analysis of a nationwide inpatient sample. Chest. 2013;144:903–14. doi: 10.1378/chest.12-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after bariatric surgery: analysis of a nationwide inpatient sample. Obes Surg. 2013;23:1842–51. doi: 10.1007/s11695-013-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora VM, Chang KL, Fazal AZ, et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59:2185–6. doi: 10.1111/j.1532-5415.2011.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meissner HH, Riemer A, Santiago SM, Stein M, Goldman MD, Williams AJ. Failure of physician documentation of sleep complaints in hospitalized patients. Western J Med. 1998;169:146–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Goring K, Collop N. Sleep disordered breathing in hospitalized patients. J Clin Sleep Med. 2008;4:105–10. [PMC free article] [PubMed] [Google Scholar]
- 13.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 14.Meltzer D, Manning WG, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137:866–74. doi: 10.7326/0003-4819-137-11-200212030-00007. [DOI] [PubMed] [Google Scholar]
- 15.Lin JS, O'Connor E, Rossom RC, et al. Screening for cognitive impairment in older adults: A systematic review of the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:601–12. doi: 10.7326/0003-4819-159-9-201311050-00730. [DOI] [PubMed] [Google Scholar]
- 16.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin Questionnaire and American Society of Anesthesiologists Checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108:822–30. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 17.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57:423–38. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 18.Fedson, Annette C, Pack A, Gislason T. Frequently used sleep questionnaires in epidemiological and genetic research for obstructive sleep apnea: a review. Sleep Med Rev. 2012;16:529–37. doi: 10.1016/j.smrv.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Nagel CL, Markie MB, Richards KC, Taylor JL. Sleep promotion in hospitalized elders. Medsurg Nurs. 2003;12:279–89. [PubMed] [Google Scholar]
- 20.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 21.Keklund G, Akerstedt T. Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6:217–20. doi: 10.1111/j.1365-2869.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- 22.Adachi M, Staisiunas PG, Knutson KL, Beveridge C, Meltzer DO, Arora VM. Perceived control and sleep in hospitalized older patients: a sound hypothesis? J Hosp Med. 2013;8:184–90. doi: 10.1002/jhm.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey AG, Stinson K, Whitaker KL, et al. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31:383–93. doi: 10.1093/sleep/31.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989;28:193–13. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Murphy SL. Review of physical activity measurement using accelerometers in older adults: Considerations for research design and conduct. Prev Med. 2009;48:108–14. doi: 10.1016/j.ypmed.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papp KK, Penrod CE, Strohl KP. Knowledge and attitudes of primary care physicians toward sleep and sleep disorders. Sleep Breath. 2002;6:103–9. doi: 10.1007/s11325-002-0103-3. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 284:861–8. doi: 10.1001/jama.284.7.861. 200. [DOI] [PubMed] [Google Scholar]
- 30.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 31.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 32.Chung SA, Yuan H, Chung F. A systematic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543–63. doi: 10.1213/ane.0b013e318187c83a. [DOI] [PubMed] [Google Scholar]
- 33.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 34.Lockhart EM, Willingham MD, Abdallah AB, et al. Obstructive sleep apnea screening and postoperative mortality in a large surgical cohort. Sleep Med. 2013;14:407–15. doi: 10.1016/j.sleep.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ. Sleep monitoring in the intensive care unit: comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med. 2008;34:2076–83. doi: 10.1007/s00134-008-1180-y. [DOI] [PubMed] [Google Scholar]
- 36.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36:1747–55. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]


