Abstract
Study Objective:
Fluid displacement from the legs during recumbency while in bed might narrow the upper airway (UA) in association with nuchal fluid accumulation that may contribute to the pathogenesis of obstructive sleep apnea (OSA). The aim of this study was to test the hypothesis that rostral fluid displacement from the legs causes a greater decrease in UA cross-sectional area (UA-XSA) and a greater increase in UA mucosal water content (UA-MWC) and internal jugular venous volume (IJVVol) in subjects with OSA than in those without OSA.
Methods:
Subjects underwent baseline assessment of leg fluid volume (LFV) measured by bio-electrical impedance, as well as UA-XSA and UA-MWC by magnetic resonance imaging. They were then randomly assigned to a 20-min period either with or without application of lower body positive pressure (LBPP) of 40 mm Hg, followed by a 15-min washout period, after which they crossed over to the other arm of the study. Measurements of LFV, UA-MWC, and UA-XSA were repeated after each arm of the study.
Results:
In 12 subjects without sleep apnea, UA-XSA increased and UA-MWC decreased significantly, whereas in 12 subjects with OSA, UA-XSA decreased and UA-MWC increased significantly in response to LBPP. The changes in UA-XSA and UA-MWC in response to LBPP differed significantly between the 2 groups (p = 0.006 and p < 0.001, respectively), despite similar changes in LFV and IJVVol.
Conclusions:
Our results suggest that rostral fluid shift may contribute to the pathogenesis of OSA at least partly through narrowing of the UA due to transudation of fluid into the UA mucosa.
Citation:
Kasai T, Motwani SS, Elias RM, Gabriel JM, Taranto Montemurro L, Yanagisawa N, Spiller N, Paul N, Bradley TD. Influence of rostral fluid shift on upper airway size and mucosal water content. J Clin Sleep Med 2014;10(10):1069-1074.
Keywords: fluid displacement, lower body positive airway pressure, obstructive sleep apnea, upper airway cross-sectional area, upper airway mucosal water
Increased body mass index (BMI), and increased neck circumference (NC)—indicators of obesity—are reported to be important risk factors for obstructive sleep apnea (OSA) in the general population.1 However, BMI and NC only account for approximately 4% and 29%, respectively, of the variability in OSA severity, as quantified by the frequency of apneas and hypopneas per hour of sleep (apnea-hypopnea index [AHI]).2 Therefore, other factors must be involved in the pathogenesis of upper airway (UA) obstruction in OSA patients.
One such factor might be fluid displacement from the legs into the neck while lying recumbent at night. An increase in neck fluid volume could increase tissue pressure thereby narrowing the UA and increasing its propensity to collapse. Shepard and colleagues first tested the effects of shifting fluid into the neck by raising the legs, and of reducing venous return to the upper body by applying venous occlusive tourniquets around the thighs in OSA patients.3 Using computed tomography, they found a tendency for UA cross-sectional area (UAXSA) to decrease and increase in response to leg raising and tourniquet application, respectively; but these changes were not significant at functional residual capacity, probably because these interventions did not cause sufficient fluid displacement to alter UA size. In addition, they did not confirm that fluid accumulated in nuchal structures in response to leg raising, as changes in leg fluid volume (LFV), NC, jugular venous volume, and nuchal soft tissue fluid content were not measured.
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is a strong relationship between frequency of apneas and hypopneas per hour of sleep (apnea-hypopnea index [AHI]) and both the amount of fluid displaced from the legs and increase in neck circumference overnight, suggesting that nuchal fluid accumulation may contribute to obstructive sleep apnea (OSA) pathogenesis. However, it is not known whether such nuchal fluid accumulation occurs mainly in the jugular veins or upper airway (UA) mucosa, and whether this is related to narrowing of the UA.
Study Impact: This study demonstrates that UA cross-sectional area decreases, and UA mucosal water content increases more in subjects with OSA than in those without OSA in response to fluid displacement from the legs by application of lower body positive pressure, whereas jugular venous volume does not change in either group. Therefore, rostral fluid shift may contribute to the pathogenesis of OSA, at least partly through narrowing of the UA due to transudation of fluid into the UA mucosa.
