Abstract
Bosutinib is an orally active, dual Src/Abl tyrosine kinase inhibitor for treatment of chronic myeloid leukemia (CML) following resistance/intolerance to prior therapy. Here, we report the data from the 2-year follow-up of a phase 1/2 open-label study evaluating the efficacy and safety of bosutinib as second-line therapy in 288 patients with chronic phase CML resistant (n = 200) or intolerant (n = 88) to imatinib. The cumulative response rates to bosutinib were as follows: 85% achieved/maintained complete hematologic response, 59% achieved/maintained major cytogenetic response (including 48% with complete cytogenetic response), and 35% achieved major molecular response. Responses were durable, with 2-year estimates of retaining response >70%. Two-year probabilities of progression-free survival and overall survival were 81% and 91%, respectively. The most common toxicities were primarily gastrointestinal adverse events (diarrhea [84%], nausea [45%], vomiting [37%]), which were primarily mild to moderate, typically transient, and first occurred early during treatment. Thrombocytopenia was the most common grade 3/4 hematologic laboratory abnormality (24%). Outcomes were generally similar among imatinib-resistant and imatinib-intolerant patients and did not differ with age. The longer-term results of the present analysis confirm that bosutinib is an effective and tolerable second-line therapy for patients with imatinib-resistant or imatinib-intolerant chronic phase CML. http://ClinicalTrials.gov Identifier: NCT00261846. Am. J. Hematol. 89:732–742, 2014. © 2014 The Authors American Journal of Hematology Published by Wiley Periodicals, Inc.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasia characterized by the presence in proliferating cells of the Philadelphia chromosome (Ph), a balanced translocation between chromosomes 9 and 22 that results in production of a Bcr-Abl fusion oncoprotein [1]. Currently, the most frequently used first-line therapy for patients with chronic phase (CP) CML is the Bcr-Abl tyrosine kinase inhibitor (TKI) imatinib [2,3]. Unfortunately, development of resistance and intolerance represent a limitation of imatinib treatment [2,4]. The second-generation TKIs dasatinib and nilotinib have demonstrated efficacy in patients with CP CML in the first-line setting and as second-line therapy following imatinib resistance/intolerance [5–12]. However, resistance or intolerance to these second-generation TKIs may occur in some patients [13,14]. Thus, alternative treatment options are needed for patients with CP CML resistant or intolerant to available TKIs.
Bosutinib (SKI-606) is an orally active, dual Src and Abl TKI with minimal activity against platelet-derived growth factor receptor or c-KIT [15–17]; it has been suggested that inhibition of these enzymes may be associated with some of the adverse events (AEs) reported with imatinib [16,18] and dasatinib [19,20] treatment. In preclinical studies, bosutinib demonstrated potent Bcr-Abl inhibition of imatinib-resistant CML cell lines and most imatinib-resistant Bcr-Abl kinase domain mutations, except T315I and V299L [16,21].
Initial reports from the open-label, phase 1/2 trial in patients with previously treated Ph+ leukemia indicated good clinical activity and tolerability with oral bosutinib 500 mg/day. Durable hematologic and cytogenetic responses were observed among patients with CP CML in the second-line setting after imatinib [22] and third-/fourth-line settings after prior imatinib plus dasatinib and/or nilotinib [23]. Responses have also been observed in accelerated phase (AP) and blast phase (BP) CML [24]. Frequent toxicities observed with bosutinib include gastrointestinal symptoms (ie, diarrhea, nausea, vomiting, and abdominal pain), rash, fatigue, and pyrexia; grade 3/4 hematologic toxicities and liver function test abnormalities have also been reported [22–24]. The current analysis of this phase 1/2 trial provides a 24-month update of bosutinib as second-line therapy for patients with CP CML and resistance or intolerance to imatinib and no exposure to other TKIs.
Methods
The study design and eligibility criteria have been previously described [22–24]. The current analysis included patients aged ≥18 years with CP CML and resistance to prior imatinib ≥600 mg/day or intolerance to any dose of imatinib who had no previous exposure to other TKIs; an Eastern Cooperative Oncology Group Performance Status score of 0 or 1; adequate bone marrow (imatinib-resistant patients), hepatic, and renal function; ≥7 days since any prior antiproliferative treatment except for hydroxyurea and anagrelide; and ≥3 months postallogeneic hematopoietic stem cell transplant [22]. All patients provided written informed consent before study enrollment.
This was a phase 1/2, open-label, multicenter, 2-part study of bosutinib in patients with Ph+ leukemias. Part 1 was a dose-escalation study that determined a recommended phase 2 dose of bosutinib 500 mg/day in patients with CP CML [22]. Part 2, described in this report, evaluated the efficacy and safety of continued oral daily dosing of bosutinib at this dose. Dose escalation was allowed for lack of efficacy (no complete hematologic response [CHR] by week 8 or no complete cytogenetic response [CCyR] by week 12) in the absence of grade 3/4 treatment-related toxicity. Doses could be held or reduced by 100-mg increments to a minimum dose of 300 mg/day based on the severity and duration of treatment-related toxicities. Treatment could continue until disease progression (defined as transformation to AP/BP CML, increased white blood cell count [i.e., doubling occurring over ≥1 month with the second count >20 × 109/L and confirmed ≥1 week later], or loss of previously attained major cytogenetic response [MCyR] or any hematologic response), unacceptable toxicity (including intolerance to bosutinib ≤300 mg/day), or withdrawal of consent. Long-term follow-up continued for 2 years after treatment discontinuation to determine patient-reported progression, initiation of new anticancer treatment, and survival.
