Abstract
Introduction
Although lung cancer is the leading cause of cancer death in women, few studies have investigated the hormonal influence on survival after a lung cancer diagnosis and results have been inconsistent. We evaluated the role of reproductive and hormonal factors in predicting overall survival in women with non–small-cell lung cancer (NSCLC).
Methods
Population-based lung cancer cases diagnosed between November 1, 2001 and October 31, 2005 were identified through the Metropolitan Detroit Surveillance, Epidemiology, and End Results Registry. Interview and follow-up data were collected for 485 women. Cox proportional hazard regression models were used to determine hazard ratios (HRs) for death after an NSCLC diagnosis associated with reproductive and hormonal variables.
Results
Use of hormone therapy (HT) was associated with improved survival (HR, 0.69; 95% confidence interval, 0.54–0.89), adjusting for stage, surgery, radiation, education level, pack-years of smoking, age at diagnosis, race, and a multiplicative interaction between stage and radiation. No other reproductive or hormonal factor was associated with survival after an NSCLC diagnosis. Increased duration of HT use before the lung cancer diagnosis (132 months or longer) was associated with improved survival (HR, 0.54; 95% confidence interval, 0.37–0.78), and this finding remained significant in women taking either estrogen alone or progesterone plus estrogen, never smokers, and smokers.
Conclusion
These findings suggest that HT use, in particular use of estrogen plus progesterone, and long-term HT use are associated with improved survival of NSCLC.
Keywords: Non-small-cell lung cancer, Hormone use, Survival
In the United States, lung cancer is the leading cause of cancer death in both men and women. It is estimated that in 2012, 116,470 men and 109,690 women were diagnosed with lung cancer and 87,750 men and 72,590 women died from the disease.1 Since 1990, lung cancer mortality in men has been decreasing, whereas in women mortality has only seen a slight decrease since 2004.2
Many studies have explored sex differences in lung cancer incidence. Although differences in smoking patterns in men and women contribute to the variations in lung cancer risk, these differences do not fully explain incidence rate variation.3 That women are more likely to develop adenocarcinomas and develop cancer at a younger age suggests alternative biological explanations.4 Reproductive and hormonal factors contributing to the risk of lung cancer have been studied, particularly the role of estrogens in cancer development, with inconsistent results.5–13
Sex differences also exist in survival; female lung cancer patients have better survival compared with the survival in male lung cancer patients.14–16 The influence of reproductive factors on lung cancer survival has not been extensively studied. Moore et al. investigated the influence of menopausal status on outcomes of lung cancer and found that even though premenopausal women had tumors of an advanced clinical stage, overall survival was not significantly different from survival of postmenopausal women. The authors suggest that lifelong exposure to estrogen may offer a protective effect in lung cancer progression.17
Research on hormone therapy (HT) use and outcomes from lung cancer is limited, and results have been mixed. In a retrospective study, Ganti et al.18 found that women taking HT had decreased survival compared with women who had never used HT. The Women’s Health Initiative (WHI) randomized control trial study showed that the use of estrogen-only HT was not associated with incidence or mortality from lung cancer.8 In the WHI study, combined estrogen and progesterone HT use was associated with an increased mortality, but not associated with incidence of lung cancer among postmenopausal women.9 Two other studies did not reveal an association between HT use and lung cancer outcomes.19,20
The purpose of our study was to investigate whether reproductive factors are associated with overall survival in female non–small-cell lung cancer (NSCLC) patients and to further explore the role of HT use on survival of women with NSCLC.
MATERIALS AND METHODS
Study Participants
Lung cancer cases diagnosed between November 1, 2001 and October 31, 2005 were identified through the population-based Metropolitan Detroit Cancer Surveillance System, a member of the National Cancer Institute’s Surveillance, Epidemiology, and End Results program. Women aged 18 to 74, diagnosed with NSCLC, and residing in Wayne, Macomb, and Oakland were eligible to participate. Eligibility criteria were originally restricted to adenocarcinoma cases, but were extended after November 1, 2004 to include all other types of NSCLC as many histologic diagnoses were not more specific.
Detailed in-person interviews were completed for 577 women (60%); 273 women refused participation and 129 reported being too ill to participate. Participation rates were 58% in white and 63% in African American women. Cases with reported race other than African American or white (n = 13), women with unknown menstrual status or those whose menses were possibly affected by previous lung cancer treatment (n = 20), and women with a previous breast cancer diagnosis (n = 59) were excluded. After applying these exclusion criteria, 485 women with NSCLC were included in our analysis.