Another potentially more effective, noninvasive means of displacing fluid from the lower extremities to the upper body is the application of lower body positive pressure (LBPP) by inflatable anti-shock trousers. LBPP displaces fluid from the legs into the upper body and increases central venous pressure.4,5 In our previous studies, we applied LBPP to healthy euvolemic subjects to mimic the effects of fluid redistribution from the lower to the upper body upon assuming the recumbent position in patients with fluid retention in their legs. In those studies, we showed that LBPP displaced fluid from the legs and caused increases in NC and UA resistance, decreases in UA-XSA, and increases in UA collapsibility.6–8 If such nuchal fluid redistribution contributes to UA narrowing and/or collapsibility, it could accumulate either in the vascular compartment and distend the jugular veins, or in the interstitial compartment of the soft tissues surrounding the UA, or both. Therefore, we tested the hypotheses, first, that LBPP decreases UA-XSA to a greater extent in subjects with than in those without OSA, and second, that such narrowing is related to distension of the internal jugular veins (IJV) and/or fluid accumulation in the peripharyngeal tissues as assessed by magnetic resonance imaging (MRI).
MATERIALS AND METHODS
Subjects
Subjects referred for suspected sleep apnea were recruited from our sleep disorders clinic. Inclusion criteria were men and women, 18 to 80 years of age with no history of cardiovascular, renal, or neurological disease, and no relevant use of medication. Exclusion criteria were tonsillar hypertrophy, a current history of smoking or alcohol abuse, and treated OSA. Demographic characteristics were recorded at the time of experiments. The protocol was approved by the Research Ethics Boards of the University Health Network, and all subjects provided written consent before participation.
Polysomnography
All subjects underwent overnight polysomnography (PSG) using standard techniques and scoring criteria for sleep stages and arousals.9,10 Thoracoabdominal motion was monitored by respiratory inductance plethysmography, and nasal airflow by nasal pressure cannulae. Oxyhemoglobin saturation (SpO2) was monitored by oximetry. Obstructive apnea was defined as > 90% reduction of tidal volume for ≥ 10 s in the presence of out-of-phase thoracoabdominal motion; and obstructive hypopnea was defined as a 50% to 90% reduction in tidal volume from baseline for ≥ 10 sec with out-of-phase thoracoabdominal motion or airflow limitation on nasal pressure.11 The AHI was quantified. Signals were recorded on a computerized sleep recording system (Sandman, Nellcor Puritan Bennett Ltd, Ottawa, Ontario, Canada) and scored by technicians blinded to clinical characteristics of subjects. Subjects were defined as having OSA if their AHI was ≥ 15/h of sleep. Subjects with AHI < 10 h of sleep were defined as having no sleep apnea (NSA).
Lower Body Positive Pressure
With subjects lying supine, deflated medical anti-shock trousers (MAST III-AT; David Clark, Inc., Worcester, MA) were applied to both legs from the ankles to the upper thighs at the beginning of the baseline period. LBPP was applied by rapidly inflating the trousers to 40 mm Hg for 20 min after which the trousers were deflated.
Leg Fluid Volume
Total fluid volume of both legs was measured using a bio-electric impedance spectrum analyzer (model 4200; Xitron Technologies, Inc., San Diego, CA).6–8,12–18 Two pairs of electrodes were applied to each leg: one pair to the upper thigh and the other to the ankle. This well-validated technique uses impedance to electric current within a body segment to measure its fluid content.19,20 Alterations in fluid content of tissues cause proportional changes in impedance.
Upper Airway Magnetic Resonance Imaging
Following PSG, all subjects underwent MRI of their UA. Following localizer scans in 3 orthogonal planes, T2 axial images of the UA from the hard palate to the glottis were acquired using an 8-channel high definition neurovascular phased array coil. A respiratory bellows was placed around the lower chest to gate images to end-expiration when the UA is narrowest.21 Patients manually activated a buzzer every 2 min on command to ensure that they remained awake throughout. Patients lay supine in the scanner breathing normally through their noses, with their heads fixed in the neutral position in a holding frame.22,23
Using fast recovery FSE (FRFSE) sequences with a repetition time (TR) of 6,000 ms and echo time (TE) of 86 ms, field of view, 200 mm and acquisition matrix, 256 × 256, contiguous 6-mm thick axial slices were acquired. Axial images of the internal jugular veins adjacent to the UA were acquired by 2-dimensional non-contrast MRI venography in 2-mm thick slices using gradient echo sequences with the following parameters: TR/TE, 35-50/9 ms; flip angle, 60°; field of view, 200 mm; matrix size, 256 × 160; and acquisition time, 9 min 10 s. UA mucosal water content (UA-MWC), was assessed by a short Tau inversion recovery sequence, as previously described23 with the following parameters: TR, 2,500-3,500 ms/TE, 45 ms/inversion time (TI), 125 ms; field of view, 200 mm; matrix size, 256 × 192, in 6 mm thick slices with an acquisition time of 3 min 48 s. Short Tau inversion recovery images were examined using a manually prescribed region of interest around the UA mucosa to quantify high intensity signal which reflects UA-MWC. A syringe filled with water was positioned beside the patient's neck during scans, as a reference for water content. The values were expressed in arbitrary units. The total scan time was 20 minutes.
