Abstract
Study Objective:
To evaluate multiple doses of gabapentin 250 mg on polysomnography (PSG) and participant-reported sleep assessments in a 5-h phase advance insomnia model.
Methods:
Adults reporting occasional disturbed sleep received gabapentin 250 mg (n = 128) or placebo (n = 128). On Days 1 and 28, participants received medication 30 min before bedtime and were in bed from 17:00 to 01:00, ∼5 h before their habitual bedtime. Sleep was assessed by PSG, a post sleep questionnaire, and the Karolinska Sleep Diary. Next-day residual effects and tolerability were evaluated. On Days 2-27, participants took medication at home 30 min before their habitual bedtime.
Results:
Treatment-group demographics were comparable. Gabapentin resulted in significantly less PSG wake after sleep onset (WASO) compared with placebo on Day 1 (primary endpoint, mean: 107.0 versus 149.1 min, p ≤ 0.001) and Day 28 (113.6 versus 152.3 min, p = 0.002), and significantly greater total sleep time (TST; Day 1: 347.6 versus 283.9 min; Day 28: 335.3 versus 289.1 min) (p ≤ 0.001). Participant-reported WASO and TST also showed significant treatment effects on both days. Gabapentin was associated with less %stage1 on Day 1, and greater %REM on Day 28, versus placebo. During home use, gabapentin resulted in significantly less participant-reported WASO and higher ratings of sleep quality. Gabapentin was well tolerated (most common adverse events: headache, somnolence) with no evidence of next-day impairment.
Conclusion:
Gabapentin 250 mg resulted in greater PSG and participant-reported sleep duration following a 5-h phase advance on Day 1 and Day 28 of use without evidence of next-day impairment, and greater sleep duration during at-home use.
Citation:
Furey SA, Hull SG, Leibowitz MT, Jayawardena S, Roth T. A randomized, double-blind, placebo-controlled, multicenter, 28-day, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med 2014;10(10):1101-1109.
Keywords: Gabapentin, insomnia, transient, polysomnography, sleep phase advance
Gabapentin interacts with α2δ subunits of voltage-gated calcium channels1 and is approved for the management of postherpetic neuralgia and restless legs syndrome (RLS), and as adjunctive therapy for the treatment of partial seizures with and without secondary generalization (with maximal daily doses ranging from 600 to 1,800 mg, depending on indication and drug formulation).2,3 In addition to these approved indications, gabapentin has been investigated in a variety of patient populations, and evidence from clinical studies suggests that it may have a positive effect on sleep. In clinical studies of pain-related disorders, gabapentin significantly improved self-reports of disturbed sleep in patients with postherpetic neuralgia, painful diabetic neuropathy, fibromyalgia, painful neuropathies associated with human immunodeficiency virus and hemodialysis, and traumatic nerve injury pain.4–10 Menopausal women with hot flashes also reported improved sleep quality following gabapentin treatment.11
Studies using polysomnography (PSG) further demonstrate positive effects of gabapentin on sleep, particularly sleep maintenance. In studies using healthy volunteers, gabapentin increased slow wave sleep,12,13 and in healthy individuals administered alcohol prior to bedtime (known to disrupt sleep), gabapentin increased slow wave sleep, reduced stage 1 sleep, and decreased number of awakenings.14 Gabapentin, as add-on or monotherapy, produced similar changes in PSG parameters in patients with seizure disorders.15,16 Finally, in patients with RLS, gabapentin increased total sleep time, sleep efficiency, and slow wave sleep, but did not consistently affect sleep latency.17–19
BRIEF SUMMARY
Current Knowledge/Study Rationale: Adults commonly report occasional disturbed sleep. Using a 5-h phase advance transient insomnia model known to impair sleep maintenance, the present study evaluated the effects of single and multiple daily doses of gabapentin 250 mg on sleep measures assessed by polysomnography (PSG) and participant reports.
Study Impact: Gabapentin 250 mg resulted in greater sleep duration as assessed by PSG and participant reports following a 5-h phase advance on the first and 28th day of treatment, with no evidence of next-day impairment. During the intervening weeks of at-home use, gabapentin 250 mg resulted in greater sleep duration and higher ratings of sleep quality, compared with placebo.
Gabapentin has been studied in individuals with existing sleep disturbances who were otherwise healthy. One small, open-label clinical study evaluated gabapentin in participants with insomnia as defined by complaints of difficulty initiating and/or maintaining sleep for more than 3 months. Gabapentin significantly improved sleep efficiency, decreased wake after sleep onset (WASO), and increased slow wave sleep relative to baseline.20
Occasional difficulty sleeping through the night is commonly reported among adults in the United States.21,22 These individuals often seek treatment options by consulting with their healthcare provider and/or turning to over-the-counter (OTC) products (for “occasional sleeplessness” as indicated on product labels in the United States23). These individuals are rarely studied in clinical drug trials, and the development of new therapeutics to target occasional difficulties with sleep maintenance may be worthwhile given the tolerability and safety concerns associated with some prescription and OTC sleep products.24,25 Low-dose gabapentin (250 mg) is being investigated as a potential therapy for occasional disturbed sleep and has been evaluated in this self-identified population. In a randomized, controlled clinical trial, single doses of gabapentin 250 and 500 mg were shown to significantly improve measures of sleep maintenance compared with placebo, using a 5-h phase advance model to induce transient insomnia in individuals with occasional disturbed sleep.26 To our knowledge, there are no published studies evaluating repeated doses of gabapentin using a randomized, placebo-controlled trial design in individuals experiencing disturbed sleep. This was the primary objective of the present study and included sleep assessments in the sleep laboratory and during home use over the course of 28 days of gabapentin nightly treatment. While in the sleep laboratory, we used a 5-h sleep phase advance to induce transient insomnia, a model that has been shown to disrupt multiple sleep parameters, most reliably those associated with sleep maintenance, including WASO, total sleep time, and sleep efficiency.27–29 The dose of gabapentin 250 mg (elimination half-life of 5-7 h30) was used in the present study since it was shown to be effective following single-dose administration.26
The specific objectives of this study were to evaluate: (1) the acute effects of gabapentin on sleep maintenance measures assessed by PSG and participant reports in transient insomnia induced by a 5-h sleep phase advance; (2) the durability of these effects in the 5-h phase advance model following 28 days of nightly use; (3) the effects of gabapentin on sleep during at-home use between Days 1 and 28; and (4) the potential residual, next-day effects of gabapentin following first and last treatments.
METHODS
Study Design
This was a randomized, double-blind, placebo-controlled, multiple-dose study conducted at 3 United States-based research sites. The study (A9451155; Clinicaltrials.gov identifier: NCT00163046) was approved by an institutional review board or independent ethics committee at each investigational center and was conducted in compliance with the Declaration of Helsinki and all International Conference on Harmonization Good Clinical Practice Guidelines. Written informed consent was obtained before any study procedures were performed.