Patients recruited in Part 1 were further analyzed along with patients from Part 2 for both efficacy and long-term safety. The primary endpoint of Part 2 was the rate of MCyR at week 24 in patients with imatinib-resistant CP CML and has been previously reported [22]; thus, only cumulative endpoints are reported in the current manuscript. Key secondary and exploratory efficacy endpoints included cumulative cytogenetic, hematologic, and molecular response, time to and duration of response, response by baseline Bcr-Abl kinase domain mutation status, progression-free survival (PFS), and overall survival (OS).
Response was determined as described previously [22]. Cytogenetic response assessments were performed every 3 months through 2 years and every 6 months thereafter during treatment. Additionally, peripheral blood was collected at weeks 1, 2, 3, 4, 8, and 12 for analysis of complete blood cell count and Bcr-Abl transcript levels (performed monthly) and thereafter was collected on the same schedule as cytogenetic response assessments. Efficacy endpoints were summarized using descriptive statistics, cumulative incidence, the Kaplan–Meier method, response rates, and confidence intervals (CIs). AEs were reported at each study visit through 30 days after the last bosutinib dose; physical examinations, vital signs, and laboratory tests were also performed routinely. Additional details of cytogenetic, hematologic, and molecular response assessments and efficacy and safety endpoints are provided in the Supporting Information.
The protocol was approved by the central or institutional review board for each study site, and the study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Results
Patients
Overall, 288 patients with imatinib-resistant (n = 200) or imatinib-intolerant (n = 88) CP CML were enrolled and treated with bosutinib in Part 2 of the study, including patients from Part 1 who were enrolled in Part 2. Patient demographics and baseline disease characteristics were previously reported [22] and are provided in Supporting Information Table SI. Briefly, the median age was 53 years (range, 18–91 years), with 224 (78%) patients aged <65 years; 153 (53%) patients were male. The median (range) time since CML diagnosis was 4.0 years (0.1–15.1 years) for imatinib-resistant patients and 2.8 years (0.1–13.6 years) for imatinib-intolerant patients. The median duration of prior imatinib treatment was 2.5 years (0.4–8.8 years) for imatinib-resistant patients and 1.5 years (<0.01–8.3 years) for imatinib-intolerant patients.
As of the data snapshot (March 28, 2011, based on an unlocked database for this interim manuscript), 92 of 200 (46%) imatinib-resistant patients and 37 of 88 (42%) imatinib-intolerant patients were still receiving treatment. The most common reasons for treatment discontinuation included an AE (22%), disease progression (14%), unsatisfactory response/lack of efficacy (7%), and patient request (6%; Supporting Information Table SII). The median (range) duration of bosutinib treatment was 22.1 months (0.2–60.8 months). Median follow-up was 30.5 months (0.6–66.0 months) for imatinib-resistant patients and 35.1 months (0.7–58.0 months) for imatinib-intolerant patients; time from the last enrolled patient's first visit to the data snapshot in the imatinib-resistant cohort (primary study cohort) was ≥24 months (96 weeks). Three imatinib-intolerant patients with CCyR at their month 21 visit had not reached their month 24 visit as of the data snapshot but were subsequently assessed, with all 3 retaining their CCyR at month 24.
Efficacy
Evaluable patients had received at least 1 dose of bosutinib and had a valid baseline assessment for the respective endpoint. A total of 85% of evaluable patients either newly achieved a confirmed CHR or maintained their baseline CHR for ≥5 weeks, with response rates being similar between imatinib-resistant and imatinib-intolerant patients (Table I). Median (range) time to a confirmed CHR among responders was 2.0 weeks (0.3–72.4 weeks) for imatinib-resistant patients and 1.7 weeks (0.9–36.3 weeks) for imatinib-intolerant patients. The cumulative incidence curve for CHR is displayed in Figure 1A. Of the 141 patients with no CHR at baseline, 109 (77%) achieved a confirmed CHR.
TABLE I.
Best Overall Response
| Response | Imatinib-resistant (n = 200) | Imatinib-intolerant (n = 88) | Total (n = 288) |
|---|---|---|---|
| Median (range) treatment duration, mo | 22.1 (0.2–60.8) | 20.7 (0.3–52.3) | 22.1 (0.2–60.8) |
| Cytogenetic response,a n (%) [95% CI] | |||
| Evaluable patients | 186 | 80 | 266 |
| MCyR | 108 (58) [51–65] | 49 (61) [50–72] | 157 (59) [53–65] |
| CCyR | 85 (46) [38–53] | 43 (54) [42–65] | 128 (48) [42–54] |
| Evaluable patients without a CCyR at baseline | 181 | 69 | 250 |
| MCyR | 103 (57) [49–64] | 39 (57) [44–68] | 142 (57) [50–63] |
| CCyR | 80 (44) [37–52] | 34 (49) [37–62] | 114 (46) [39–52] |
| Molecular response,b n (%) [95% CI] | |||
| Evaluable patients | 132 | 68 | 200 |
| MMR | 45 (34) [26–43] | 24 (35) [24–48] | 69 (35) [28–42] |
| CMR | 33 (25) [18–33] | 22 (32) [22–45] | 55 (28) [21–34] |
| Hematologic response,c n (%) [95% CI] | |||
| Evaluable patients | 199 | 88 | 287 |
| CHR | 170 (85) [80–90] | 74 (84) [75–91] | 244 (85) [80–89] |
| Evaluable patients without a CHR at baseline | 100 | 41 | 141 |
| CHR | 76 (76) [66–84] | 33 (81) [65–91] | 109 (77) [70–84] |
Abbreviations: CCyR, complete cytogenetic response; CHR, complete hematologic response; CMR, complete molecular response; FISH, fluorescence in situ hybridization; MCyR, major cytogenetic response; MMR, major molecular response; PCR, polymerase chain reaction; PCyR, partial cytogenetic response; Ph+, Philadelphia chromosome-positive.