Data Collection
The study was approved by the institutional review board and all participants signed a written informed consent. Surveys collected demographic information, medical history, smoking history, reproductive history, and environmental tobacco exposure. Reproductive history included age at first birth, age of menarche, age of menopause, oral contraceptive use and duration before lung cancer diagnosis, and hormone use and duration before lung cancer diagnosis. Details of HT type (estrogen only, estrogen and progesterone combined, and progesterone only) and dose were collected. Data collected on a number of risk factors for NSCLC have been published previously.21–24 NSCLC diagnoses dates, histology, and treatment data were collected through the Metropolitan Detroit Cancer Surveillance System.
Statistical Analysis
Statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC). Student’s t tests were used to compare means of continuous variables, whereas comparisons of categorical variables were performed using χ2 tests. A Wilcoxon rank-sum test was used to compare medians between groups. All demographic, treatment, reproductive, and hormonal variables were first included in a univariate Cox proportional hazards regression model to assess the influence of the variable on survival. Nonhormonal and nonreproductive factors such as stage, treatment with surgery, treatment with radiation, income, age at diagnosis, pack-years, and race were included in all models. Stepwise regression methods were used to identify reproductive and hormonal variables associated with outcome. Reproductive and hormonal factors that met significance at the p value less than 0.05 level remained in the model. Additional models included HT duration (categorized: 0 months, 1–41 months, 42–131 months, 132 months, or longer before lung cancer diagnosis). HT duration was selected as the measure of exposure for additional analyses because dose data were missing for 41% of women reporting progesterone use and 45% of women reporting estrogen use. Of those women reporting doses, the majority reported using doses of 2.5 mg of progesterone and 0.625 mg of estrogen. With little variation in reported dose, duration of use was used to evaluate cumulative exposure. Models stratifying on menopausal status, HT type, and smoking status were also developed. No interaction between ever smoking and hormone use was found in any of the models. A level of 0.05 was used to assess the statistical significance of p values in all analyses.
RESULTS
Subject characteristics are listed in Table 1. Of the 485 women, 76.9% of the cases were white, and most were current or former smokers (92.3%); 91.6% of the women were post-menopausal and 72.0% had used oral contraceptives; 72.8% of the cases presented with an adenocarcinoma histology. Stage of diagnosis was balanced with about one-third of the women diagnosed in each of the local, regional, and distant stages.
TABLE 1.
Subject Characteristics and Hormone Therapy Status
| Variable | All Women N = 485 n (%)a |
Hormone Therapyb
|
p | Type of Hormone Therapy
|
p | ||
|---|---|---|---|---|---|---|---|
| Yes N = 230 n (%)a |
No N = 254 n (%)a |
Estrogen Only N = 99 n (%)a |
Combined Estrogen + Progesterone N = 85 n (%)a |
||||
| Median survival time, mo | 50.0 | 80.0 | 37.5 | <0.001 | 83.0 | 87.0 | 0.83 |
| Age, mean ± SD | 60.1 ± 9.0 | 61.8 ± 7.6 | 58.6 ± 9.8 | <0.001 | 62.3 ± 7.2 | 59.6 ± 7.4 | 0.01 |
| Race | <0.001 | 0.33 | |||||
| White | 373 (76.9) | 198 (86.0) | 174 (68.5) | 81 (81.8) | 74 (87.1) | ||