Study Population
Eligible participants were men or women (non-pregnant, non-lactating) ≥ 18 years of age who reported occasional disturbed sleep over the past month (defined by a participant's report of at least one night with problems sleeping, trouble falling or staying asleep, or waking in the middle of the night).
Participants were excluded if they reported any of the following: (1) a recent history of, or current treatment for, a sleep disorder or a chronic painful condition that would interfere with sleep; (2) current use or a known sensitivity to gabapentin; (3) expected use of any other medications to promote sleep (including OTC medications) during the study; or (4) expected travel across one or more time zones or shift work during the study. Medications for chronic medical conditions other than sleep disturbance were allowed, provided the participants expected to remain on a stable dose throughout the trial. Additional exclusion criteria were applied at randomization (Visit 2, see below).
Procedures
The following procedures were conducted at the screening visit (Visit 1): routine physical; medical and sleep history; and urine samples collected and processed for pregnancy and drug screening. Diaries were dispensed and participants were asked to complete the diary each morning at home, providing bedtime, wake time, and estimates of the previous night's sleep.
Eligible participants returned to the facility for Visit 2 (randomization visit) 4-16 days after the screening visit. Diaries were collected and reviewed. Participants completed the Ep-worth Sleepiness Scale (ESS),31 Pittsburgh Sleep Quality Index (PSQI),32 and the Profile of Mood States (POMS).33 Participants were required to meet the following randomization criteria: (1) negative urine drug screen result (Visit 1); (2) negative breathalyzer test result (Visit 2) and no alcohol use within 48 h of Visit 2; (3) bedtime between 22:00 and 01:00 and rise time between 05:00 and 10:00; (4) 7 to 9 h spent in bed for each of the 3 days prior to Visit 2; (5) a score ≤ 10 on the ESS at Visit 2; (6) no travel across time zones or shift work since Visit 1; (7) no use of other medications to promote sleep 3 days prior to Visit 2; and no use of amphetamines, benzodiazepines, cocaine, marijuana, methadone, opiates, propoxyphene, barbiturates, and phencyclidine since Visit 1.
Participants who met all criteria at Visits 1 and 2 were randomized to 250 mg/day gabapentin or matching placebo in a 1:1 ratio. Study medication was taken 30 min prior to bedtime on Days 1 and 28 (Visits 2 and 4, respectively) in the research facility. Participants took study medication at approximately 16:30, went to bed with lights out at 17:00, and were awakened with lights on at 01:00. PSG was conducted during the 8-h lights-out period. Participant-reported sleep measures were collected approximately 15 min (8.75 h post drug ingestion) after awakening. For evaluation of next-day residual effects, participants completed the multiple sleep latency test (MSLT),34 the Stanford Sleepiness Scale (SSS),35 and the psychomotor vigilance task (PVT).36 The MSLT was conducted at 2, 4, 6, and 8 h after awakening on Day 1 (10.5, 12.5, 14.5, and 16.5 h post drug ingestion, respectively); the SSS at approximately 15 min, 2.5 h, and 5 h after awakening on Days 1 and 28 (8.75, 11, and 13.5 h post drug ingestion, respectively); and the PVT was conducted within 1 h of awakening and again at 2.5 and 5 h after awakening on Days 1 and 28 (∼9.5, 11, and 13.5 h post drug ingestion, respectively). Participants also completed the ESS, PSQI, and POMS on Day 28 at approximately 14:00 (prior to the sleep phase advance period).
Between Days 1 and 28, participants took study medication 30 min prior to their regular bedtime (at-home use). Participant-reported sleep assessments were recorded each morning. An interim visit (Visit 3) occurred 12-16 days after Visit 2, during which vital signs, reports of adverse events (AEs) and any other medications were collected, and a POMS assessment completed. The sleep diary was reviewed by the site staff to ensure proper completion and clarity of entries. Treatment compliance was assessed by daily diary entries and confirmed by collection and counting of returned trial medication.
Assessments
Efficacy during Sleep Phase Advance Periods
The prespecified primary endpoint was PSG-quantified WASO during the 8-h time in bed on Day 1. Secondary endpoints included WASO on Day 28 and the remaining PSG parameters on Days 1 and 28: latency to persistent sleep, number of awakenings, total wake time plus stage 1 sleep, total sleep time, and percentage of each sleep stage (1, 2, stage 3+4, and REM). Participant-reported sleep assessments upon awakening on Days 1 and 28 were also collected (sleep latency, number of awakenings, WASO, total sleep time, sleep refreshment, and sleep quality). Sleep refreshment was based on the question, “How refreshed do you feel?”: 0 = not at all, 1 = a little, 2 = some, 3 = a lot, 4 = completely. Sleep quality was based on the question, “What was the quality of your sleep last night?”: 0 = very poor, 1 = poor, 2 = fair, 3 = good, 4 = very good. Participants also completed the Karolinska Sleep Diary (KSD). The KSD consists of 7 questions, and the KSD sleep quality index (SQI) is the subset that consists of the mean score for the following 4 items: sleep quality, calm sleep, ease of falling asleep, and slept throughout.37
Efficacy during Home Use
Participant-reported sleep assessments upon awakening were collected and included sleep latency, number of awakenings, WASO, total sleep time, sleep refreshment, and sleep quality. Also assessed was ease of awakening, phrased as “Rate your ease of awakening this morning”: 1 = very difficult, 2 = rather difficult, 3 = neither difficult nor easy, 4 = rather easy, 5 = very easy.
Next-Day Residual Effects
Participants completed the MSLT, SSS, and PVT at prespecified time intervals after awakening from the sleep phase advance periods (see details in Procedures) to assess sleepiness and psychomotor performance.
Safety and Tolerability
Safety and tolerability were assessed by monitoring AEs and measuring vital signs (pulse, blood pressure, and respiratory rate) at Visit 1 screening, and before dosing with study medication and after awakening from the phase advance on Days 1 and 28. Vital signs were also measured at Visit 3 (12-16 days after Visit 2). The AEs were coded using Medical Dictionary for Regulatory Activities (Version 9.0), categorized by system organ class and preferred term, and evaluated by the investigator for severity (mild, moderate, or severe) and relation to study medication (related or not related).
Statistical Analysis
A planned sample size of 250 participants (125 in each treatment group) was sufficient to provide at least 85% power to detect a difference of 35 min in PSG-derived WASO (primary endpoint), assuming a standard deviation of 80.4 min and a 2-sided level of significance of 0.05. All efficacy analyses were based on the intent-to-treat (ITT) population, which included all randomized participants who received at least one dose of study drug and had any subsequent efficacy evaluation. The safety population included all participants who received at least one dose of study medication. The ITT and safety populations consisted of all 256 randomized participants, 128 in each treatment group.
An analysis of variance with treatment and clinical site terms in the model was used to compare treatments for most endpoints (PSG WASO, number of awakenings, total wake time + stage 1 sleep, participant-reported sleep quality and refreshment, KSD, SSS, MSLT, PVT, POMS, and PSQI). The remaining endpoints were analyzed using the Cochran-Mantel-Haenszel test, stratifying by site, using modified ridit scores.