A radiologist blinded to study conditions analyzed the UA data. Using MATLAB (MATrix LABoratory), the radiologist used electronic calipers to manually trace the UA lumen and calculate the XSA of each 6 mm slice from the soft palate to the glottis. These were averaged to provide mean UA-XSA. The smallest UA-XSA was also noted. A similar procedure was used to delineate the UA mucosa on the short Tau inversion recovery sequences in order to quantify UA-MWC.
A technologist, blinded to study conditions, analyzed jugular vein volumes. The XSA of each 2-mm slice of the internal jugular veins was calculated at the same level using dedicated vascular 3-D post processing software (VITREA, Vital Images Inc.), from which the volume for each slice was derived, and summed to provide right and left internal jugular vein volumes (IJVVol).24
Experimental Protocol
A randomized double crossover design was used. Subjects lay supine on the MRI table and were initially randomized to either LBPP of 40 mm Hg for 20 min, or to a 20 min control period during which the anti-shock trousers were wrapped around the legs in a deflated state with no pressure applied. After baseline measurement of the fluid volume of both legs while supine with the trousers deflated, MRI scanning was performed either with or without LBPP according to the randomization. Immediately after MRI scanning, LFV was measured while supine. Subjects were then seated upright with the anti-shock trousers removed for 15 min as a washout period. Subjects then crossed over to the other arm of the study for 20 min during which a second MRI scan was performed.
Statistical Analysis
Values are expressed as mean ± SD unless indicated otherwise. Baseline characteristics of the NSA and OSA groups were compared by using the Student t-test for continuous variables. Fisher exact test was used to compare nominal variables between the 2 groups. All measured variables during LBPP periods were also compared between NSA and OSA groups using a linear mixed model. The model included treatments, groups, a group-treatment interaction, and order effect as fixed effects and subject as a random effect. A significant interaction effect indicates a difference in treatment effect between groups. Because the distribution of both UA-XSA and UA-MWC values were skewed, the log-transformed values were used for all statistical analyses. Relationships between the 2 variables were examined by the Spearman correlation coefficient. Differences in UA-XSA, IJVVol, and UA-MWC between control and LBPP periods (i.e., [values during LBPP period] – [values during control period]) were computed and used for correlation analyses. A two-sided p value < 0.05 was considered significant. Analyses were performed by SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Subjects
We recruited 12 subjects without sleep apnea and 12 subjects with OSA whose characteristics are shown in Table 1. Subjects with OSA had greater AHIs than subjects without sleep apnea by design. There were no significant differences in other characteristics between the 2 groups.
Table 1.
Characteristics of the subjects.
Magnetic Resonance Images of the Upper Airway
Representative MRI images of the UA in a subject with and a subject without OSA are shown in Figure 1. Note that the UA-XSA during control is smaller in the subject with OSA than in the subject without OSA. In addition, during LBPP, there was an increase in UA-XSA in the subject from the NSA group compared to control, whereas there was a decrease in UA-XSA in the subject from the OSA group compared to control.
Figure 1. Magnetic resonance images of the upper airway (UA) of subjects.