| African American | 112 (23.1) | 32 (13.9) | 80 (31.5) | 18 (18.2) | 11 (12.9) | ||
| Smoking statusc | 0.03 | 0.06 | |||||
| Never | 37 (7.6) | 17 (7.4) | 20 (7.9) | 12 (12.1) | 3 (3.5) | ||
| Ex-smoker | 155 (32.0) | 87 (37.8) | 68 (26.9) | 42 (42.4) | 33 (38.8) | ||
| Current | 292 (60.3) | 126 (54.8) | 165 (65.2) | 45 (45.5) | 49 (57.7) | ||
| Histology | 0.13 | 0.23 | |||||
| Squamous cell | 42 (8.7) | 19 (8.3) | 23 (9.1) | 4 (4.0) | 10 (11.8) | ||
| Adenocarcinoma | 353 (72.8) | 178 (77.4) | 174 (68.5) | 80 (80.8) | 61 (71.8) | ||
| Large cell | 13 (2.7) | 5 (2.2) | 8 (3.2) | 3 (3.0) | 2 (2.4) | ||
| NSCLC, NOS | 77 (15.9) | 28 (12.2) | 49 (19.29) | 12 (12.1) | 12 (14.1) | ||
| Pack-years of smokingd, mean ± SD | 47.5 ± 29.5 | 49.3 ± 28.3 | 45.8 ± 30.5 | 0.22 | 49.2 ± 27.8 | 47.1 ± 27.9 | 0.62 |
| Stage at diagnosise | 0.01 | 0.47 | |||||
| Local | 162 (33.6) | 88 (38.4) | 74 (29.4) | 42 (42.4) | 30 (35.3) | ||
| Regional | 161 (33.4) | 81 (35.4) | 79 (31.4) | 37 (37.4) | 32 (37.7) | ||
| Distant | 159 (33.0) | 60 (26.2) | 99 (39.3) | 20 (20.2) | 23 (27.1) | ||
| Hormone therapy duration, mof | 0.78 | ||||||
| 1–41 | 73 (33.3) | N/A | N/A | 31 (31.6) | 21 (26.9) | ||
| 42–131 | 72 (32.8) | 34 (34.7) | 28 (35.9) | ||||
| 132 | 74 (33.8) | 33 (33.7) | 29 (37.2) | ||||
| Age at menopause, mean ± SD | 44.2 ± 8.1 | 43.6 ± 8.1 | 44.7 ± 8.2 | 0.15 | 41.4 ± 7.6 | 46.0 ± 7.9 | <0.001 |
| Education levelg | <0.001 | 0.62 | |||||
| <High school | 20 (4.1) | 3 (1.3) | 17 (6.7) | 1 (1.0) | 1 (1.2) | ||
| High school | 266 (55.0) | 116 (50.7) | 149 (58.7) | 52 (52.5) | 38 (45.2) | ||
| >High school | 198 (40.9) | 110 (48.0) | 88 (34.7) | 46 (46.5) | 45 (53.6) | ||
| Treatment with surgeryh | <0.001 | 0.06 | |||||
| No | 195 (40.3) | 74 (32.3) | 120 (47.2) | 25 (25.3) | 32 (38.1) | ||
| Yes | 289 (59.7) | 155 (67.7) | 134 (52.8) | 74 (74.8) | 52 (61.9) | ||
| Treatment with radiationi | 0.15 | 0.23 | |||||
| No | 278 (58.2) | 140 (61.7) | 138 (55.2) | 61 (62.9) | 46 (54.1) | ||
| Yes | 200 (41.8) | 87 (38.3) | 112 (44.8) | 36 (37.1) | 39 (45.9) | ||
| Vital status | <0.01 | 0.58 | |||||
| Alive | 175 (36.1) | 106 (46.1) | 69 (27.2) | 46 (46.5) | 43 (50.6) | ||
| Dead | 310 (63.9) | 124 (53.9) | 185 (72.8) | 53 (53.5) | 42 (49.4) | ||
Percentages may not add to 100.0 because of rounding.
Hormone use was missing for one case.
Smoking status was missing for one case.
Pack-years were missing for two cases.
Stage was missing for three cases.
Duration of use was missing for 11 cases.
Education level was missing for one case.
Surgery information was missing for one case.
Radiation information was missing for seven cases.
NSCLC, non–small-cell lung cancer; NOS, not otherwise specified; N/A, not applicable.
Of the 485 women, 230 women (47.4%) had taken HT. Women who had taken HT were more likely to be white (p < 0.001), to have quit smoking (p = 0.03), had a higher education level (p < 0.001), and were at a higher income level (p = 0.02) compared with those who had never taken HT. HT type was determined for 187 women of the 230 who used hormones. Of those who used hormones, 99 women had taken estrogen only, three had taken progesterone only, and 85 had taken the combined estrogen and progesterone formulation. The women taking the combined therapy were older at menopause (mean years 46 versus 41.4; p < 0.001), but there were no other significant differences between the users of each hormone formulation in race, smoking status, pack-years, HT duration, or education level.