Evaluable patients must have had an adequate baseline cytogenetic assessment. Cytogenetic response [27] was determined using standard cytogenetics (G-band karyotype) with ≥20 metaphases counted for postbaseline assessments; if <20 metaphases were available postbaseline, FISH analysis of bone marrow aspirate with ≥200 cells for the presence of Bcr-Abl fusion gene was used. MCyR included PCyR (1–35% Ph+ metaphases) and CCyR (0% Ph+ metaphases; <1% if using FISH). Cytogenetic response could be achieved during the study or maintained from baseline for ≥4 weeks, unless otherwise noted within the table.
Patients enrolled in China, India, Russia, and South Africa could not be evaluated for molecular response due to logistical constraints; treated patients not from these four countries were evaluable for molecular response. Molecular response was assessed at a central laboratory (Quest Diagnostics) using nonnested real time PCR for the ratio of Bcr-Abl to Abl transcripts. MMR was categorized as a ≥3-log reduction from standardized baseline and included CMR (undetectable Bcr-Abl transcript with a PCR sensitivity of ≥5 logs). To be considered a responder for MMR/CMR, the patient should also have had detectable Bcr-Abl transcript levels at baseline or any time postbaseline, and have achieved/maintained a CCyR; patients with cytogenetic assessments not showing CCyR on the same day of molecular assessment were not considered to have an MMR/CMR at that time. MMR was not assessed using the International Scale because it was not widely available when the study was initiated.
Evaluable patients must have had an adequate baseline hematologic assessment. The definition of CHR was standard [22]; hematologic response was required to be confirmed and to last for ≥4 weeks, with peripheral blood and/or bone marrow documentation, and could be achieved during the study or maintained from baseline for ≥5 weeks, unless otherwise noted within the table.
Figure 1.
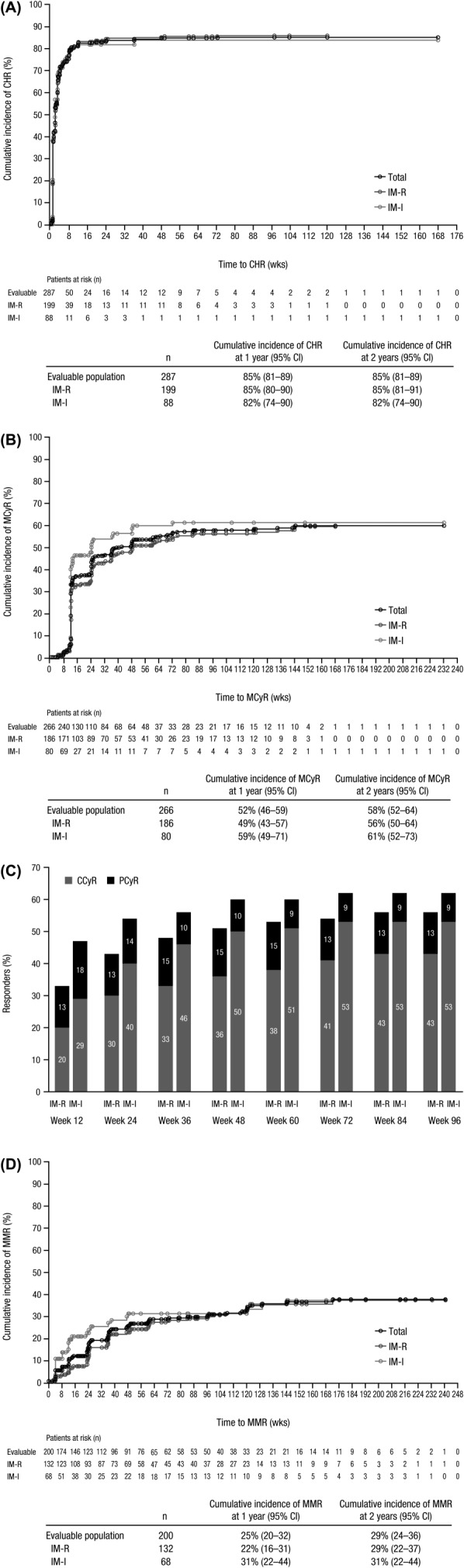
Cumulative incidence curve for time to response adjusting for the competing risk of treatment discontinuation without response. Time to CHR (A), MCyR (B), and MMR (D) was calculated among evaluable patients with a valid baseline assessment from the start date of therapy until the first date of attained/maintained response (confirmed for CHR and unconfirmed for MCyR and MMR) or last nonmissing assessment date for those without a response or discontinuation. All treated patients were evaluable for MMR except patients from sites in China, India, Russia, and South Africa, who were not assessed for molecular response. (C) Rates of MCyR, including PCyR and CCyR, were cumulative by the defined time points for evaluable patients (IM-R, n = 186; IM-I, n = 80) who had an adequate baseline cytogenetic assessment and maintained/achieved their response. Abbreviations: CCyR, complete cytogenetic response; CHR, complete hematologic response; IM-I, imatinib intolerant; IM-R, imatinib resistant; MCyR, major cytogenetic response; MMR, major molecular response; PCyR, partial cytogenetic response.
Among evaluable patients with a valid baseline cytogenetic assessment, 59% newly achieved an MCyR or maintained their baseline MCyR for ≥4 weeks, including 58% of imatinib-resistant patients and 61% of imatinib-intolerant patients (Table I). The CCyR rate was 48%. Median (range) time to MCyR among responders was 12.3 weeks (4.0–144.0 weeks) for imatinib-resistant patients and 12.1 weeks (8.0–72.1 weeks) for imatinib-intolerant patients. The cumulative incidence curve for MCyR is displayed in Figure 1B. Nearly all patients who maintained/achieved an MCyR did so during the first year (Fig. 1C). Among patients without a CCyR at baseline, the MCyR rate was 57% for imatinib-resistant and imatinib-intolerant patients. The MCyR and CCyR rates were 53% (95% CI, 47–60) and 43% (95% CI, 37–49), respectively, when patients with a CCyR at baseline were considered non-responders.