No imputation of missing data was performed, except in the following situations. For PSG measures, if persistent sleep onset was not reached, latency to persistent sleep was set to equal the value of time in bed for those observations that PSG recording was ≥ 450 min; if the PSG recording was < 450 min, non-latency-related PSG variables were set to missing. For participant-reported sleep measures, if a participant reported not falling asleep for a treatment night, sleep latency was set to 480 min, WASO and number of awakenings were set to missing, total sleep time was set to 0 min, and the assessment of sleep refreshment and the assessment of sleep quality were set to the worst possible score, 0. For MSLT, if sleep was not reached, a score of 20 minutes was assigned.
RESULTS
Participants and Treatment
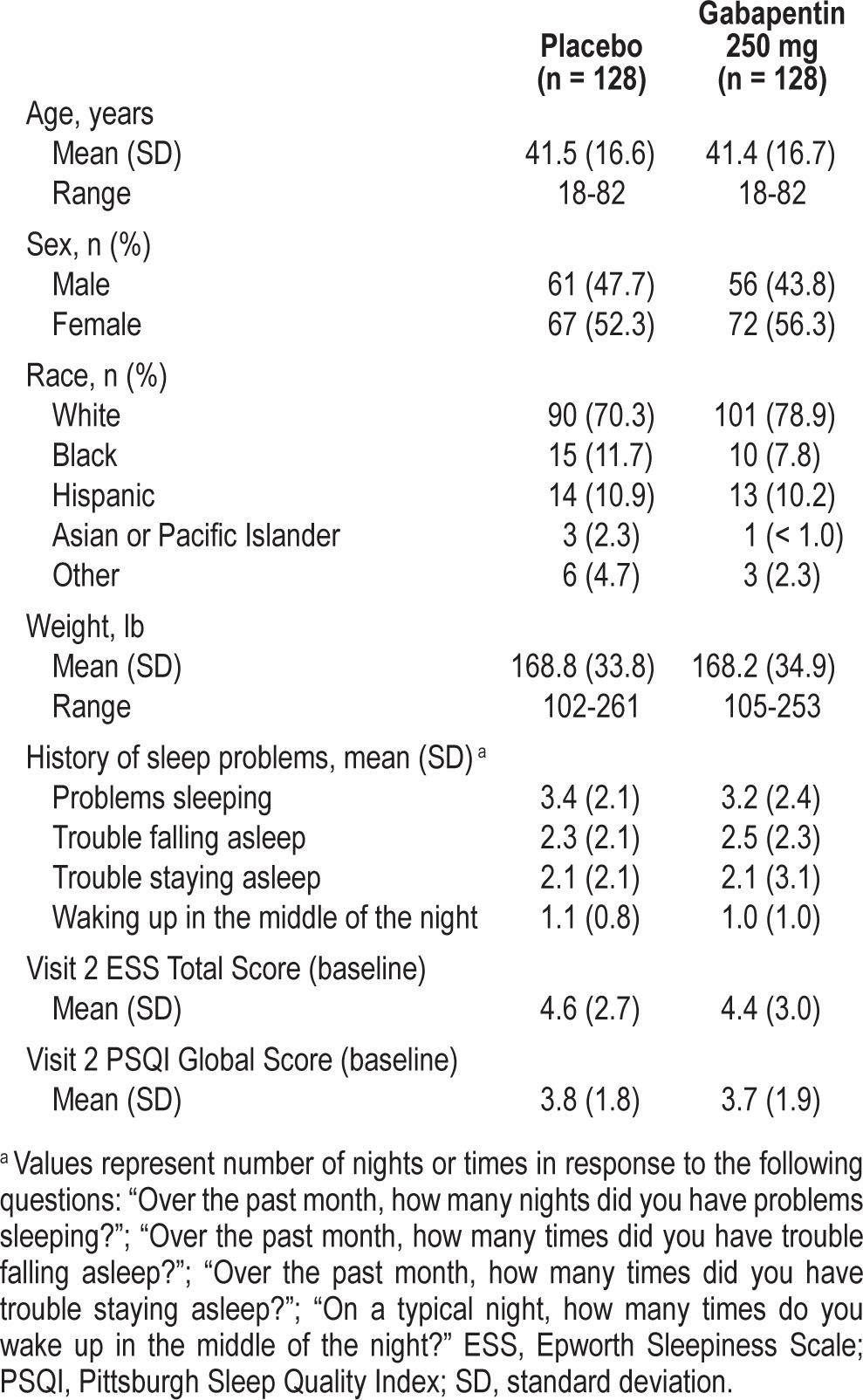
There were 256 participants randomized, of whom 237 completed the study (placebo: n = 115; gabapentin: n = 122) (Table 1). Nineteen participants discontinued for the following reasons: withdrew consent (n = 10), protocol violations (n = 5), AEs (n = 2), and other (n = 2). Treatment groups did not differ significantly with respect to demographic or baseline measures (Table 2), including history of sleep problems and sleepiness (as measured by the ESS), and the 8 sub-indices of the PSQI and the 7 categories of the POMS (data not shown). Based on diary entries, the majority of participants took 15-28 days of study drug in the placebo (89.5%) and gabapentin (91.4%) groups.
Table 1.
Participant disposition.
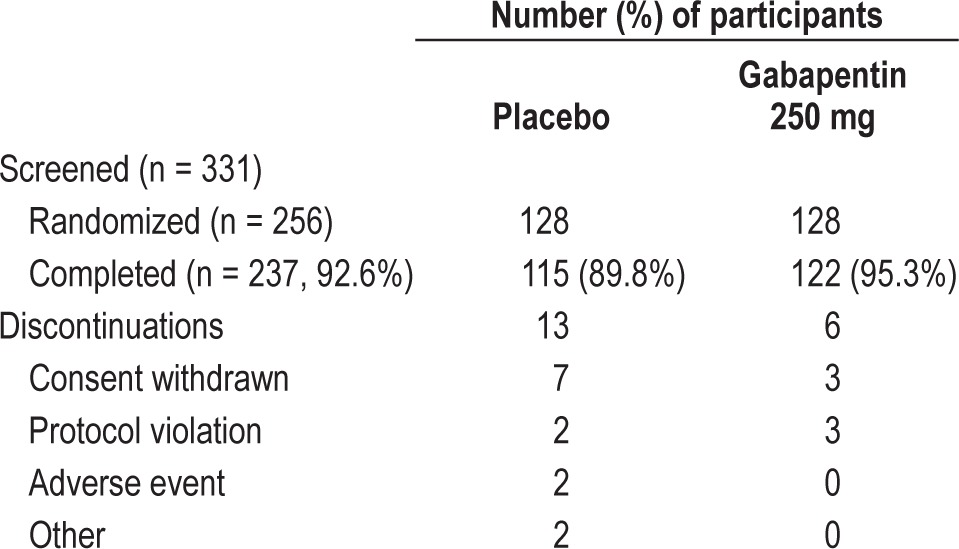
Table 2.