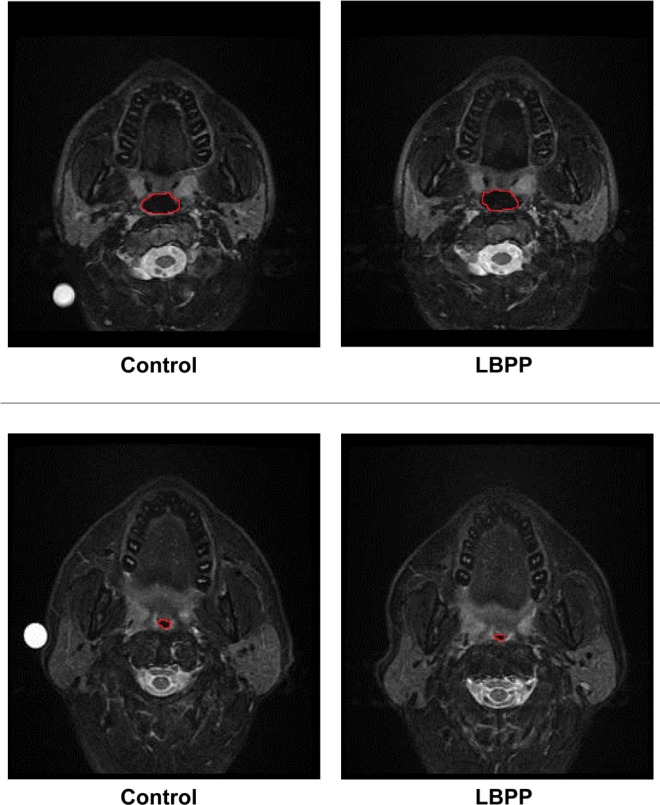
top: In the NSA subject, LBPP caused UA-XSA to increase compared to control (from 3.01 to 3.71 cm2). bottom: In the OSA subject, LBPP caused UA-XSA to decrease compared to control (from 2.04 to 1.72 cm2). LBPP, lower body positive pressure; NSA, no sleep apnea; OSA, obstructive sleep apnea; UA, upper airway; UA-XSA, upper airway cross-sectional area.
Upper Airway Cross-Sectional Area, Internal Jugular Vein Volume, and Upper Airway Mucosal Water Content
Although log-transformed UA-XSA tended to be smaller during the control period in the OSA than in the NSA group, this difference did not reach statistical significance (0.26 ± 0.23 versus 0.51 ± 0.38, p = 0.070). Similarly, neither mean IJVVol (6.39 ± 2.67 versus 7.65 ± 2.04 cm3, p = 0.207) nor mean of log-transformed UA-MWC during the control period (9.5 ± 0.4 versus 9.6 ± 0.5, p = 0.659) differed significantly between the OSA and NSA groups. In addition, there was a significant inverse correlation between the AHI and log-transformed UA-XSA during control (r = -0.412, p = 0.045). However, there was no significant correlation between the AHI and control IJVVol (r = -0.268, p = 0.205) or control UA-MWC (log-transformed) (r = -0.106, p = 0.623).
Effects of Lower Body Positive Pressure
Similar reductions in LFV in response to LBPP were observed in both groups (p = 0.922, Table 2). In the NSA group, mean UA-XSA increased significantly during LBPP compared to control. In contrast, in the OSA group, mean UA-XSA decreased significantly during LBPP compared to control. The change in mean UA-XSA in response to LBPP differed significantly between the 2 groups (p = 0.006, Figure 2). There was no significant change in IJVVol in response to LBPP in either group compared to control, and the change in IJVVol in response to LBPP did not differ between the 2 groups (p = 0.478, Table 2). In the NSA group, UA-MWC decreased significantly during LBPP compared to control, whereas in the OSA group, UA-MWC increased significantly during LBPP. The increase in UA-MWC in response to LBPP was significantly greater in the OSA than in the NSA group (p < 0.001, Figure 3).
Table 2.
Effect of LBPP on ΔLFV and IJVVol.
Figure 2. Comparisons of mean UA-XSA during control and LBPP in the NSA and OSA groups.