After adjusting for stage at diagnosis, surgery, radiation, education level, pack-years, age, race, and a multiplicative interaction between stage and radiation, the only reproductive or hormonal factor that predicted survival after NSCLC diagnosis was hormone use (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.54–0.89) (Table 2). Although HRs were reduced for all duration of use categories, hormone use of 132 months (11 years) or more before lung cancer diagnosis was significantly associated with better survival (HR, 0.54; 95% CI, 0.37–0.78). Age at first birth, age of first menstrual period, age of last menstrual period, number of pregnancies, number of children, oral contraceptive use and duration, infertility, or average length of days between menstrual cycles did not predict survival.
TABLE 2.
Survival of NSCLC by HT Use and Duration of Use
| All Women HRa (95% CI) |
Postmenopausal Women HRa (95% CI) |
Estrogen-Only HT Users HRa (95% CI) |
Estrogen + Progesterone HT Users HRa (95% CI) |
Never Smokers HRa (95% CI) |
Former and Current Smokers HRa (95% CI) |
|
|---|---|---|---|---|---|---|
| Ever HT use | 0.69 (0.54–0.88) | 0.72 (0.56–0.92) | 0.79 (0.59–1.04) | 0.64 (0.47–0.86) | 0.19 (0.05–0.73) | 0.73 (0.57–0.94) |
| Duration of HT use, mo | ||||||
| 0–41 | 0.82 (0.57–1.17) | 0.87 (0.60–1.25) | 0.79 (0.52–1.21) | 0.80 (0.51–1.26) | 0.20 (0.02–1.75) | 0.87 (0.60–1.25) |
| 42–131 | 0.81 (0.57–1.15) | 0.83 (0.58–1.19) | 0.98 (0.64–1.48) | 0.70 (0.43–1.14) | 0.78 (0.08–7.71) | 0.83 (0.58–1.19) |
| ≥132 | 0.54 (0.37–0.78) | 0.56 (0.38–0.82) | 0.58 (0.37–0.92) | 0.50 (0.30–0.83) | 0.12 (0.02–0.72) | 0.75 (0.60–0.94) |
Adjusted for age at diagnosis, race, pack-years (continuous), education, stage at diagnosis, treatment with surgery, treatment with radiation, and the multiplicative interaction between stage and radiation.
NSCLC, non–small-cell lung cancer; HT, hormone therapy; HR, hazard ratio; CI, confidence interval.
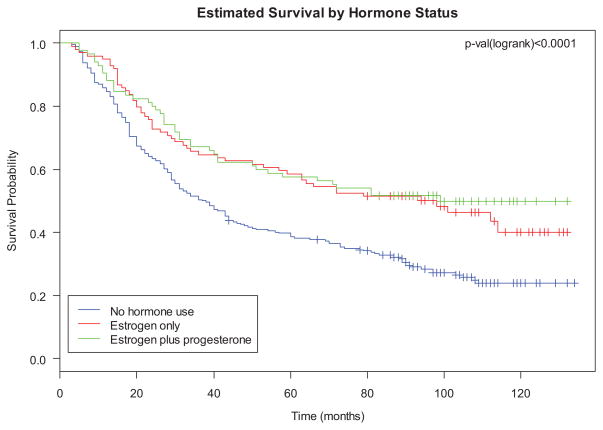
Ever use of HT did not significantly predict survival for women who took estrogen-only HT (HR, 0.79; 95% CI, 0.59–1.04), unless use of estrogen was for 11 years or longer in which case survival was improved (HR, 0.58; 95% CI, 0.37–0.92). Hormone use for those taking combined estrogen and progesterone was associated with significantly improved survival (HR, 0.64; 95% CI, 0.47–0.86). Although HRs were reduced for all duration of use categories, duration of HT was only statistically significantly associated with survival among those with 11 years or more of combined estrogen and progesterone use (HR, 0.50; 95% CI, 0.30–0.83) (Fig. 1).
FIGURE 1.
Survival by hormone therapy type.
The significance of hormone use did not change when restricting our analyses to postmenopausal women (n = 444). As in our analysis of all women, duration of more than 11 years of HT use was significant in this subgroup analysis. Hormone use was also predictive of survival in both never smokers and in ever smokers, and duration of HT use of 11 years was significantly protective in both groups.