Demographic characteristics and baseline data.
Efficacy: Sleep Phase Advance Periods (Days 1, 28)
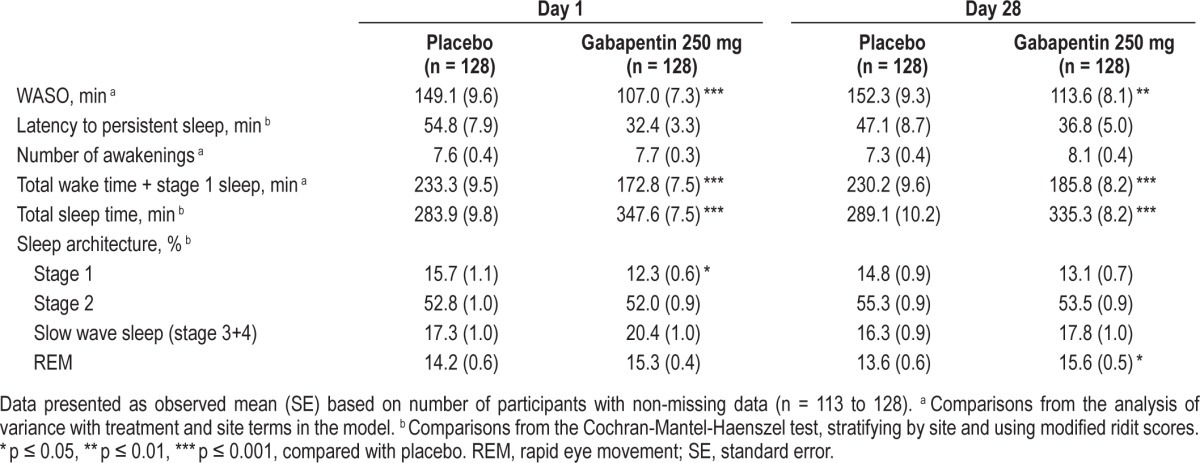
Gabapentin 250 mg resulted in significantly less PSG-derived WASO than placebo on Day 1 (primary endpoint, 149.1 versus 107.0 min for placebo and gabapentin, respectively; p ≤ 0.001) and Day 28 (152.3 versus 113.6 min, p ≤ 0.01) (Figure 1). All PSG endpoints are summarized in Table 3. In addition to less WASO, gabapentin 250 mg resulted in significantly less wake time plus stage 1 sleep (p ≤ 0.001) and greater total sleep time (p ≤ 0.001), compared with placebo on Days 1 and 28. Although there was a shorter latency to persistent sleep in the gabapentin 250 mg group on Days 1 and 28, the effects were not statistically significant from placebo. Gabapentin was associated with significantly less % stage 1 sleep on Day 1 (p ≤ 0.05), and significantly greater % REM sleep on Day 28 (p ≤ 0.05).
Figure 1. PSG-derived WASO (min) at Days 1 and 28 in participants receiving placebo or gabapentin.
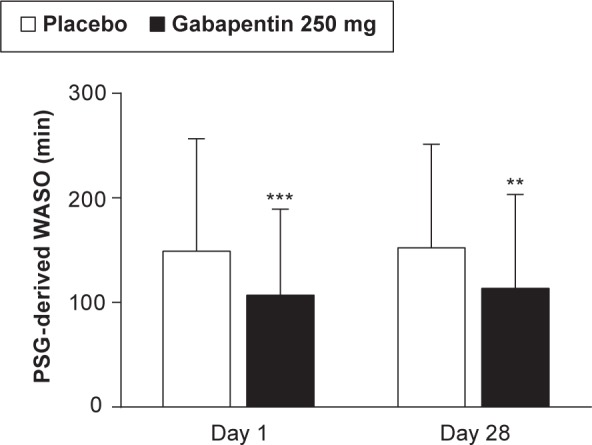
Data shown are mean (SD). *** p ≤ 0.001 versus placebo on Day 1 and ** p ≤ 0.01 versus placebo at Day 28. Day 1 sample size: placebo (n = 126); gabapentin (n = 128). Day 28: placebo (n = 113); gabapentin (n = 122). PSG, polysomnography; SD, standard deviation; WASO, wake after sleep onset.
Table 3.
PSG sleep assessments on Days 1 and 28.
On participant-reported sleep assessments, gabapentin significantly affected all Day 1 sleep parameters, with the exception of sleep latency (versus placebo, p ≤ 0.01) (Table 4). On Day 28, gabapentin was associated with significantly less WASO, greater total sleep time, and higher ratings of sleep quality, compared with placebo (p ≤ 0.01) (Table 4).
Table 4.
Participant-reported sleep assessments on Days 1 and 28.
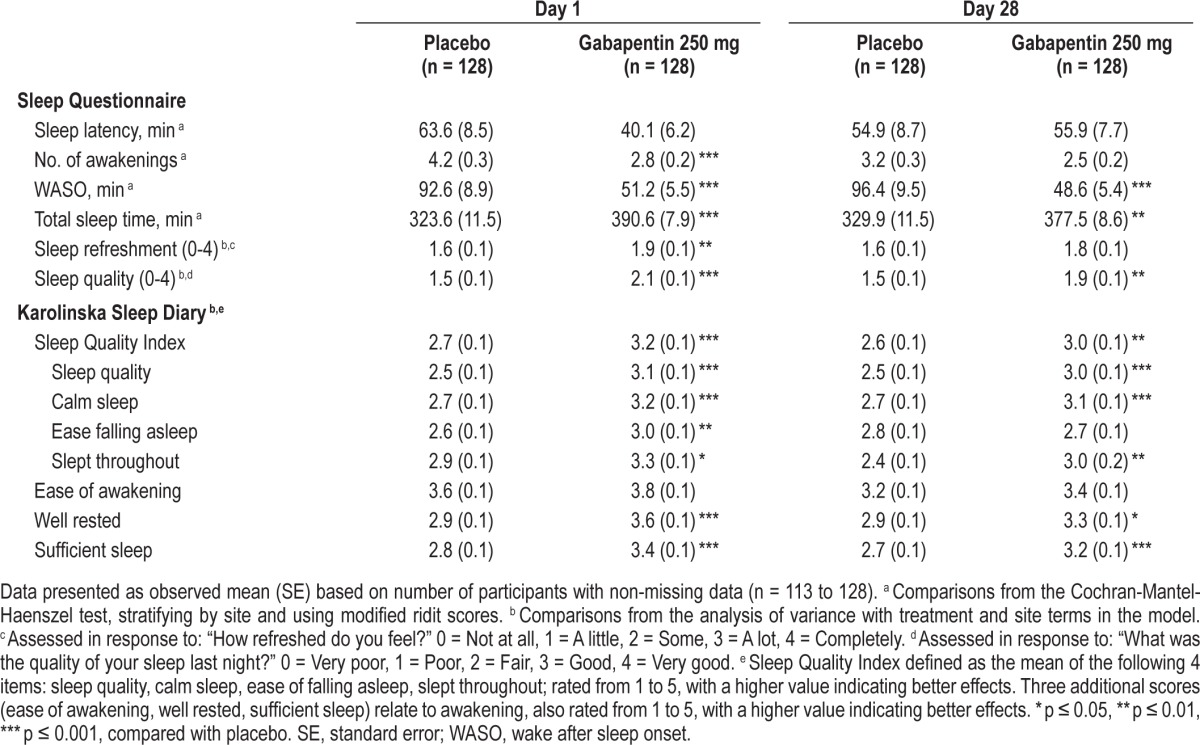
Gabapentin resulted in a significantly higher score on the KSD-SQI (and most of the individual items) on Days 1 and 28 (versus placebo, p ≤ 0.01) (Table 4). Significantly higher ratings of feeling well rested (p ≤ 0.05) and having sufficient sleep (p ≤ 0.001) were also reported by gabapentin-treated participants, relative to placebo (Table 4).