A major molecular response (MMR; not assessed on the International Scale) was achieved in 35% of treated patients (analysis excluded patients from China, India, Russia, and South Africa), including 28% of patients who achieved a complete molecular response, with the proportion of patients who achieved an MMR being similar for imatinib-resistant (34%) and imatinib-intolerant (35%) patients (Table I). Median (range) time to MMR among responders was 35.9 weeks (3.1–172.0 weeks) for imatinib-resistant patients and 12.2 weeks (4.0–144.1 weeks) for imatinib-intolerant patients. The cumulative incidence of MMR is displayed in Fig. 1D. Among 104 patients who achieved/maintained a CCyR and were evaluable for molecular response, 69 (66%; 95% CI, 56–75) patients achieved an MMR.
Responses were durable, with Kaplan–Meier median durations of CHR, MCyR, and MMR not being reached for imatinib-resistant or imatinib-intolerant patients (Fig. 2). The 2-year Kaplan–Meier estimates of retaining a response remained >70% in the overall population for all three response types, although estimates were generally higher for imatinib-intolerant versus imatinib-resistant patients.
Figure 2.
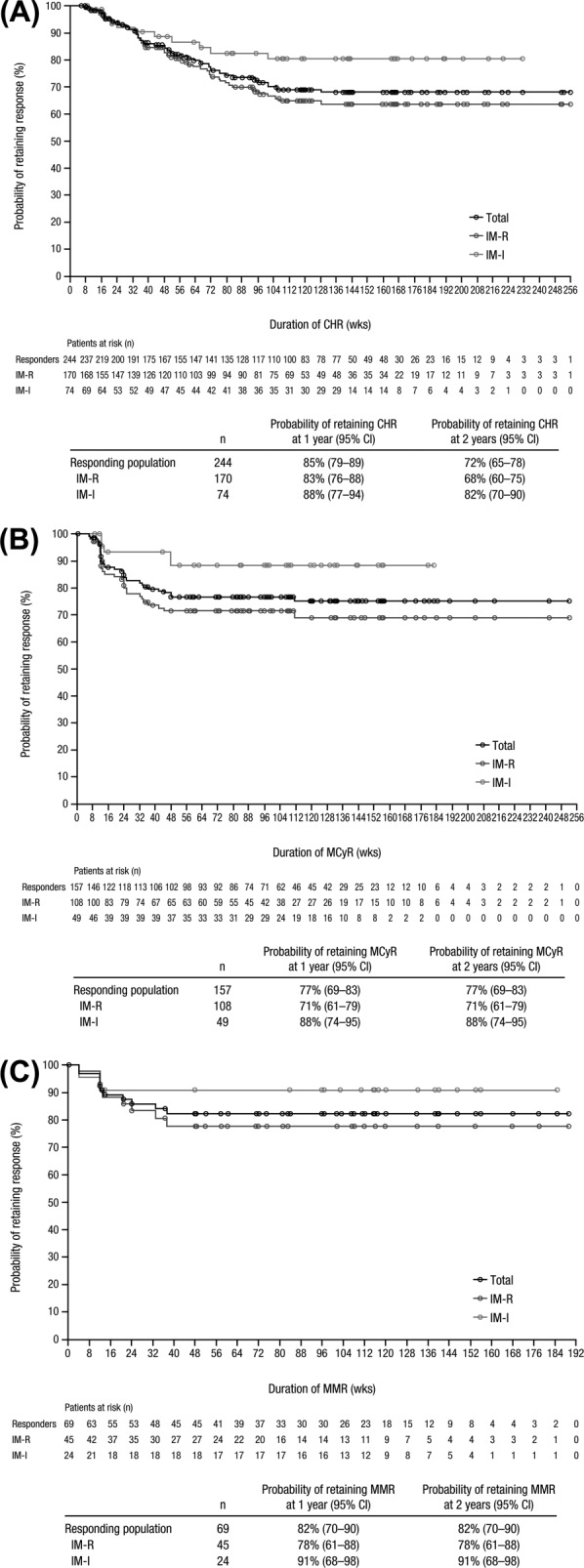
Duration of CHR (A), MCyR (B), and MMR (C). Duration of response was calculated among responders from the first date of response until confirmed loss of response, treatment discontinuation due to progressive disease or death, or death within 30 days of the last dose; patients without events were censored at their last assessment visit. The probability of retaining response at 2 years was based on Kaplan–Meier estimates. Abbreviations: CHR, complete hematologic response; IM-I, imatinib intolerant; IM-R, imatinib resistant; MCyR, major cytogenetic response; MMR, major molecular response.
CHR and MCyR response rates were comparable between older (aged ≥65 years) and younger (aged <65 years) patients, as were Kaplan–Meier estimates of retaining either response at 2 years (≥65%; Table II).
TABLE II.
