Abstract
Objective:
Disturbed sleep during pregnancy is associated with adverse obstetric outcomes and less mental well-being. In pregnant women with a mental disorder, who frequently suffer from sleep problems, it is unknown whether predominantly objective or subjective sleep quality is more affected. To clarify this, we compared objective and subjective parameters of sleep quality between patients and healthy controls during pregnancy.
Methods:
This observational study was embedded in an ongoing study among pregnant women with a mental disorder at the department of Psychiatry of Erasmus University Medical Center Rotterdam, the Netherlands. We compared 21 pregnant women with a confirmed mental disorder with 33 healthy controls (gestational age, 23-29 weeks). To measure objective parameters of sleep quality, all participants continuously wore a wrist actigraph for 7 days and nights. Subjective sleep quality was retrospectively assessed using the Pittsburgh Sleep Quality Index (PSQI) and on a daily basis with the Subjective Sleep Quality-scale (SSQ). Differences in parameters of sleep between patients and controls were tested using a multivariate linear regression analysis adjusted for parity, gestational age, educational level, and employment status.
Results:
Objective parameters of sleep quality and subjective sleep quality as assessed by the PSQI did not differ significantly between patients and controls. Daily sleep reports showed that, relative to controls, patients had a significantly worse average SSQ-score (5.2 vs. 7.6, adjusted β = 0.12, 95%CI = 0.03-0.53, p < 0.01).
Conclusions:
Our exploratory study suggests that perceived sleep quality reported on a daily basis by pregnant women with a mental disorder is worse than the sleep quality as measured by wrist actigraphy.
Citation:
Van Ravesteyn LM, Tulen JH, Kamperman AM, Raats ME, Schneider AJ, Birnie E, Steegers EA, Hoogendijk WJ, Tiemeier HW, Lambregtse–van den Berg MP. Perceived sleep quality is worse than objective parameters of sleep in pregnant women with a mental disorder. J Clin Sleep Med 2014;10(10):1137-1141.
Keywords: mental disorder, pregnancy, actigraphy, sleep quality
Approximately one-third of all pregnant women report sleeping problems.1 Poor sleep quality and the persistence of disturbed sleep is associated with less mental well-being and adverse obstetric outcomes.2–4 However, little is known about the causal pathways that explain the association between poor sleep quality and adverse obstetric outcomes. For example, disrupted sleep can be a (prodromal) symptom of a mental disorder or a consequence of the mental disorder.5 From studies in non-pregnant psychiatric patients compared to healthy controls, it is known that a mental disorder is associated with prolonged sleep onset latency, increased wake after sleep onset, and reduced sleep efficiency.6–8 Moreover, having a mental disorder itself is associated with adverse birth outcomes.9 A first step that could help to clarify these underlying causal pathways is to investigate whether objective and subjective parameters of sleep quality during pregnancy differ between patients and healthy controls. Previous studies showed that pregnant women with a depressive disorder report more fragmented sleep, as reflected in longer sleep latencies and poorer sleep efficiency, than pregnant women without a depressive disorder.10,11 Non-pregnant patients with depression and sleep problems also showed discrepancy between subjective and objective sleep measurements12—e.g., objective sleep quality—as measured by actigraphy more closely approximated those of the golden standard (polysomnography) than subjective measurements in depressed insomniacs.13 It is unclear whether sleep quality in pregnant women is objectively worse in a sense of reduced or fragmented sleep, or whether their perception of it is altered, possibly as a result of co-occurring psychiatric symptoms. The interpretation of perceived poor sleep quality in pregnant women with a mental disorder could help clinicians to determine whether they should primarily focus on the perception of sleep quality and treatment of the underlying mental disorder, or whether they should intervene on the objective parameters of sleep. To identify sleep quality in pregnant women with a mental disorder, we studied both objective (wrist actigraph) and subjective (questionnaires) indicators of sleep in patients and in healthy controls during the second trimester of their pregnancy.
BRIEF SUMMARY
Current Knowledge/Study Rationale: In pregnant women with a mental disorder it is unknown whether predominantly objective or subjective sleep quality is more affected. More insight in this distinction could help clinicians to target their interventions.
Study Impact: Perinatal health care professionals should be aware that perceived, and not objective, parameters of sleep quality are significantly worse in pregnant women with a mental disorder compared to pregnant controls, and cautious use of sleep medication is warranted.