Lung cancer was the recorded cause of death for 84.5% of the cases who died. Ever use of hormones remained significant when restricting the analyses to women with a lung cancer–specific cause of death (HR, 0.69; 95% CI, 0.53–0.90). Ever use of hormones was also significantly associated with survival when the analysis was restricted to women with adenocarcinoma of the lung (HR, 0.65; 95% CI, 0.49–0.87).
DISCUSSION
In this retrospective study, several reproductive factors were tested for their influence on survival after an NSCLC diagnosis. Age at first birth, number of children born, number of pregnancies, oral contraceptive use and duration, age of first or last menstrual period, infertility, and average length of days between menstrual cycles did not predict survival of NSCLC. However, increased duration of HT use was associated with a decreased risk of death after an NSCLC diagnosis. These findings suggest a complex relationship between exposure to exogenous hormones and lung cancer outcomes.
Research examining the relationship between reproductive factors and lung cancer survival in women has been limited. Skuladottir and Olsen25 examined whether reproductive patterns could predict outcomes of lung cancer in both men and women. They observed that women without children had worse prognoses than did parous women. The authors found a similar result in men without children, and concluded that the finding was not the result of hormones, but likely the result of lifestyle factors such as socioeconomic status. A more recent cohort study explored the impact of parity on the risk of death from lung cancer in Taiwan. With increasing parity, there was an increased risk of death although this trend was not significant (p= 0.25).26 That study was limited to premenopausal women, whereas our study is composed mainly of postmenopausal women. We did not find a significant association between parity and increased risk of death. Plasma estrogen levels are increased during pregnancy,27 but the precise role of estrogens in lung cancer development and progression is still not fully understood.28 To our knowledge, to date, there have been no other findings on reproductive factors and effect on survival outcomes for lung cancer.
Observational studies on HT use and lung cancer outcomes have been few and have resulted in conflicting findings. Table 3 summarizes the literature in this area. Our study supports the findings of an observational study that reported reduced lung cancer mortality among long-term hormone users (defined as use of 15 or more years) (relative risk, 0.22; 95% CI, 0.04–1.15).29 Huang et al. observed that postmenopausal women with lung cancer and a history of HT use had an increased survival time, but the difference was not statistically significant (p = 0.12). The authors did find significantly better survival among smokers who had taken HT compared with smokers who had not taken HT (median survival, 16.2 versus 10.4 months; p = 0.04); however, this result was not significant in a multivariate analysis.20 In our study, hormone use and longer duration of use were associated with an increased survival of NSCLC among both smokers and never smokers in multivariate analysis. Conversely, another more recent study found that women with lung cancer taking HT were at significantly greater risk of death compared with women with lung cancer who had never taken HT (HR = 1.97; 95% CI, 1.14–3.39), and this relationship was even more dramatic among those with a smoking history.18 None of these studies reported HT duration or distinguish between the types of HT taken.
TABLE 3.
Studies of HT and Survival after a Lung Cancer Diagnosis
| Author | Objective | Participants | Results | Comments |
|---|---|---|---|---|
| Ettinger et al.29 | Compare specific-cause mortality rates in women with and without long-term (≥5 yr) postmenopausal estrogen replacement therapy | 232 postmenopausal estrogen users; 222 postmenopausal nonusers; follow-up averaged 18 yr | Estrogen use associated with reduced risk of death from lung cancer (RR, 0.22; 95% CI, 0.04–1.15) | Although overall mortality in this group was reduced most for women with longer duration of use, duration of use data was not provided for lung cancer mortality. Treatment and stage information were not included. |
| Ganti et al.18 | Determine the impact of hormone replacement therapy on lung cancer survival | 498 women with lung cancer (86 HT users); follow-up was presented for 10 years | Hormone use associated with increased risk of death from lung cancer (HR, 1.97; 95% CI, 1.14–3.39); this effect was stronger in smokers; there was no association between HT use and survival in never smokers | No HT type, dose, or duration of use data were available; included women with small-cell lung cancer (24%). |