Summary of Results for Older versus Younger Patients
| Parameter | Older patients (≥65 y) (n = 64) | Younger patients (<65 y) (n = 224) |
|---|---|---|
| Median (range) age, y | 70 (65–91) | 48 (18–64) |
| ECOG performance status,a n (%) | ||
| 0 | 39 (61) | 181 (81) |
| 1 | 25 (39) | 41 (18) |
| 2 | 0 | 1 (<1) |
| Median (range) time since CML diagnosis, y | 5.5 (0.1–13.7) | 3.2 (0.1–15.1) |
| Key baseline medical conditions, n (%) | ||
| Gastrointestinal disorders | 38 (59) | 77 (34) |
| Vascular disorders | 31 (48) | 52 (23) |
| Metabolism disorders | 27 (42) | 70 (31) |
| Diabetes mellitus | 2 (3) | 7 (3) |
| Musculoskeletal disorders | 27 (42) | 60 (27) |
| Blood/lymphatic disorders | 26 (41) | 67 (30) |
| Cardiac disordersb | 25 (39) | 23 (10) |
| Nervous system disorders | 23 (36) | 31 (14) |
| Respiratory disorders | 19 (30) | 31 (14) |
| Endocrine disorders | 11 (17) | 16 (7) |
| Hepatobiliary disorders | 4 (6) | 19 (9) |
| Median (range) no. of baseline medications | 4 (1–14) | 2 (1–16) |
| Median (range) duration of bosutinib, mo | 13.8 (0.3–48.9) | 22.1 (0.2–60.8) |
| Median (range) follow-up, mo | 33.8 (1.0–53.0) | 31.7 (0.6–66.0) |
| Cytogenetic response,c n (%) [95% CI] | ||
| Evaluable patients | 62 | 204 |
| MCyR | 33 (53) [40–66] | 124 (61) [54–68] |
| CCyR | 29 (47) [34–60] | 99 (49) [42–56] |
| Probability of retaining MCyR at 2 yearsd | 72% [52–85] | 78% [69–84] |
| Hematologic response,e n (%) [95% CI] | ||
| Evaluable patients | 64 | 223 |
| CHR | 52 (81) [70–90] | 192 (86) [81–90] |
| Probability of retaining CHR at 2 yearsd | 65% [48–77] | 74% [67–80] |
| Non-hematologic TEAEs with ≥8% difference between age groups | ||
| Vomiting | 29 (45) | 77 (34) |
| Fatigue | 23 (36) | 44 (20) |
| Decreased appetite | 17 (27) | 23 (10) |
| Weight decreased | 14 (22) | 9 (4) |
| Asthenia | 13 (20) | 23 (10) |
| Nasopharyngitis | 12 (19) | 24 (11) |
| Dyspnea | 12 (19) | 13 (6) |
| Peripheral edema | 10 (16) | 13 (6) |
| Increased ALT | 9 (14) | 53 (24) |
| Pleural effusion | 9 (14) | 6 (3) |
| Increased AST | 8 (13) | 46 (21) |
| Increased lipase | 8 (13) | 11 (5) |
| Chills | 8 (13) | 8 (4) |
| Increased blood creatinine | 8 (13) | 5 (2) |
| Abdominal pain | 7 (11) | 60 (27) |
| Influenza | 1 (2) | 22 (10) |
| Dose interruption due to a TEAE, n (%) | 49 (77) | 153 (68) |
| Dose reduction due to a TEAE, n (%) | 36 (56) | 101 (45) |
| Discontinuation due to an AE, n (%) | 19 (30) | 47 (21) |
| Death within 30 days of last dose due to an AE, n (%) | 1 (2) | 2 (1) |
| Transformation to AP/BP CML, n | 2 | 9 |
| PFS at 2 yearsd [95% CI] | 76% [60–87] | 82% [75–87] |
| OS at 2 yearsd [95% CI] | 87% [75–93] | 92% [87–95] |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AP, accelerated phase; AST, aspartate aminotransferase; BP, blast phase; CCyR, complete cytogenetic response; CHR, complete hematologic response; CML, chronic myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization; MCyR, major cytogenetic response; OS, overall survival; PCyR, partial cytogenetic response; PFS, progression-free survival; Ph+, Philadelphia chromosome-positive; TEAE, treatment-emergent adverse event.
ECOG Performance Status was missing for 1 younger, imatinib-intolerant patient.
The most common cardiac events at baseline (≥3 patients) were coronary artery disease (older, n = 6; younger, n = 1), myocardial infarction (n = 5; n = 1, respectively), acute myocardial infarction (n = 2; n = 3), arrhythmia (n = 3; n = 2), cardiomyopathy (n = 3; n = 2), and palpitations (n = 2; n = 2).
Evaluable patients must have had an adequate baseline cytogenetic assessment. Cytogenetic response [27] was determined using standard cytogenetics (G-band karyotype) with ≥20 metaphases counted for postbaseline assessments; if <20 metaphases were available post-baseline, FISH analysis of bone marrow aspirate with ≥200 cells for the presence of Bcr-Abl fusion gene was used. MCyR included PCyR (1–35% Ph+ metaphases) and CCyR (0% Ph+ metaphases; <1% if using FISH). Cytogenetic response could be achieved during the study or maintained from baseline for ≥4 weeks.
Probabilities at 2 years were based on Kaplan–Meier estimates.
Evaluable patients must have had an adequate baseline hematologic assessment. The definition of CHR was standard [22]; hematologic response was required to be confirmed and to last for ≥4 weeks, with peripheral blood and/or bone marrow documentation, and could be achieved during the study or maintained from baseline for ≥5 weeks.
A total of 212 patients were assessed for Bcr-Abl kinase domain mutations at baseline: 79 (37%) patients had ≥1 mutation, including 11 (5%) patients who had ≥2 mutations. Forty-two unique point mutations were identified, several of which have been associated with resistance to imatinib in the clinical setting [25]; the most frequent mutations were M35IT, F359V, and T315I (n = 9 each). As a whole, patients with ≥1 mutation had response rates (CHR, 83%; MCyR, 58%) that were similar to those observed for patients without baseline mutations (CHR, 90%; MCyR, 59%). When patients with the T315I mutation were excluded, the response rates for patients with a mutation were 91% for CHR and 62% for MCyR.