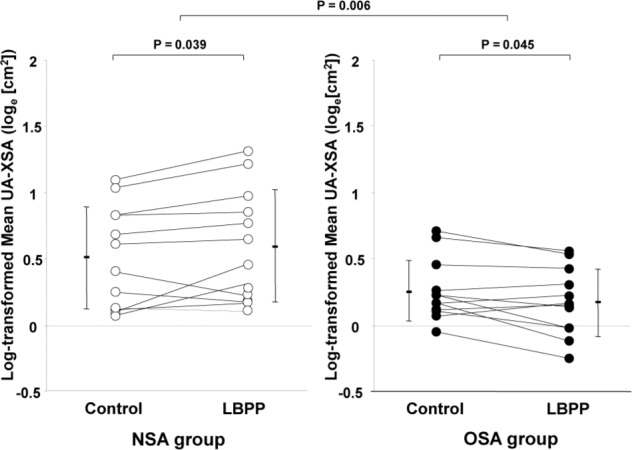
In the NSA group, mean UA-XSA during LBPP increased significantly compared to control (log-transformed UA-XSA, 0.60 ± 0.42 versus 0.51 ± 0.38, respectively [median (interquartile range); absolute UA-XSA, 1.67 (1.17) versus 1.74 (1.36) cm2, respectively], whereas in the OSA group mean UA-XSA decreased significantly during LBPP compared to control (log transformed UA-XSA, 0.18 ± 0.25 versus 0.26 ± 0.23, respectively) [median (interquartile range); absolute UA-XSA, 1.17 (0.52) versus 1.23 (0.40) cm2, respectively]. The change in mean UA-XSA in response to LBPP differed significantly between the 2 groups (p = 0.006). Note that data are plotted on a log scale. LBPP, lower body positive pressure; NSA, no sleep apnea; OSA, obstructive sleep apnea; UA-XSA, upper airway cross-sectional area.
Figure 3. Comparisons of UA-MWC during control and LBPP in both the NSA and OSA groups.
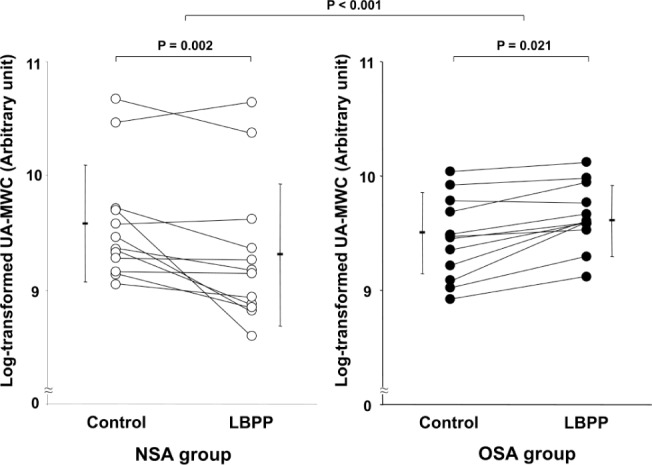
In the NSA group, log-transformed UA-MWC during LBPP decreased significantly compared to control (9.3 ± 0.6 versus 9.6 ± 0.5, respectively), whereas in the OSA group, log-transformed UA-MWC during LBPP increased significantly compared to control (9.6 ± 0.3 versus 9.5 ± 0.4, respectively). The change in UA-XSA in response to LBPP differed significantly between the 2 groups (p < 0.001). Note that data are plotted on a log scale. LBPP, lower body positive pressure; NSA, no sleep apnea; OSA, obstructive sleep apnea; UA-MWC, upper airway mucosal water content.
There were significant correlations between the AHI and change in mean UA-XSA (r = -0.465, p = 0.022) and change in UA-MWC (r = 0.624, p = 0.001), whereas there was no significant relationship between AHI and change in IJVVol (r = 0.246, p = 0.246). In addition, there was a significant correlation between change in mean UA-XSA and change in UA-MWC (r = -0.497, p = 0.014). However, there was no significant correlation between change in IJVVol and changes in mean UA-XSA (r = -0.045, p = 0.834) or UA-MWC (r = -0.097, p = 0.654). Furthermore, there were no relationships between change in ΔLFV and AHI (r = 0.026, p = 0.904), mean UA-XSA (r = -0.123, p = 0.566), IJVVol (r = -0.168, p = 0.432) or UAMWC (r = 0.196, p = 0.360).