Efficacy during Home Use
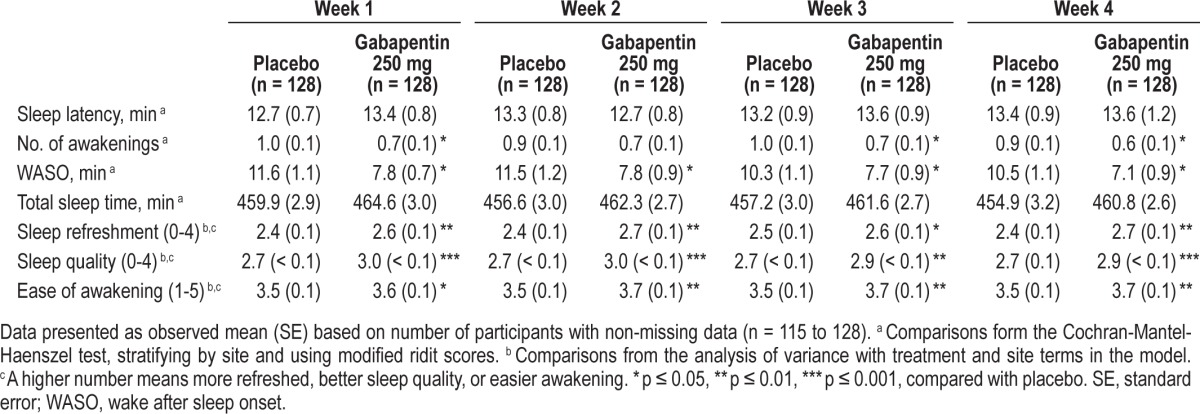
Participant-reported sleep assessments during at-home use are summarized weekly for all parameters (Table 5). Participants taking gabapentin reported significantly less WASO, fewer awakenings, higher ratings of sleep refreshment, sleep quality, and ease of awakening during most weeks of at-home use. There were no significant differences in sleep latency or total sleep time.
Table 5.
Participant-reported sleep assessments during at-home use: Weeks 1 to 4.
Other Evaluations
Gabapentin was associated with significantly improved ratings of sleep quality relative to baseline (p = 0.03) compared with placebo, as assessed by the PSQI global score on Day 28 (mean change from baseline [SE] for placebo and gabapentin: -0.3 [0.1] and -0.6 [0.2], respectively). The individual components of the PSQI were consistent with the global score, but did not show significant differences (data not shown).
The POMS was conducted at baseline and on Days 14 and 28; there were no significant treatment differences in the POMS total score and 6 subscale scores (tension, depression, anger, vigor, fatigue, and confusion), with the exception of the confusion subscale on Day 14 in which participants receiving gabapentin reported being less confused (mean change from baseline [SE] for placebo and gabapentin: 0 [0.2] and -0.4 [0.1], respectively; p ≤ 0.05).
Next-Day Residual Effects
Gabapentin did not significantly affect next-day sleepiness as measured by the MSLT. The test was administered following the first sleep phase advance at 2, 4, 6, and 8 h after awakening; the mean score of latencies from these 4 tests was used for the analysis (mean [SE]: 8.8 [0.4] versus 9.0 [0.4] min for placebo and gabapentin, respectively). Gabapentin also did not affect post-awakening sleepiness as measured by the mean SSS, except that participants taking gabapentin reported being less sleepy upon awakening (8.75 h post drug ingestion) and only following the second sleep phase advance (mean [SE] for placebo and gabapentin: 3.3 [0.1] and 2.9 [0.1], respectively, p ≤ 0.05). Gabapentin did not significantly affect any of the variables of the PVT upon awakening (median reaction time, Day 1: 237 versus 236 ms; Day 28: 245 versus 245 ms for placebo and gabapentin, respectively) and at 2.5 and 5 h post awakening on Days 1 and 28 (data not shown).
Safety and Tolerability
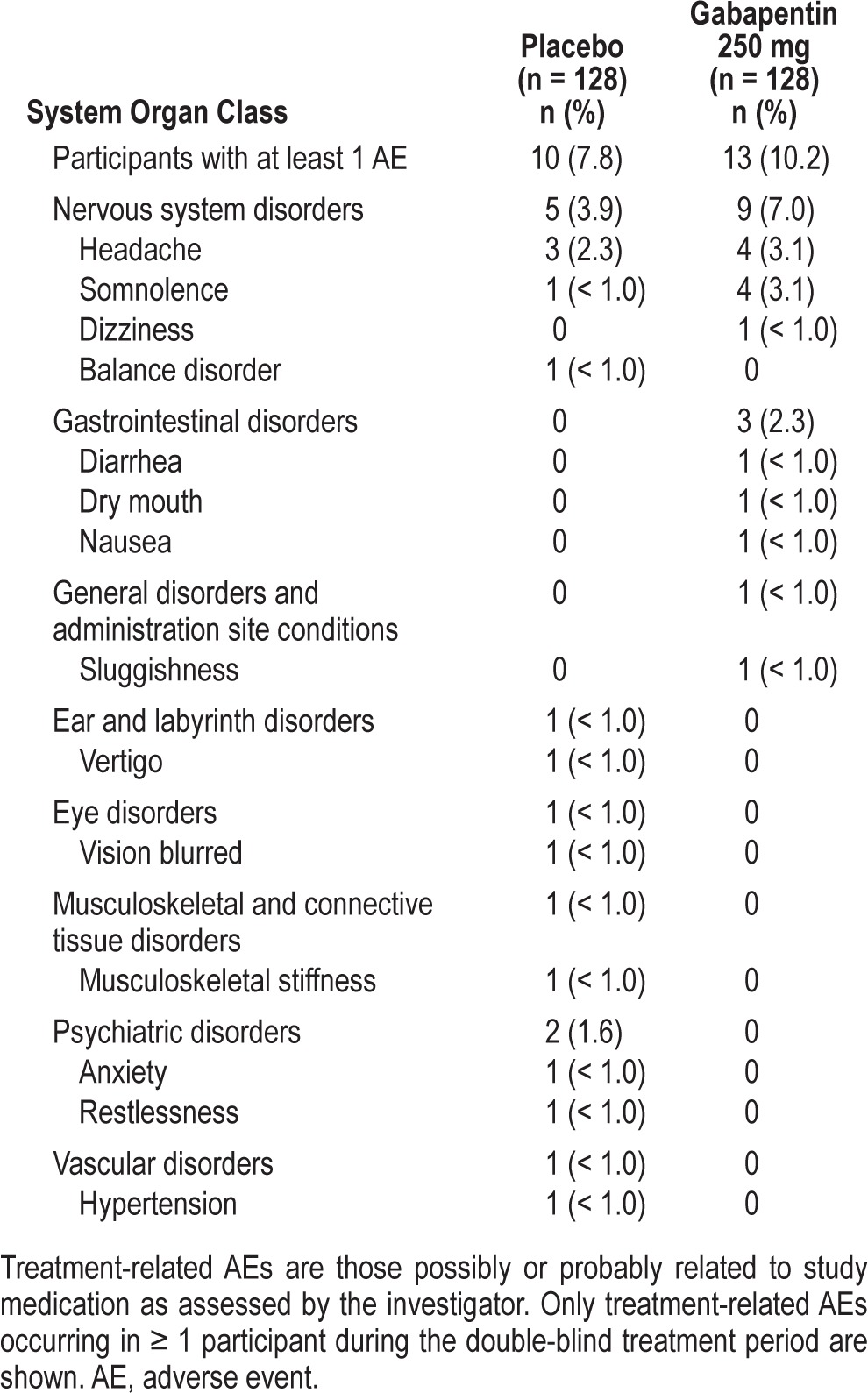
Of the 256 randomized participants, 57 (22.3%) reported at least one AE after dosing: 30 (23.4%) and 27 (21.1%) participants in the placebo and gabapentin groups, respectively. There were no serious AEs or deaths. Two participants in the placebo group discontinued due to an AE (moderate restlessness in one participant and moderate anxiety in the other). Most events were of mild severity. The most common treatment-related AE was headache, which was reported by 3 (2.3%) and 4 (3.1%) participants in the placebo and gabapentin groups, respectively. Somnolence was reported by 1 (< 1.0%) participant in the placebo group and 4 (3.1%) participants in the gabapentin group. All other treatment-related AEs were reported once (Table 6).
Table 6.
Summary of treatment-related adverse events.
Gabapentin was not associated with any clinically or statistically meaningful changes in vital signs, and there were no discontinuations due to vital sign changes.
DISCUSSION
Using a 5-h sleep phase advance model of transient sleep disturbance in participants with a history of occasional disturbed sleep, gabapentin 250 mg had significant benefits on PSG and participant-reported assessments, compared with placebo. Beneficial effects were seen on sleep maintenance and quality, but not sleep latency, and occurred on the first day of treatment and after 28 days of nightly use. Although it is not expected that individuals with occasional sleep disturbances would require nightly administration of a sleep aid over a prolonged period, it was important to confirm the durability of the sleep maintenance benefit in the event of continued use. These results are consistent with a previous single-dose study of similar design26 and with a small open-label study in individuals diagnosed with primary insomnia treated with gabapentin for 4 weeks, in which 540 mg/day significantly improved sleep efficiency, decreased WASO, and increased slow wave sleep relative to baseline results.20 Alongside the beneficial effects of gabapentin on sleep, the AEs following single or multiple (28-day) doses were infrequent and mild to moderate in severity, with the most common AEs being headache and somnolence (3.1% each in gabapentin 250 mg).