Safety and tolerability
All 288 patients received ≥1 dose of bosutinib and were included in the safety population. The most common nonhematologic treatment-emergent AEs (TEAEs) were gastrointestinal (i.e., diarrhea, nausea, vomiting, and abdominal pain); rash, pyrexia, fatigue, and increased alanine aminotransferase (ALT) were also commonly observed (Table III). Diarrhea, rash, and elevated ALT represent the most common grade 3/4 nonhematologic TEAEs, although the incidence of grade 4 events was low (diarrhea, 0%; rash, 1%; elevated ALT, 1%). The incidences of pleural effusion (all grades, 5%; grade 3, n = 2; grade 4, n = 1) and pancreatitis (all grades, 1%) AEs were low among imatinib-resistant and imatinib-intolerant patients. Only 3% of patients experienced a pleural effusion AE considered related to study drug.
TABLE III.
Treatment-Emergent Adverse Events and Laboratory Abnormalities
| Imatinib-resistant (n = 200) | Imatinib-intolerant (n = 88) | Total (n = 288) | ||||
|---|---|---|---|---|---|---|
| Event, n (%) | All grades | Grade 3/4 | All grades | Grade 3/4 | All grades | Grade 3/4 |
| Nonhematologic TEAEsa | ||||||
| Diarrhea | 168 (84) | 17 (9) | 75 (85) | 11 (13) | 243 (84) | 28 (10) |
| Nausea | 84 (42) | 0 | 45 (51) | 4 (5) | 129 (45) | 4 (1) |
| Vomiting | 70 (35) | 3 (2) | 36 (41) | 8 (9) | 106 (37) | 11 (4) |
| Rash | 63 (32) | 17 (9) | 36 (41) | 10 (11) | 99 (34) | 27 (9) |
| Pyrexia | 54 (27) | 1 (1) | 14 (16) | 0 | 68 (24) | 1 (<1) |
| Abdominal pain | 45 (23) | 2 (1) | 22 (25) | 2 (2) | 67 (23) | 4 (1) |
| Fatigue | 45 (23) | 1 (1) | 22 (25) | 2 (2) | 67 (23) | 3 (1) |
| Elevated ALT | 41 (21) | 13 (7) | 21 (24) | 8 (9) | 62 (22) | 21 (7) |
| Upper abdominal pain | 40 (20) | 1 (1) | 17 (19) | 0 | 57 (20) | 1 (<1) |
| Cough | 44 (22) | 1 (1) | 13 (15) | 0 | 57 (20) | 1 (<1) |
| Elevated AST | 36 (18) | 6 (3) | 18 (21) | 5 (6) | 54 (19) | 11 (4) |
| Headache | 30 (15) | 0 | 18 (21) | 0 | 48 (17) | 0 |
| Arthralgia | 27 (14) | 0 | 13 (15) | 1 (1) | 40 (14) | 1 (<1) |
| Decreased appetite | 27 (14) | 2 (1) | 13 (15) | 0 | 40 (14) | 2 (1) |
| Asthenia | 22 (11) | 5 (3) | 14 (16) | 0 | 36 (13) | 5 (2) |
| Back pain | 16 (8) | 0 | 17 (19) | 0 | 33 (12) | 0 |
| Nasopharyngitis | 24 (12) | 0 | 12 (14) | 0 | 36 (13) | 0 |
| Constipation | 18 (9) | 0 | 15 (17) | 1 (1) | 33 (12) | 1 (<1) |
| Oropharyngeal pain | 22 (11) | 0 | 8 (9) | 0 | 30 (10) | 0 |
| Hematologic laboratory abnormalitiesb | ||||||
| Thrombocytopenia | 131 (66) | 42 (21) | 62 (70) | 28 (32) | 193 (67) | 70 (24) |
| Anemia | 182 (91) | 23 (12) | 76 (86) | 16 (18) | 258 (90) | 39 (14) |
| Leukopenia | 101 (51) | 14 (7) | 46 (52) | 9 (10) | 147 (51) | 23 (8) |
| Neutropenia | 97 (49) | 28 (14) | 45 (51) | 21 (24) | 142 (49) | 49 (17) |
| Nonhematologic laboratory abnormalitiesb | ||||||
| Elevated ALT | 110 (55) | 20 (10) | 58 (66) | 10 (11) | 168 (58) | 30 (10) |
| Elevated AST | 97 (49) | 7 (4) | 48 (55) | 6 (7) | 145 (50) | 13 (5) |
| Hypophosphatemia | 89 (45) | 19 (10) | 36 (41) | 6 (7) | 125 (43) | 25 (9) |
| Hypocalcemia | 82 (41) | 5 (3) | 41 (47) | 6 (7) | 123 (43) | 11 (4) |
| Hyperglycemia | 85 (43) | 4 (2) | 27 (31) | 4 (5) | 112 (39) | 8 (3) |
| Elevated creatinine | 74 (37) | 2 (1) | 36 (41) | 0 | 110 (38) | 2 (1) |
| Elevated alkaline phosphatase | 69 (35) | 0 | 35 (40) | 0 | 104 (36) | 0 |
| Low bicarbonate | 58 (29) | 0 | 30 (34) | 1 (1) | 88 (31) | 1 (<1) |
| Elevated lipase | 52 (26) | 16 (8) | 31 (35) | 8 (9) | 83 (29) | 24 (8) |
| Hypermagnesemia | 50 (25) | 15 (8) | 27 (31) | 18 (21) | 77 (27) | 33 (12) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TEAE, treatment-emergent adverse event.
Toxicities were graded for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Includes TEAEs reported for ≥10% of patients.
Includes on-treatment laboratory abnormalities reported for ≥30% of patients (all grades) and grade 3/4 laboratory abnormalities reported for ≥5% of patients.