METHODS
The protocol of this cohort study was, as part of a larger randomized controlled trial (DAPPER, NTR3015 http://www.trialregister.nl), approved by the Medical Ethical Committee at Erasmus MC Rotterdam. In short, the aim of the DAPPER study is to evaluate the effectiveness of a group-based multi-component psychotherapy intervention for pregnant women with a mental disorder, compared to individual counselling. Eligible participants were pregnant women diagnosed with a mental disorder and/or personality disorder, confirmed by a Structured Clinical Interview for DSM-IV diagnosis by one trained medical doctor.14 We chose to recruit participants at the end of their second trimester (23-29 weeks of gestation), when sleep quality seems to be less affected by the pregnancy itself or by the routine 20-week fetal ultrasound examination in Dutch antenatal care, which can be potentially stressful.
Through contact with 4 community midwifery practices in Rotterdam, we recruited pregnant women (1) without current psychiatric symptoms on the Brief Symptom Inventory15 (BSI, global severity index score: < 0.71) and (2) without psychotropic medication use. Participants were excluded if they suffered from a tremor or medical conditions that could affect sleep (e.g., sleep apnea) or were unable to read or write in Dutch. Once written informed consent had been obtained, all participants provided demographic information. A low educational level was defined as: no education, only attending primary school or finished secondary school on the lowest level.
Objective parameters of sleep were measured using the Actiwatch Actigraphy model AW4 (Cambridge Neurotechnology Ltd, UK). Agreement of the Actiwatch Actigraphy model AW4 with the golden standard (polysomnography) has been demonstrated,16 though not in a population of pregnant women. When placed on the non-dominant wrist, the Actiwatch measures the number of movements above a certain threshold per 60-s epoch, and provides the following indices: total sleep time (TST); sleep latency (time until asleep); sleep efficiency (percentage of time spent asleep while in bed); and the fragmentation index (percentage immobility phases of one minute). Sleep data were analyzed using the Actiwatch Sleep Analysis program (Version 1.16, Cambridge Neurotechnology Ltd, UK). Although all participants wore an Actiwatch for 7 consecutive days and nights, only the weekdays (≥ 3 days and nights) were used for analysis because of the increase in variability during the weekends and also in agreement with recent literature.17–19 The precision of the 5-weekday assessment period was better than for 7 days and did not differ between the groups. Correlation of the 5-weekday assessment period was moderate for the actigraphically measured TST (ICC = 0.60) and sleep latency (ICC = 0.51), and good for sleep efficiency (ICC = 0.76) and sleep fragmentation (ICC = 0.68) for all participants. The correlation for the subjective parameters was weaker than the actigraphically measured parameters; the ICCs for TST, sleep latency, and SSQ were respectively 0.48, 0.46, and 0.46.
Subjective sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI), a self-rating questionnaire that measures sleep quality and disturbances retrospectively over a 1-month period. The PSQI scoring method produces an overall score between 0 and 21, with higher scores indicating poorer sleep quality.20 A PSQI-score ≥ 5 is considered the cutoff point, which discriminates a good sleeper (< 5) from a poor one. Relative to a combination of clinical interviews and polysomnographic measures, the threshold of ≥ 5 yields a diagnostic sensitivity of 89.6% and a specificity of 86.5%.21 The PSQI has good validity in depressed and pregnant populations.22 By analogy with the actigraphically measured parameters, we included two specific PSQI-domains: sleep latency and sleep efficiency.
Participants kept a daily sleep diary for one week and received a text message to remind them to fill it in after breakfast. The diary included questions on total sleep time, sleep latency, and the Subjective Sleep Quality scale (SSQ), an 11-item true-or-false questionnaire that culminates in a sum score of between 0 and 11, with higher scores indicating good sleep quality.23 By analogy with the actigraphically measured parameters, a minimum of 3 weekday dairies of each participant were averaged, based on a threshold setting of 20 activity counts within a 1-min epoch for the analysis of the Actiwatch data. The number and length of naps during the day were not included in the TST calculations of the diary or in the actigraphically measured TST.
Data Analysis
Differences on demographic characteristics (age, gestational age, parity, family status, ethnicity, educational level, employment and the percentage of available weekday data) between patients and controls were tested using T-tests (continuous variables) or χ2 tests (categorical variables). Actigraphically measured parameters and SSQ-scores were averaged over ≥ 3 weekday nights for each participant. All parameters of sleep were tested for normality using Q-Q plots and histograms; variables that violated the assumption of normality were log-transformed to resemble a normal distribution. Differences in sleep parameters between patients and controls were tested using univariate and multivariable linear regression analyses, adjusting for parity, gestational age, education level, and employment status. Beta values (95% CIs) are reported and a two-sided p-value of < 0.05 was taken as statistically significant. A total sample size of 52 participants allows for the detection of large effect sizes (≥ 0.8) with a significance level of 0.05 and a power of 0.80.24 Data were analyzed using SPSS (version 20) for Windows.