In the present study, gabapentin produced only modest changes in sleep architecture that were directionally consistent across the 2 sleep phase advance periods. In terms of statistical significance, during the first phase advance, gabapentin was associated with significantly less stage 1 sleep, whereas during the second phase advance, gabapentin resulted in greater REM sleep, compared with placebo. Gabapentin also significantly increased stage 4 sleep in the first phase advance (data not shown), but did not produce an overall significant increase in slow wave sleep (stage 3+4). Other studies have shown decreased stage 1 with increased slow wave sleep in both healthy volunteers and clinical populations (RLS, seizure disorders),12-14,16,17,19,26,38 with some studies showing increases in REM sleep.19,38 The reason for seeing inconsistent effects over days may be related to the power to detect these relatively small and variable effects of this dose of gabapentin (250 mg) on sleep architecture.
Consistent with effects observed during the phase advance periods on Days 1 and 28, gabapentin showed significant benefits on participant-reported sleep maintenance, quality, and refreshment during at-home use (Days 2-27). This is the first randomized, controlled clinical study to our knowledge that evaluated gabapentin's effects on sleep during home use in individuals who reported occasional sleep disturbances, but were otherwise healthy. In a variety of clinical populations, individuals taking gabapentin at home reported beneficial effects on sleep disturbances,5,7,11,17 but whether these may have been secondary to improvements in comorbid symptoms (pain, hot flashes, and limb movements) is not completely understood. A recent review of the mechanistically similar medication, pregabalin, on sleep disturbances across multiple pain-related clinical conditions suggests that it has a direct effect on sleep (independent of its pain-relieving properties).39 Likewise, although fewer studies were described, a similar conclusion was drawn for gabapentin.39 Thus, data from both clinical and healthy populations are converging to suggest that gabapentin has beneficial effects on sleep maintenance, with parallel improvements in sleep quality and feeling refreshed and well rested upon awakening.
Gabapentin 250 mg did not produce next-day effects on sleepiness and psychomotor performance on either Day 1 or 28, measured both objectively and subjectively. These observations are consistent with the single-dose study mentioned earlier.26 Across these 2 studies, the consistent lack of impairment using physiological (MSLT) and performance (PVT, DSST) measures, subjective rating scales (POMS, SSS), and spontaneous reports of somnolence (AEs) suggests a lack of next-day residual sleepiness and/or psychomotor/cognitive impairment with gabapentin. This is important given the common next-day effects associated with some currently prescribed hypnotics and OTC sleep aids, and possibly offers a unique differentiation.
The US Food and Drug Administration (FDA) is requiring manufacturers of zolpidem to lower the recommended bedtime dose, owing to morning blood levels in some patients that may contribute to impaired performance on activities requiring alertness. Additionally, the FDA is urging healthcare providers to caution patients on the risks associated with next-day impairment and will continue to assess these risks with other prescribed hypnotics and OTC sleep aids (http://www.fda.gov/Drugs/DrugSafety/ucm334033.htm). Although the evidence is encouraging, whether gabapentin 250 mg possesses a unique profile (beneficial effects on sleep maintenance without next-day residual effects) awaits specifically designed and adequately powered studies of next-day effects.