Although gastrointestinal AEs (diarrhea, nausea, vomiting) were common, they were typically of low severity, had an early onset (median [range] time to first event, 2.0 [1–594] days, 5.0 [1–878] days, and 8.0 [1–1,141] days, respectively), and were generally transient (median [range] duration, 1.0 [1–574] days, 2.0 [1–446] days, and 1.0 [1–165] days). Patients with diarrhea were primarily managed with loperamide and/or diphenoxylate/atropine (69%), and less frequently with temporary bosutinib dose interruptions (15%) and reductions (6%). Few (n = 6) patients discontinued bosutinib due to diarrhea. Antiemetics were used in 45% and 33% of patients with nausea and vomiting, respectively.
Cardiac TEAEs (i.e., cardiac disorders and electrocardiogram investigations) were reported in 39 (14%) patients, including 6% with a grade ≥3 cardiac event; few (n = 13 [5%]) had an event considered treatment related by the investigator. The most common cardiac events, irrespective of relationship, were atrial fibrillation and palpitations (n = 7 each). Two patients discontinued treatment due to a cardiac event, including grade 2 cardiac failure (considered drug related) and grade 2 coronary artery disease, and 1 additional patient died of unrelated cardiac failure 3 days after the patient's last bosutinib dose. During treatment, an increase from baseline in QTcF interval (i.e., corrected using Fridericia's formula) of more than 60 msec (grade 2 toxicity) was detected in 1 imatinib-resistant patient, although the patient's QTcF interval remained within the normal range. A QTcF interval exceeding 500 msec (grade 3 toxicity) was registered in a different imatinib-resistant patient on two separate occasions; the QTcF interval returned to normal without treatment modification.
Maximum grade 3/4 hematologic laboratory abnormalities were common among imatinib-resistant and imatinib-intolerant patients (Table III). The median (range) time to first myelosuppression laboratory value was 8 days (2–589 days) for anemia, 21 days (2–841 days) for thrombocytopenia, and 29 days (2–845 days) for neutropenia. Of note, although 70 (24%) patients experienced grade 3/4 on-treatment laboratory abnormalities of thrombocytopenia, only 3 imatinib-resistant patients experienced hemorrhagic AEs (grade 1 conjunctival hemorrhage lasting 8 days, grade 1 epistaxis lasting 1 day, and grade 3 subarachnoid hemorrhage lasting 16 days) in the context of grade 3/4 thrombocytopenia.
The most common nonhematologic laboratory abnormalities were ALT and aspartate aminotransferase (AST) elevations (Table III), with 82% and 91% of patients with events, respectively, experiencing a maximum toxicity grade of 1/2. The median (range) duration of ALT elevation from grade 3/4 to grade 0/1 was 36 days (11–596 days) for imatinib-resistant patients versus 19 days (15–170 days) for imatinib-intolerant patients; the duration from grade 2 to grade 0/1 was 29 days (3–288 days) versus 23.5 days (5–211 days), respectively. Median (range) duration of AST elevation from grade 3/4 to grade 0/1 was 22 days (5–52 days) for imatinib-resistant patients versus 15 days (7–170 days) for imatinib-intolerant patients; the duration from grade 2 to grade 0/1 was 15 days (7–169 days) versus 16 days (8–92 days).
Dose modifications due to TEAEs were common, with 65% of imatinib-resistant patients and 83% of imatinib-intolerant patients experiencing a temporary treatment interruption and 44% and 57%, respectively, receiving a dose reduction. Thrombocytopenia was the TEAE most frequently leading to treatment interruption (n = 66 [55% of patients with thrombocytopenia]) and dose reduction (n = 43 [36% of patients with thrombocytopenia]). The AEs most frequently leading to bosutinib discontinuation were thrombocytopenia (5%), diarrhea (2%), neutropenia (2%), and ALT elevation (2%; Supporting Information Table SII).
The majority of both older (aged ≥65 years) and younger (aged <65 years) patients experienced only maximum grade 1/2 events, although certain types of TEAEs were reported more frequently among older patients, particularly vomiting, constitutional symptoms, pleural effusions, and dyspnea (Table II). In contrast, aminotransferase elevations, influenza, and abdominal pain were more common among younger patients. Grade 3/4 hematologic laboratory abnormalities among older and younger patients were thrombocytopenia (20% and 25%, respectively), lymphopenia (20% and 13%), anemia (19% and 12%), and neutropenia (13% and 18%). Dose interruptions and reductions were observed among older (77% and 56%, respectively) and younger (68% and 45%) patients; 30% of older patients and 20% of younger patients discontinued bosutinib due to an AE (Table II). Few patients in either group died within 30 days of their last dose because of an AE (older, n = 1; younger, n = 2).
Long-term outcomes
Median PFS was not reached; the 2-year Kaplan–Meier estimate of PFS was 81% (Fig. 3A). Disease progression included transformation to AP/BP CML, which occurred in 11 patients during bosutinib treatment. Among imatinib-resistant patients, 4 patients transformed to AP with a time to transformation ranging from 415 to 630 days after bosutinib initiation and 6 patients transformed to BP with a time to transformation ranging from 42 to 476 days after bosutinib initiation. One imatinib-intolerant patient transformed to AP 246 days after bosutinib initiation; with continued bosutinib treatment, this patient returned to CP and regained a confirmed CHR.
Figure 3.