DISCUSSION
The findings of the present study suggest that in awake subjects without sleep apnea, acute fluid displacement from the legs induces an increase in UA-XSA in association with a reduction in peripharyngeal water content. The reason for this is not clear. One possibility is that LBPP and rostral fluid shift induce reflex activation of UA dilator muscles similar to that induced by application of negative airway pressure.25 Muscle contraction could, in turn, lead to extrusion of fluid from the peripharyngeal tissues.26 However, since we could not measure genioglossus muscle activity during MRI scanning, we were unable to determine whether activation of dilator muscles contributed to increased UA-XSA during LBPP. Another possibility was that LBPP and rostral fluid displacement caused sympathetically mediated peripharyngeal vasoconstriction with reduced UA-MWC and UA dilation.27 However, we were unable to measure sympathetic activity during MRI scanning, and in previous studies it has been shown that LBPP does not affect indices of sympathetic activity.28 Further studies will be required to determine whether LBPP has any effect on genioglossus or sympathetic activity that might affect UA-XSA.
In contrast to subjects without SA, in those with OSA, the increase in UA-MWC and reduction in UA-XSA arising from LBPP indicated some degree of UA obstruction had been induced. During sleep, such an effect of rostral fluid displacement from the legs would predispose to OSA.14,15 However, it was not possible to perform experiments in the MRI scanner with confirmation that subjects were asleep under all conditions. It is therefore possible that the present findings may not be entirely applicable to the sleeping state. However, responses to fluid shift during sleep are unlikely to be qualitatively different. The pharynx narrows and pharyngeal resistance invariably increases at the transition from wakefulness to sleep in patients with OSA and in healthy subjects.29,30 Thus, one would anticipate that a given degree of fluid shift into the neck during sleep would cause an even greater increase in pharyngeal airflow obstruction in patients with OSA, because resistance would increase inversely to the fourth power of the change in the radius of the pharyngeal lumen.
These findings provide evidence of a differential response to fluid displacement from the legs that could predispose to OSA by inducing UA obstruction on the one hand or prevent OSA by inducing UA dilatation on the other. This may be one explanation for why subjects in the NSA group did not have OSA despite a BMI similar to those who did. Furthermore, the combination of findings from the present and our previous studies6–8 suggest that rostral fluid shift when moving from the upright to the recumbent position at bedtime could predispose to UA collapse and OSA in susceptible individuals at least partially through fluid accumulation in peripharyngeal soft tissue. In keeping with these possibilities, surgical specimens from uvulopalatopharyngoplasty from OSA patients revealed vascular congestion and diffuse interstitial edema,31 while peripharyngeal water content assessed by MRI scanning before treatment in OSA patients decreased following chronic application of continuous positive airway pressure.23
In another study involving hemodialyzed patients with end-stage renal disease (ESRD), we demonstrated significant direct relationships between their AHI and both their baseline IJVVol, and UA-MWC.24 Those support a role for both intra- and extra-vascular peripharyngeal fluid accumulation in the pathogenesis of OSA in ESRD patients. However, in the present study we did not find any relationship between AHI and either baseline IJVVol or UA-MWC. One possible explanation for this difference is that ESRD patients generally have much greater fluid retention than healthy subjects. Consequently, when they lie down, a large amount of fluid accumulates immediately in IJV and UA mucosa, whereas in subjects without ESRD or other fluid retaining states, such as in the subjects described herein, fluid does not accumulate to the same extent in IJV and UA mucosa immediately upon lying down. However, when LBPP was applied and fluid was extruded from the legs, we found that the degree of the ensuing increase in UA-MWC was related to the AHI, whereas the degree of change in IJVVol was not. In addition, we found that the degree of decrease in UA-XSA in response to LBPP was inversely related to the concomitant increase in UA-MWC. Therefore, it appeared that the main factor contributing to UA narrowing in response to rostral fluid shift during LBPP in OSA subjects was accumulation of extravascular fluid in the UA mucosa rather than intravascular fluid accumulation in the IJV.
In a previous study involving non-obese subjects, LBPP for 5 min caused a narrowing of the UA measured by acoustic pharyngometry.7 The reason why the NSA group in the present study experienced dilation rather than narrowing of the UA in response to LBPP is not clear. In our previous study, we applied LBPP of 40 mm Hg for only 5 min, whereas we applied it for 20 min in the present study. As shown in our previous study,7 maximum narrowing of the UA occurred after 1 min and was sustained until 5 min. However, we do not know the time course of UA-XSA change between 0 and 20 min in the present study, nor do we know what happened to UA-XSA after 5 min in the previous study. For example, it could be that in the NSA group UA dilation in response to LBPP was a relatively late response that occurred sometime between 5 and 20 min. In addition, acoustic pharyngometry only assesses XSA of the oropharyngeal and hypopharyngeal part of the UA, whereas MRI scanning assesses the entire UA, including the retropalatal pharynx not included in acoustic pharyngometry. Nevertheless, the decrease in UA-MWC that accompanied the increase in UA-XSA in the NSA group is internally consistent. This observation supports the concept that alternations in UA-MWC contribute to alterations in UA-XSA that are related to the severity of OSA.