The consistent effects of gabapentin on participant-reported sleep assessments during the phase advance and during at-home use in the same study support the validity of the circadian phase advance as a model of transient disturbed sleep. This model is used frequently to evaluate the hypnotic potential of drugs, although the length of the phase advance varies (3 to 12 h) among studies. Only one study, to our knowledge, has evaluated the effects of different phase advance lengths on sleep; as the phase advance was lengthened from 3 to 6 h, greater disruptions in PSG-derived sleep maintenance parameters were observed, with no significant effects on sleep latency.27 In other studies in which the effects of phase advance were compared with a baseline night (habitual bedtimes), sleep maintenance endpoints appeared to be consistently disrupted, whereas the sleep latency effects were more inconsistent, irrespective of phase advance length.28,29,40,41 Despite these differences, hypnotic drugs consistently mitigate the disruptive effects of phase advance (on maintenance and/or latency), supporting the use of this model to evaluate effects on transient sleep disturbance.28,29,42
The clinical significance and economic burden (direct and indirect costs) of occasional disturbed sleep are not well understood, because most research focuses on chronic insomnia. Some studies include individuals with mild or transient insomnia, but definitions are not standardized across studies, thus limiting interpretation and generalization. Nonetheless, there are some data suggesting that mild or occasional insomnia symptoms pose significant health and economic burdens. A large Canadian epidemiological study demonstrated increased work-related costs (in terms of increased absenteeism and reduced productivity) with individuals presenting insomnia symptoms who otherwise did not fulfill diagnostic criteria for insomnia disorder.43 Additionally, as part of the same study, a proportion of these individuals (10% to 14%) went on to develop chronic insomnia at 1-, 2-, and 3-year follow-ups.44 Finally, a health plan study found that insomnia severity was associated with comorbid chronic medical and psychiatric illnesses, and that the odds of having at least one psychiatric diagnosis was greater in the group categorized as “subthreshold” insomnia, compared with the “no insomnia” group.45 Individuals with mild, transient, or occasional sleep disturbances often self-treat; unfortunately, evidence from controlled clinical studies substantiating efficacy and safety of OTC sleep aids is minimal. Given the potential value of short-term treatment intervention for occasional sleep disturbances (on an as-needed basis consistent with product labeling), the development of safe and efficacious sleep aids, including both prescription and OTC agents, is important.
Study Limitations
To our knowledge, this is the first randomized, controlled clinical trial to evaluate single and multiple doses of gabapentin 250 mg on PSG and participant-reported sleep assessments in healthy volunteers who report occasional disturbed sleep. However, the study is not without limitations. First, only one dose of gabapentin was evaluated; a higher dose (500 mg) was tested in the phase advance study by Rosenberg et al.,26 but with only single-dose administration. Evaluation of both lower and higher doses of gabapentin would provide a fuller understanding of the dose-response relationship in this population. Second, although there were no significant impairing effects of gabapentin on next-day measures, confirmation awaits well-designed clinical studies that include active comparators (hypnotic agents with known next-day effects) and assess multiple next-day effects (e.g., driving) as primary endpoints.
CONCLUSIONS
Our data, gathered from a model of transient disturbed sleep and at-home use, demonstrate that gabapentin 250 mg effectively may improve sleep maintenance and quality following single and multiple doses (up to 28 days), without evidence of residual, next-day impairment.
DISCLOSURE STATEMENT
This study was sponsored by Pfizer Inc. Drs. Furey and Jayawardena are full-time employees of Pfizer Inc and receive salary and other compensation. Drs. Hull and Leibowitz were clinical investigators of the study. Dr. Hull has served as a consultant or member of a speaker's bureau for Jazz Pharmaceuticals, Pfizer, Purdue, and Transcept. Dr. Roth has received research funding from Cereve and Procter and Gamble, and has acted as a consultant and/or speaker for Intec, Merck, Novartis, Pfizer, Procter and Gamble, Shire, Somaxon, and Xenoport. Off-label Disclosure: This study evaluated the effects of multiple doses of 250 mg gabapentin on transient insomnia induced by sleep phase advance in participants reporting occasional disturbed sleep, which is not an approved indication. Results were presented at SLEEP 2013, the 27th Annual Meeting of the Associated Professional Sleep Societies:
Hull SG, Leibowitz MT, Furey S, Jayawardena S, Roth T. A randomized, double-blind, placebo-controlled, multicenter, 28-day, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. Sleep 2013;36(Abstract Suppl):A221.
ACKNOWLEDGMENTS
Medical writing support was provided by Diane Hoffman, Ph.D., of Engage Scientific Solutions and was funded by Pfizer Inc. Support consisted of research (scientific literature), writing under the direction of the authors, copyediting, and the design and production of graphics.
ABBREVIATIONS
- AE
adverse event
- DSST
Digit Symbol Substitution Test
- ESS
Epworth Sleepiness Scale
- FDA
Food and Drug Administration
- ITT
intent-to-treat
- KSD-SQI
Karolinska Sleep Diary Sleep Quality Index
- MSLT
multiple sleep latency test
- OTC
over-the-counter
- POMS
Profile of Mood States
- PSG
polysomnography, polysomnographic
- PSQI
Pittsburgh Sleep Quality Index
- PVT
psychomotor vigilance task
- REM
rapid eye movement
- RLS
restless legs syndrome
- SSS
Stanford Sleepiness Scale
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–76. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 2.Neurontin (gabapentin) Capsules, Tablets, Oral Solution. New York: Pfizer Inc; 2012. Jul, [Accessed July 24, 2013]. U.S. prescribing information. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=630. [Google Scholar]
- 3.Horizant (gabapentin enacarbil) Extended-release Tablets. California: XenoPort, Inc.; 2013. [Accessed July 24, 2013]. U.S. Prescribing Information. Available at: http://www.horizant.com/#legal_notice. [Google Scholar]
- 4.Rice AS, Maton S. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–24. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 5.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–42. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 6.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–6. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 7.Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56:1336–44. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 8.Hahn K, Arendt G, Braun JS, et al. A placebo-controlled trial of gabapentin for painful HIV-associated sensory neuropathies. J Neurol. 2004;251:1260–6. doi: 10.1007/s00415-004-0529-6. [DOI] [PubMed] [Google Scholar]