| Huang et al.20 | Determine whether history of HT use (either E only or E + P) is associated with lung cancer survival | 648 postmenopausal women (114 HT users) | Non-significant improved survival among HT users (median survival, 16.4 vs. 10.5 mo; p = 0.12); in analyses adjusted for age, stage, and smoking, there was no association between HT use and survival | Smoking data were missing for 25% of the cases; stage of diagnosis was available; no HT type, dose, or duration of use data were available. |
| Ayeni et al.19 | Determine whether HT is associated with survival after a lung cancer diagnosis | 397 women with lung cancer (115 HT users); 6-yr follow-up | HT not associated with outcomes of lung cancer in women even after adjustment for stage, age, treatment type, performance status, and weight loss | No HT type, dose, or duration of use data were available. |
| Chlebowski et al.9 | Compare incidence and mortality from lung cancer in women enrolled in a randomized clinical trial of estrogen plus progesterone vs. placebo | 8506 postmenopausal women assigned to combined therapy (70 deaths); 8102 postmenopausal women assigned to placebo (40 deaths); mean of 5.6 yr of treatment and 2.4 yr of follow-up | Combined estrogen plus progesterone use was associated with risk of death from non–small-cell lung cancer (HR, 1.71; 95% CI, 1.16–2.52) | Randomized, controlled trial so that dose and duration of use are controlled; no association with lung cancer incidence; no treatment information for women with lung cancer; women in the treatment arm were more likely to have lung cancer with distant metastases. |
| Chlebowski et al.8 | Compare incidence and mortality from lung cancer in women enrolled in a randomized clinical trial of estrogen vs. placebo | 5310 postmenopausal women assigned to conjugated equine estrogen (34 deaths); 5439 postmenopausal women assigned to placebo (33 deaths); mean follow-up of 7.9 yr | Estrogen use alone was not associated with risk of death from lung cancer (HR, 1.07; 95% CI, 0.66–1.72) | Randomized, controlled trial so that dose and duration of use are controlled; no association with lung cancer incidence; no treatment information for women with lung cancer. |
HT, hormone therapy; RR, relative risk; CI, confidence interval.
In our study, HT use did not predict survival among those who took estrogen only, except among long-term users. HT use was associated with better survival among women who had taken combined estrogen plus progesterone and this result was pronounced for women taking combined HT for 11 years or longer. Our results contrast those of the WHI’s randomized controlled trial study which assessed use of estrogen alone among postmenopausal women who had a hysterectomy and combined estrogen plus progesterone in postmenopausal women with no previous hysterectomy.8,9 These studies concluded that although estrogen-only therapy did not influence lung cancer incidence or mortality, combined estrogen plus progesterone use increased the number of deaths from lung cancer. There are advantages to the WHI trial including the randomized double-blind design with standardized dosing and centralized review of lung cancer outcomes. The clinical trial design more effectively controls for potential confounding associated with factors that affect HT use and outcomes such as race and smoking history. There are also limitations to the WHI studies such as a lack of treatment data and limited time of HT use.
The epidemiologic literature as described remains inconsistent. None of the studies directly address the same question because timing of use, type used, dose and duration of use, and unmeasured confounders vary between studies limiting the conclusions that can be drawn. The role of hormone exposures 11 or more years before a lung cancer diagnosis may impact outcomes differently than shorter exposures closer to the time of diagnosis, or use after diagnosis. Women using HT may also be different from women not using HT in terms of unmeasured confounders such as comorbidities, socioeconomic status, body mass index, or differential interactions with the medical system that affect outcomes.
The mechanisms underlying the association between estrogens and lung cancer are being evaluated in preclinical and clinical settings. Estrogens promote both cell proliferation and the transcription of estrogen-responsive genes. Estrogen action is through two distinct receptors, estrogen receptor (ER)-α and ER-β, both of which are expressed in the lung and localized in the cytoplasm and in the nucleus.30,31 Estrogen can be synthesized in the lung by aromatase, and high aromatase expression has been associated with poor prognosis in post-menopausal women with early lung cancer.32 Progesterone action, mediated by progesterone receptor (PR), is thought to stimulate tissue differentiation and inhibit cell proliferation.31 Progesterone supplementation has been shown to inhibit the growth of PR-positive lung tumors in mice.33 The interplay between estrogen and progesterone in lung carcinogenesis is not well understood and, in the epidemiologic literature, combined HT seems to be driving findings.