CONCLUSIONS
The present study provides novel evidence in OSA subjects that fluid displacement from the legs into peripharyngeal soft tissue can constrict the UA lumen and influence the AHI. Fluid displacement from the lower to the upper body while recumbent may therefore contribute to pharyngeal narrowing and predispose to its collapse during sleep in OSA patients. On the other hand, in subjects without sleep apnea, there appears to be mechanisms that induce UA dilation in response to rostral fluid shift that could protect against development of OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was supported by Canadian Institutes of Health Research operating grant MOP-82731. Dr. Kasai was supported by an unrestricted research fellowship from Fuji-Respironics Inc. Dr. Motwani was supported by Toronto Rehabilitation Institute, Dr. Elias was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, Toronto Rehabilitation Institute, and Toronto General Hospital. Dr. Taranto Montemurro was supported by fellowships from the Chair of Respiratory Medicine, University of Brescia, Brescia, Italy and from Toronto Rehabilitation Institute. Mr. Gabriel was supported by Ontario Student Opportunity Trust Fund Awards from the Toronto Rehabilitation Institute and the Cardiovascular Sciences Collaborative Program of the University of Toronto. Dr. Bradley was supported by the Clifford Nordal Chair in Sleep Apnea and Rehabilitation Research. The other authors have indicated no financial conflicts of interest. The work was performed in Toronto General Hospital University Health Network., Toronto, Ontario, Canada.
REFERENCES
- 1.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3:509–14. [PubMed] [Google Scholar]
- 2.Katz I, Stradling J, Slutsky AS, Zamel N, Hoffstein V. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141:1228–31. doi: 10.1164/ajrccm/141.5_Pt_1.1228. [DOI] [PubMed] [Google Scholar]
- 3.Shepard JW, Jr., Pevernagie DA, Stanson AW, Daniels BK, Sheedy PF. Effects of changes in central venous pressure on upper airway size in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;153:250–4. doi: 10.1164/ajrccm.153.1.8542124. [DOI] [PubMed] [Google Scholar]
- 4.Bivins HG, Knopp R, Tiernan C, dos Santos PA, Kallsen G. Blood volume displacement with inflation of antishock trousers. Ann Emerg Med. 1982;11:409–12. doi: 10.1016/s0196-0644(82)80036-x. [DOI] [PubMed] [Google Scholar]
- 5.Shi X, Crandall CG, Raven PB. Hemodynamic responses to graded lower body positive pressure. Am J Physiol. 1993;265:H69–73. doi: 10.1152/ajpheart.1993.265.1.H69. [DOI] [PubMed] [Google Scholar]
- 6.Chiu KL, Ryan CM, Shiota S, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174:1378–83. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 7.Shiota S, Ryan CM, Chiu KL, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62:868–72. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su MC, Chiu KL, Ruttanaumpawan P, et al. Lower body positive pressure increases upper airway collapsibility in healthy subjects. Respir Physiol Neurobiol. 2008;161:306–12. doi: 10.1016/j.resp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 9.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 10.Rechtschaffen A, Kales A. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. A Manual of Standardized Terminology, Techniques and Scoring for Sleep Stages of Human Subjects. [Google Scholar]
- 11.Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail. 2009;15:279–85. doi: 10.1016/j.cardfail.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56:1077–82. doi: 10.1161/HYPERTENSIONAHA.110.154427. [DOI] [PubMed] [Google Scholar]
- 13.Su MC, Chiu KL, Ruttanaumpawan P, et al. Difference in upper airway collapsibility during wakefulness between men and women in response to lower-body positive pressure. Clin Sci (Lond) 2009;116:713–20. doi: 10.1042/CS20080321. [DOI] [PubMed] [Google Scholar]