- 9.Biyik Z, Solak Y, Atalay H, Gaipov A, Guney F, Turk S. Gabapentin versus pregabalin in improving sleep quality and depression in hemodialysis patients with peripheral neuropathy: a randomized prospective crossover trial. Int Urol Nephrol. 2013;45:831–37. doi: 10.1007/s11255-012-0193-1. [DOI] [PubMed] [Google Scholar]
- 10.Gordh TE, Stubhaug A, Jensen TS, et al. Gabapentin in traumatic nerve injury pain: a randomized, double-blind, placebo-controlled, cross-over, multi-center study. Pain. 2008;138:255–66. doi: 10.1016/j.pain.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Yurcheshen ME, Guttuso T, Jr., McDermott M, Holloway RG, Perlis M. Effects of gabapentin on sleep in menopausal women with hot flashes as measured by a Pittsburgh Sleep Quality Index factor scoring model. J Womens Health (Larchmt) 2009;18:1355–60. doi: 10.1089/jwh.2008.1257. [DOI] [PubMed] [Google Scholar]
- 12.Foldvary-Schaefer N, De Leon Sanchez I, Karafa M, Mascha E, Dinner D, Morris HH. Gabapentin increases slow-wave sleep in normal adults. Epilepsia. 2002;43:1493–7. doi: 10.1046/j.1528-1157.2002.21002.x. [DOI] [PubMed] [Google Scholar]
- 13.Rao ML, Clarenbach P, Vahlensieck M, Krätzschmar S. Gabapentin augments whole blood serotonin in healthy young men. J Neural Transm. 1988;73:129–34. doi: 10.1007/BF01243384. [DOI] [PubMed] [Google Scholar]
- 14.Bazil CW, Battista J, Basner RC. Gabapentin improves sleep in the presence of alcohol. J Clin Sleep Med. 2005;1:284–7. [PubMed] [Google Scholar]
- 15.Placidi F, Diomedi M, Scalise A, Marciani MG, Romigi A, Gigli GL. Effect of anticonvulsants on nocturnal sleep in epilepsy. Neurology. 2000;54:S25–32. [PubMed] [Google Scholar]
- 16.Legros B, Bazil CW. Effects of antiepileptic drugs on sleep architecture: a pilot study. Sleep Med. 2003;4:51–5. doi: 10.1016/s1389-9457(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59:1573–9. doi: 10.1212/wnl.59.10.1573. [DOI] [PubMed] [Google Scholar]
- 18.Winkelman JW, Bogan RK, Schmidt MH, Hudson JD, DeRossett SE, Hill-Zabala CE. Randomized polysomnography study of gabapentin enacarbil in subjects with restless legs syndrome. Mov Disord. 2011;26:2065–72. doi: 10.1002/mds.23771. [DOI] [PubMed] [Google Scholar]
- 19.Saletu M, Anderer P, Saletu-Zyhlarz GM, et al. Comparative placebo-controlled polysomnographic and psychometric studies on the acute effects of gabapentin versus ropinirole in restless legs syndrome. J Neural Transm. 2010;117:463–73. doi: 10.1007/s00702-009-0361-3. [DOI] [PubMed] [Google Scholar]
- 20.Lo HS, Yang CM, Lo HG, Lee CY, Ting H, Tzang BS. Treatment effects of gabapentin for primary insomnia. Clin Neuropharmacol. 2010;33:84–90. doi: 10.1097/WNF.0b013e3181cda242. [DOI] [PubMed] [Google Scholar]
- 21.National Sleep Foundation. 2009 Sleep in America Poll Highlights and Key Findings. [Accessed March 25, 2014]. Available at: http://sleepfoundation.org/sites/default/files/2009 POLL HIGHLIGHTS.pdf.
- 22.Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS) Sleep. 2011;34:997–1011. doi: 10.5665/SLEEP.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Department of Health and Human Services. US Food and Drug Administration. Nighttime sleep-aid products for over-the-counter human use; final monograph. [Accessed July 31, 2014];Federal Register. 1989 54:6814–27. Available at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Over-the-CounterOTCDrugs/StatusofOTCRulemakings/ucm091128.pdf. [Google Scholar]
- 24.Meoli AL, Rosen C, Kristo D, et al. Oral nonprescription treatment for insomnia: an evaluation of products with limited evidence. J Clin Sleep Med. 2005;1:173–87. [PubMed] [Google Scholar]
- 25.Deak MC, Winkelman JW. Insomnia. Neurol Clin. 2012;30:1045–66. doi: 10.1016/j.ncl.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10:1093–100. doi: 10.5664/jcsm.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26:1029–36. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 28.Kanno O, Watanabe H, Kazamatsuri H. Effects of zopiclone, flunitrazepam, triazolam and levomepromazine on the transient change in sleep-wake schedule: polygraphic study, and the evaluation of sleep and daytime condition. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:229–39. doi: 10.1016/0278-5846(93)90044-s. [DOI] [PubMed] [Google Scholar]
- 29.Walsh JK, Schweitzer PK, Sugerman JL, Muehlbach MJ. Transient insomnia associated with a 3-hour phase advance of sleep time and treatment with zolpidem. J Clin Psychopharmacol. 1990;10:184–9. [PubMed] [Google Scholar]
- 30.Bockbrader HN. Clinical pharmacokinetics of gabapentin. Drugs Today. 1995;31:613–19. [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.McNair DM, Lorr M, Droppelman LF. San Diego, CA: Educational and Industrial Testing Service; 1971. Manual for the Profile of Mood States. [Google Scholar]
- 34.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 35.Herscovitch J, Broughton R. Sensitivity of the Stanford Sleepiness Scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep. 1981;4:83–91. doi: 10.1093/sleep/4.1.83. [DOI] [PubMed] [Google Scholar]
- 36.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable simple visual RT task during sustained operations. Behav Res Methods Instruments Comput. 1985;17:652–55. [Google Scholar]
- 37.Keklund G, Akerstedt T. Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6:217–20. doi: 10.1111/j.1365-2869.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- 38.Placidi F, Mattia D, Romigi A, Bassetti MA, Spanedda F, Marciani MG. Gabapentin-induced modulation of interictal epileptiform activity related to different vigilance levels. Clin Neurophysiol. 2000;111:1637–42. doi: 10.1016/s1388-2457(00)00365-5. [DOI] [PubMed] [Google Scholar]
- 39.Roth T, Arnold LM, Garcia-Borreguero D, Resnick M, Clair AG. A review of the effects of pregabalin on sleep disturbance across multiple clinical conditions. Sleep Med Rev. 2014;18:261–71. doi: 10.1016/j.smrv.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Walsh JK, Deacon S, Dijk DJ, Lundahl J. The selective extrasynaptic GABAA agonist, gaboxadol, improves traditional hypnotic efficacy measures and enhances slow wave activity in a model of transient insomnia. Sleep. 2007;30:593–602. doi: 10.1093/sleep/30.5.593. [DOI] [PubMed] [Google Scholar]
- 41.Walsh JK, Mayleben D, Guico-Pabia C, Vandormael K, Martinez R, Deacon S. Efficacy of the selective extrasynaptic GABAA agonist, gaboxadol, in a model of transient insomnia: a randomized, controlled clinical trial. Sleep Med. 2008;9:393–402. doi: 10.1016/j.sleep.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Roth T, Heith Durrence H, Jochelson P, et al. Efficacy and safety of doxepin 6 mg in a model of transient insomnia. Sleep Med. 2010;11:843–7. doi: 10.1016/j.sleep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- 44.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 45.Sarsour K, Morin CM, Foley K, Kalsekar A, Walsh JK. Association of insomnia severity and comorbid medical and psychiatric disorders in a health plan-based sample: Insomnia severity and comorbidities. Sleep Med. 2010;11:69–74. doi: 10.1016/j.sleep.2009.02.008. [DOI] [PubMed] [Google Scholar]