RESULTS
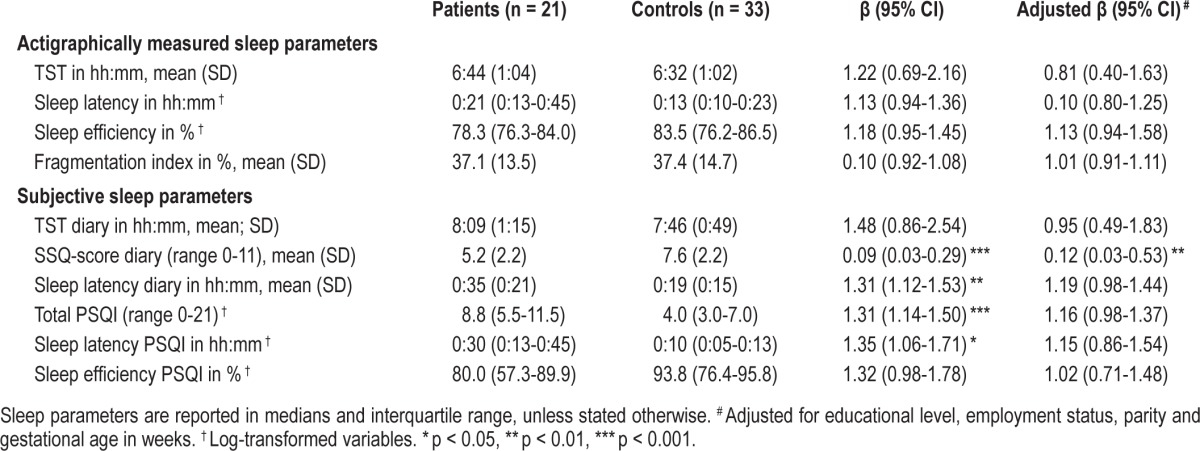
Patients were diagnosed with a current unipolar major depressive disorder (n = 12), generalized anxiety disorder (n = 6), personality disorder (n = 2), or bipolar disorder (n = 1). None of the participants used sleep medication; one patient with depression used an SSRI during pregnancy. Patients had a significantly lower educational level, and significantly more of them were unemployed than controls (Table 1). Other demographic characteristics did not differ. All participants completed the protocol. The percentage of analyzed weekday nights out of the total nights collected did not differ between the groups, not for the Actiwatch data (patients 92% vs. controls 97%, p = 0.15) nor for the sleep diaries (patients 92% vs. controls 97%, p = 0.09). There were no significant differences between patients and controls in the actigraphically measured parameters of sleep (Table 2). Patients reported longer sleep latency than controls and had a significantly poorer average score on the Subjective Sleep Quality Scale in the diaries. The SSQ-score remained significantly different between patients and controls after adjustment for parity, gestational age, educational level, and employment status. Sleep quality reported retrospectively over a 1-month period (PSQI-score) did not remain significantly different after adjustment, although in the crude analysis, a significantly larger proportion of patients scored above the non-adjusted threshold of a poor sleeper (patients 81% vs. controls 39%, p < 0.01). The disagreement between the actigraphically measured and diary-reported TST was larger in patients (mean = 1:25 h; Bland-Altman 95% limits of agreement (LA) between 0:11 to 3:01) than in controls (mean = 1:14 h; LA = 0:12 to 2:20).25 A similar trend was observed regarding sleep latency and sleep efficiency.
Table 1.
Demographic characteristics of all participants at start of study.
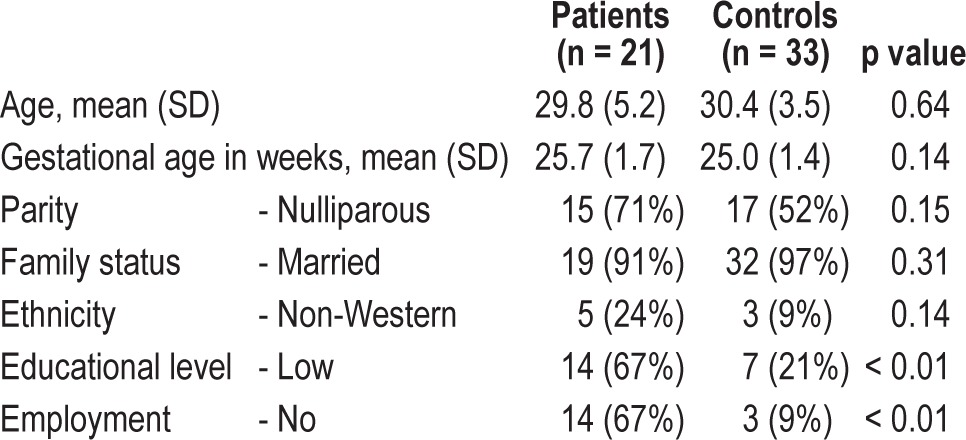
Table 2.