- 14.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–6. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 15.Yumino D, Redolfi S, Ruttanaumpawan P, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121:1598–605. doi: 10.1161/CIRCULATIONAHA.109.902452. [DOI] [PubMed] [Google Scholar]
- 16.Redolfi S, Arnulf I, Pottier M, Bradley TD, Similowski T. Effects of venous compression of the legs on overnight rostral fluid shift and obstructive sleep apnea. Respir Physiol Neurobiol. 2011:390–3. doi: 10.1016/j.resp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Redolfi S, Arnulf I, Pottier M, et al. Attenuation of obstructive sleep apnea by compression stockings in subjects with venous insufficiency. Am J Respir Crit Care Med. 2012;184:1062–6. doi: 10.1164/rccm.201102-0350OC. [DOI] [PubMed] [Google Scholar]
- 18.Elias RM, Bradley TD, Kasai T, Motwani SS, Chan CT. Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transplant. 2012;27:1569–73. doi: 10.1093/ndt/gfr605. [DOI] [PubMed] [Google Scholar]
- 19.Zhu F, Kuhlmann MK, Kotanko P, Seibert E, Leonard EF, Levin NW. A method for the estimation of hydration state during hemodialysis using a calf bioimpedance technique. Physiol Meas. 2008;29:S503–16. doi: 10.1088/0967-3334/29/6/S42. [DOI] [PubMed] [Google Scholar]
- 20.HYDRA ECF/ICF (Model 4200) San Diego, CA, USA: Operating Manual Revision 1.03 ed: Xitron Technologies, Inc; 2007. Bio-impedance spectrum analyzer for measuring intracellular and extracellular fluid volumes. [Google Scholar]
- 21.Arens R, Sin S, McDonough JM, et al. Changes in upper airway size during tidal breathing in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2005;171:1298–304. doi: 10.1164/rccm.200411-1597OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horner RL, Mohiaddin RH, Lowell DG, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J. 1989;2:613–22. [PubMed] [Google Scholar]
- 23.Ryan CF, Lowe AA, Li D, Fleetham JA. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after chronic nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1991;144:939–44. doi: 10.1164/ajrccm/144.4.939. [DOI] [PubMed] [Google Scholar]
- 24.Elias RM, Chan CT, Paul N, et al. Relationship of pharyngeal water content and jugular volume with severity of obstructive sleep apnea in renal failure. Nephrol Dial Transplant. 2013;28:937–44. doi: 10.1093/ndt/gfs473. [DOI] [PubMed] [Google Scholar]
- 25.Horner RL, Innes JA, Morrell MJ, Shea SA, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol. 1994;476:141–51. [PMC free article] [PubMed] [Google Scholar]
- 26.Goddard AA, Pierce CS, McLeod KJ. Reversal of lower limb edema by calf muscle pump stimulation. J Cardiopulm Rehabil Prev. 2008;28:174–9. doi: 10.1097/01.HCR.0000320067.58599.ac. [DOI] [PubMed] [Google Scholar]
- 27.Wasicko MJ, Leiter JC, Erlichman JS, Strobel RJ, Bartlett D., Jr Nasal and pharyngeal resistance after topical mucosal vasoconstriction in normal humans. Am Rev Respir Dis. 1991;144:1048–52. doi: 10.1164/ajrccm/144.5.1048. [DOI] [PubMed] [Google Scholar]
- 28.Fu Q, Sugiyama Y, Kamiya A, Shamsuzzaman AS, Mano T. Responses of muscle sympathetic nerve activity to lower body positive pressure. Am J Physiol. 1998;275:H1254–9. doi: 10.1152/ajpheart.1998.275.4.H1254. [DOI] [PubMed] [Google Scholar]
- 29.Skatrud JB, Dempsey JA. Airway resistance and respiratory muscle function in snorers during NREM sleep. J Appl Physiol. 1985;59:328–35. doi: 10.1152/jappl.1985.59.2.328. [DOI] [PubMed] [Google Scholar]
- 30.Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol. 2005;99:2440–50. doi: 10.1152/japplphysiol.00772.2005. [DOI] [PubMed] [Google Scholar]
- 31.Anastassov GE, Trieger N. Edema in the upper airway in patients with obstructive sleep apnea syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:644–7. doi: 10.1016/s1079-2104(98)90197-4. [DOI] [PubMed] [Google Scholar]