Stabile et al.34 report that both cytoplasmic and nuclear expression of ERs and PR predict overall survival and time to progression. High cytoplasmic ER-β is associated with reduced survival, whereas low total PR is a negative predictor of time to progression, even after adjustment for age, stage, sex, and smoking. Patients with the combined expression characteristics of low ER-β, low aromatase, low EGFR, and high PR had shorter overall survival compared with patients with the opposite pattern (HR, 6.6; 95% CI, 1.7–25.2). Expression varied by smoking status with never and former smokers having higher expression of nuclear ER-α, cytoplasmic PR, and total PR. Overall survival was higher in women compared with men, and no survival differences were noted by menopausal status. In an earlier study we conducted, which included some of the women from this current study, we evaluated the role of ER-β expression in lung tumors on survival; we also had HT information for some of the women.35 In that study, ER-β expression was not associated with use of HT before diagnosis or with any reproductive factors, but this was a small study with limited follow-up. If outcomes are truly mediated by tumor expression of ERs and PR, the role of HT in lung cancer mortality may be dependent on tumor characteristics that have not been evaluated in the epidemiologic literature.
Strengths of our study include the use of a large population-based sample, detailed information on HT type, duration of HT, the inclusion of a large proportion of cases with a history of HT use (n = 230, 47.5%), and the long-term follow-up of cases. Some limitations are of note. Reproductive and hormonal factors are based on recollection, so there is a potential for recall bias. We did use a calendar to trigger memories of important events in the woman’s life to minimize this bias. Overall, the study had 80% power to detect an HR associated with the use of HT of 0.73. Some of the stratified analyses included smaller numbers of women, so those results should be interpreted with caution. Women in the study were healthy enough to participate in the survey and this is reflected in longer overall survival than might be expected for lung cancer cases, so this study may not be generalizable to all women with NSCLC.
In conclusion, our study examines the influence of both reproductive and hormonal factors on NSCLC outcomes. Hormone use was associated with increased survival, particularly with use for 11 or more years, and especially among users of combined estrogen plus progesterone. With few consistent results in the literature, more research examining the biological significance of long-term HT use on lung cancer outcomes is needed, with better characterization of tumors in terms of ER and PR expression.
Acknowledgments
This research was funded in part by NIH grants R01-CA87895, and contracts N01-PC35145 and P30CA22453.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda: National Cancer Institute; Apr, 2013. Available at: http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88:183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 4.Donington JS, Colson YL. Sex and gender differences in non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2011;23:137–145. doi: 10.1053/j.semtcvs.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Baik CS, Strauss GM, Speizer FE, Feskanich D. Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses’ Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:2525–2533. doi: 10.1158/1055-9965.EPI-10-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinton LA, Gierach GL, Andaya A, et al. Reproductive and hormonal factors and lung cancer risk in the NIH-AARP Diet and Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:900–911. doi: 10.1158/1055-9965.EPI-10-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinton LA, Schwartz L, Spitz MR, Park Y, Hollenbeck AR, Gierach GL. Unopposed estrogen and estrogen plus progestin menopausal hormone therapy and lung cancer risk in the NIH-AARP Diet and Health Study Cohort. Cancer Causes Control. 2012;23:487–496. doi: 10.1007/s10552-012-9904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Anderson GL, Manson JE, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women’s health initiative randomized trial. J Natl Cancer Inst. 2010;102:1413–1421. doi: 10.1093/jnci/djq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chlebowski RT, Schwartz AG, Wakelee H, et al. Women’s Health Initiative Investigators. Oestrogen plus progestin and lung cancer in post-menopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374:1243–1251. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim WY, Chen Y, Chuah KL, et al. Female reproductive factors, gene polymorphisms in the estrogen metabolism pathway, and risk of lung cancer in Chinese women. Am J Epidemiol. 2012;175:492–503. doi: 10.1093/aje/kwr332. [DOI] [PubMed] [Google Scholar]