Differences of actigraphically measured and subjective parameters of sleep between patients and controls.
DISCUSSION
Our results indicate that subjective sleep quality as measured on a daily basis by the SSQ was significantly worse in pregnant women with a mental disorder than in those without a mental disorder. There was no significant difference regarding the objective parameters of sleep. In all participants, actigraphically measured TST was lower than the diary-reported TST. This is consistent with other studies reporting similar discrepancies between actigraphically measured TST and subjective perception of TST among non-pregnant insomnia sufferers and normal sleepers.26 Recently, Herring and colleagues showed that the discrepancy between actigraphically measured and self-reported TST in a majority of 80 healthy pregnant women was over one hour.27 While our study confirms this finding, it also suggests that this discrepancy is greater in patients than in controls. Despite this discrepancy, it is worth noting that all participants' average actigraphically measured TST (6:40 h) was shorter than that recorded in earlier studies in pregnant women (7.1-7.8 h).3,28,29 The suboptimal sleep quality in pregnant women is also reflected by the overall less favorable scores on the PSQI.
This study has several limitations. First, our study is subject to limited power to detect small and medium effect sizes; as a consequence of that, we cannot exclude a type II error in our findings. However, our subjective measures did reach significance within the same sample size. Secondly, due to our small sample we had to group all mental disorders together and could therefore not make a statement for each mental disorder separately during pregnancy. We also acknowledge that the Actiwatch AW4 model is not validated against polysomnography in a population of pregnant women and that there are large differences in sleep variables between different brands of actigraphs and settings.30 At last, we did not match for the daytime activities and level of education between patients and controls, although significantly more healthy controls were employed and highly educated. However, after adjusting for employment and level of education, as well as for the confounders parity and gestational age, the difference between subjective sleep quality as measured by the SSQ remained significant.
Despite these limitations, this study is one of the first in a clinical population to find that a mental disorder during pregnancy is more associated with poorer subjective sleep quality than with changes in parameters of objective sleep quality. Our results demonstrate the importance of focusing on the perception of sleep in pregnant women with a mental disorder who report sleep problems. Although this is an exploratory study, we speculate that these women might benefit from cognitive behavioral therapy, as demonstrated in studies with non-pregnant participants.31–33 Future research should focus on whether the association between perceived poor sleep quality and adverse birth outcomes that has been found in previous studies is independent or could be explained by co-occurring psychiatric symptoms. Also, the consequences of perceived poor sleep quality during pregnancy for persistence or recurrence of mental disorders in the postpartum period has to be investigated in women with a known mental disorder. For example, Park et al. recently showed that subjective perception of sleep quality of healthy pregnant women is a stronger predictor of depressive symptoms postpartum than actigraphy measures.34 Although previous research has shown a clear association between post-partum reduced sleep and the occurrence or exacerbation of affective and psychotic disorders, little is known about whether these women are already at increased risk during pregnancy.11,35
Perinatal health-care professionals should be aware that overall sleep quality is reduced during pregnancy and should explain to their patients that this particularly concerns sleep perception. Cautious use of sleep medication (e.g., benzodiazepines) is recommended because of the potential risks for the fetus and the risk of addiction of the mother. Also, additional risks exist in women with sleep apnea. One exception to this involves pregnant women with a bipolar disorder or past or current psychosis, in whom sleep plays a crucial role in the prevention of postpartum psychosis.36
DISCLOSURE STATEMENT
This was not an industry supported study. This study was performed as part of the DAPPER-study at Erasmus University Medical Center, Rotterdam, The Netherlands. Erasmus University Medical Center (MRACE) and the Coolsingel Foundation financially supported the DAPPER-study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the women who participated in this study and Monique Roggeveen and Laura Schot for their help in recruiting controls and monitoring participants.