- 11.Pesatori AC, Carugno M, Consonni D, et al. Reproductive and hormonal factors and the risk of lung cancer: the EAGLE study. Int J Cancer. 2013;132:2630–2639. doi: 10.1002/ijc.27926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz AG, Wenzlaff AS, Prysak GM, et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J Clin Oncol. 2007;25:5785–5792. doi: 10.1200/JCO.2007.13.3975. [DOI] [PubMed] [Google Scholar]
- 13.Weiss JM, Lacey JV, Jr, Shu XO, et al. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am J Epidemiol. 2008;168:1319–1325. doi: 10.1093/aje/kwn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visbal AL, Williams BA, Nichols FC, 3rd, et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78:209–215. doi: 10.1016/j.athoracsur.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Alexiou C, Onyeaka CV, Beggs D, et al. Do women live longer following lung resection for carcinoma? Eur J Cardiothorac Surg. 2002;21:319–325. doi: 10.1016/s1010-7940(01)01114-9. [DOI] [PubMed] [Google Scholar]
- 16.Kligerman S, White C. Epidemiology of lung cancer in women: risk factors, survival, and screening. AJR Am J Roentgenol. 2011;196:287–295. doi: 10.2214/AJR.10.5412. [DOI] [PubMed] [Google Scholar]
- 17.Moore KA, Mery CM, Jaklitsch MT, et al. Menopausal effects on presentation, treatment, and survival of women with non-small cell lung cancer. Ann Thorac Surg. 2003;76:1789–1795. doi: 10.1016/s0003-4975(03)01024-5. [DOI] [PubMed] [Google Scholar]
- 18.Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 19.Ayeni O, Robinson A. Hormone replacement therapy and outcomes for women with non-small-cell lung cancer: can an association be confirmed? Curr Oncol. 2009;16:21–25. doi: 10.3747/co.v16i3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang B, Carloss H, Wyatt SW, Riley E. Hormone replacement therapy and survival in lung cancer in postmenopausal women in a rural population. Cancer. 2009;115:4167–4175. doi: 10.1002/cncr.24475. [DOI] [PubMed] [Google Scholar]
- 21.Van Dyke AL, Cote ML, Wenzlaff AS, et al. Cytokine and cytokine receptor single-nucleotide polymorphisms predict risk for non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev. 2009;18:1829–1840. doi: 10.1158/1055-9965.EPI-08-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz AG, Cote ML, Wenzlaff AS, et al. Chronic obstructive lung diseases and risk of non-small cell lung cancer in women. J Thorac Oncol. 2009;4:291–299. doi: 10.1097/JTO.0b013e3181951cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cote ML, Yoo W, Wenzlaff AS, et al. Tobacco and estrogen metabolic polymorphisms and risk of non-small cell lung cancer in women. Carcinogenesis. 2009;30:626–635. doi: 10.1093/carcin/bgp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. Regular adult aspirin use decreases the risk of non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev. 2008;17:148–157. doi: 10.1158/1055-9965.EPI-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skuladottir H, Olsen JH. Can reproductive pattern explain better survival of women with lung cancer? Acta Oncol. 2006;45:47–53. doi: 10.1080/02841860500374455. [DOI] [PubMed] [Google Scholar]
- 26.Cheng MH, Tsai SS, Chen CC, et al. Parity and risk of death from lung cancer among a cohort of premenopausal parous women in Taiwan. J Epidemiol. 2012;22:364–369. doi: 10.2188/jea.JE20110123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindberg BS, Johansson ED, Nilsson BA. Plasma levels of nonconjugated oestrone, oestradiol-17beta and oestriol during uncomplicated pregnancy. Acta Obstet Gynecol Scand Suppl. 1974;32:21–36. doi: 10.3109/00016347409156390. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty S, Ganti AK, Marr A, Batra SK. Lung cancer in women: role of estrogens. Expert Rev Respir Med. 2010;4:509–518. doi: 10.1586/ers.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ettinger B, Friedman GD, Bush T, Quesenberry CP., Jr Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet Gynecol. 1996;87:6–12. doi: 10.1016/0029-7844(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 30.Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol. 2009;36:524–531. doi: 10.1053/j.seminoncol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazmi N, Márquez-Garbán DC, Aivazyan L, et al. The role of estrogen, progesterone and aromatase in human non-small-cell lung cancer. Lung Cancer Manag. 2012;1:259–272. doi: 10.2217/lmt.12.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mah V, Seligson DB, Li A, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res. 2007;67:10484–10490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishibashi H, Suzuki T, Suzuki S, et al. Progesterone receptor in non-small cell lung cancer—a potent prognostic factor and possible target for endocrine therapy. Cancer Res. 2005;65:6450–6458. doi: 10.1158/0008-5472.CAN-04-3087. [DOI] [PubMed] [Google Scholar]
- 34.Stabile LP, Dacic S, Land SR, et al. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res. 2011;17:154–164. doi: 10.1158/1078-0432.CCR-10-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz AG, Prysak GM, Murphy V, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res. 2005;11:7280–7287. doi: 10.1158/1078-0432.CCR-05-0498. [DOI] [PubMed] [Google Scholar]