REFERENCES
- 1.Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs. 2000;29:590–7. doi: 10.1111/j.1552-6909.2000.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 2.Kamysheva E, Skouteris H, Wertheim EH, Paxton SJ, Milgrom J. A prospective investigation of the relationships among sleep quality, physical symptoms, and depressive symptoms during pregnancy. J Affect Disord. 2010;123:317–20. doi: 10.1016/j.jad.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004;191:2041–6. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 4.Okun ML, Luther JF, Wisniewski SR, Sit D, Prairie BA, Wisner KL. Disturbed sleep, a novel risk factor for preterm birth? J Womens Health. 2012;21:54–60. doi: 10.1089/jwh.2010.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. discussion 69-70. [DOI] [PubMed] [Google Scholar]
- 6.Spiegelhalder K, Regen W, Nanovska S, Baglioni C, Riemann D. Comorbid sleep disorders in neuropsychiatric disorders across the life cycle. Curr Psychiatry Rep. 2013;15:364. doi: 10.1007/s11920-013-0364-5. [DOI] [PubMed] [Google Scholar]
- 7.Baglioni C, Regen W, Teghen A, et al. Sleep changes in the disorder of insomnia: A meta-analysis of polysomnographic studies. Sleep Med Rev. 2013;17:377–90. doi: 10.1016/j.smrv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Kyung Lee E, Douglass AB. Sleep in psychiatric disorders: where are we now? Can J Psychiatry. 2010;55:403–12. doi: 10.1177/070674371005500703. [DOI] [PubMed] [Google Scholar]
- 9.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–24. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okun ML, Kiewra K, Luther JF, Wisniewski SR, Wisner KL. Sleep disturbances in depressed and nondepressed pregnant women. Depress Anxiety. 2011;28:676–85. doi: 10.1002/da.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33:531–8. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotenberg VS, Indursky P, Kayumov L, Sirota P, Melamed Y. The relationship between subjective sleep estimation and objective sleep variables in depressed patients. Int J Psychophysiol. 2000;37:291–7. doi: 10.1016/s0167-8760(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 13.McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. 2012;21:122–7. doi: 10.1111/j.1365-2869.2011.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JB. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- 15.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 16.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 17.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 18.Berger AM, Wielgus KK, Young-McCaughan S, Fischer P, Farr L, Lee KA. Methodological challenges when using actigraphy in research. J Pain Symptom Manage. 2008;36:191–9. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Tsai SY, Kuo LT, Lai YH, Lee CN. Factors associated with sleep quality in pregnant women: a prospective observational study. Nurs Res. 2011;60:405–12. doi: 10.1097/NNR.0b013e3182346249. [DOI] [PubMed] [Google Scholar]
- 22.Skouteris H, Wertheim EH, Germano C, Paxton SJ, Milgrom J. Assessing sleep during pregnancy: a study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Women's Health Issues. 2009;19:45–51. doi: 10.1016/j.whi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.De Diana I. Two stochastic sleep quality scales for self-rating of subject's sleep. Sleep Res. 1976;5:101. [Google Scholar]
- 24.Cohen J. Hillside, NJ: Lawrence Erlbaum Associates; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 25.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 26.Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med. 2003;4:285–96. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 27.Herring SJ, Foster GD, Pien GW, et al. Do pregnant women accurately report sleep time? A comparison between self-reported and objective measures of sleep duration in pregnancy among a sample of urban mothers. Sleep Breath. 2013;17:1323–7. doi: 10.1007/s11325-013-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–8. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV. Effects of pregnancy on mothers' sleep. Sleep Med. 2002;3:37–42. doi: 10.1016/s1389-9457(01)00130-7. [DOI] [PubMed] [Google Scholar]
- 30.Rupp TL, Balkin TJ. Comparison of Motionlogger Watch and Actiwatch actigraphs to polysomnography for sleep/wake estimation in healthy young adults. Behav Res Methods. 2011;43:1152–60. doi: 10.3758/s13428-011-0098-4. [DOI] [PubMed] [Google Scholar]
- 31.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edinger JD, Olsen MK, Stechuchak KM, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32:499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 34.Park EM, Meltzer-Brody S, Stickgold R. Poor sleep maintenance and subjective sleep quality are associated with postpartum maternal depression symptom severity. Arch Womens Ment Health. 2013;16:539–47. doi: 10.1007/s00737-013-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorheim SK, Bondevik GT, Eberhard-Gran M, Bjorvatn B. Subjective and objective sleep among depressed and non-depressed postnatal women. Acta Psychiatr Scand. 2009;119:128–36. doi: 10.1111/j.1600-0447.2008.01272.x. [DOI] [PubMed] [Google Scholar]
- 36.Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165:830–43. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]