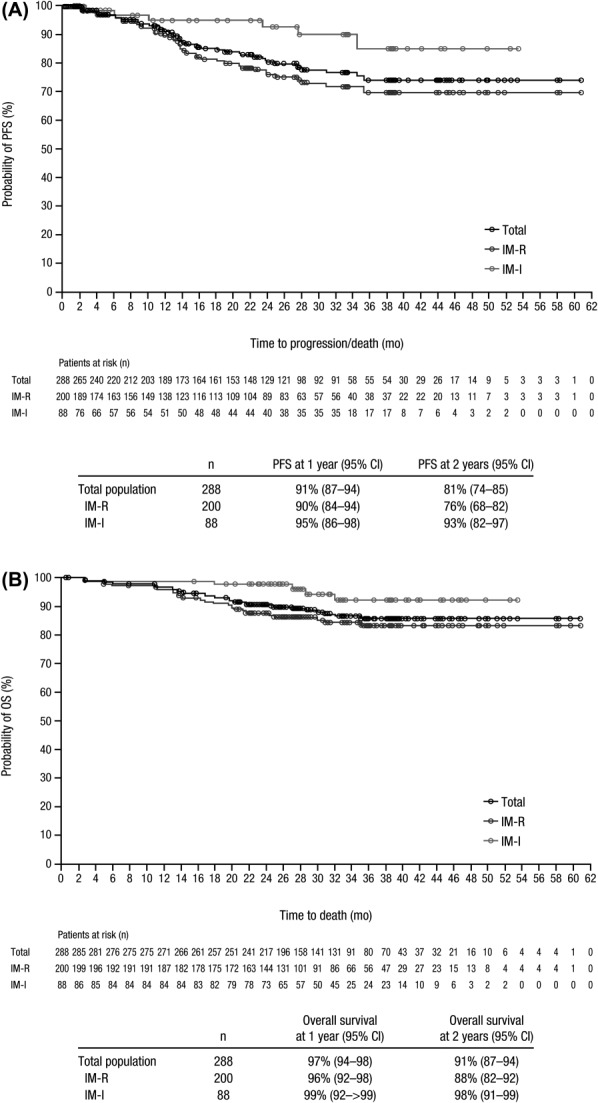
PFS (A) and OS (B). PFS was calculated for the all-treated population from the start date of therapy until treatment discontinuation due to disease progression (as assessed by the investigator; including transformation to AP or BP CML) or death, or death within 30 days of the last dose; patients without events were censored at their last assessment visit. OS was calculated for the all-treated population from the start date of therapy to the date of death due to any cause; patients without events were censored at the last contact (patients were followed up for 2 years after treatment discontinuation). PFS and OS at 1 and 2 years were based on Kaplan–Meier estimates. Abbreviations: AP, accelerated phase; BP, blast phase; CML, chronic myeloid leukemia; IM-I, imatinib intolerant; IM-R, imatinib resistant; OS, overall survival; PFS, progression-free survival.
Overall, 34 (12%) patients died during the study (ie, active treatment phase plus 2-year posttreatment follow-up period), including 29 (15%) imatinib-resistant patients and 5 (6%) imatinib-intolerant patients. Median OS was not reached; the 2-year Kaplan–Meier estimate for OS was 91% (Fig. 3B). Disease progression was the most common reason for death (n = 18 [6%]), followed by an AE (n = 10 [3%]); only one death was considered treatment-related (due to febrile neutropenia 78 days after the last bosutinib dose). Five (2%) patients (all imatinib-resistant) died within 30 days of their last bosutinib dose. Of these, three deaths were attributed to AEs unrelated to bosutinib (acute renal failure, pneumonia, cardiac failure) and two deaths were attributed to disease progression.
Transformations to AP/BP CML as well as the 2-year Kaplan–Meier estimates of PFS and OS were similar among older and younger patients (Table II).
Among patients with ≥1 baseline Bcr-Abl kinase domain mutation (n = 79) versus those without a baseline mutation (n = 133), the 2-year Kaplan–Meier estimates were generally lower for PFS (70% [95% CI, 57–80] vs 86% [95% CI, 77–91]) and OS (81% [95% CI, 70–88] vs 95% [95% CI, 89–97]).
Discussion
The current 2-year follow-up analysis of the phase 1/2 study of bosutinib in imatinib-resistant and imatinib-intolerant CP CML confirms the previously reported clinical activity and tolerability of bosutinib previously reported [22] and provides evaluation of longer-term endpoints.
Consistent with the initial report for this study cohort [22], bosutinib demonstrated high rates of cumulative MCyR in imatinib-resistant (58%; including a 46% CCyR rate) and imatinib-intolerant (61%; including a 54% CCyR rate) patients. Among patients without a CCyR at baseline, 57% of both imatinib-resistant and imatinib-intolerant patients achieved an MCyR. The rates of CHR (85%) and MMR (35%) were also high in this previously treated population. Notwithstanding differences in study design and patient populations, the response rates in the current study are similar to those observed in studies with other second-generation TKIs (dasatinib, nilotinib) after a minimum 2-year follow-up. In a phase 3 dose-optimization study, 63% of patients who had received dasatinib 100 mg/day after imatinib failure (n = 167) achieved/maintained an MCyR (including a 50% CCyR rate), and 92% of patients achieved/maintained a CHR [12]. In a phase 2 study of nilotinib 800 mg/day after imatinib failure (n = 321), MCyR was achieved by 59% of patients (including a 44% CCyR rate) [8]. Compared with the present study, responses to dasatinib and nilotinib were achieved more rapidly, with median times to MCyR <3 months [8,12]; however, this could be explained by the visit schedule, as CP CML patients in the current bosutinib study were not required to have their first cytogenetic assessment until month 3.
Responses to bosutinib were durable, with Kaplan–Meier estimates of 72% for retaining a CHR, 77% for retaining an MCyR, and 82% for retaining an MMR among all responders at 2 years; these rates were higher among imatinib-intolerant patients (82%, 88%, and 91%, respectively). The durability of response observed with bosutinib is comparable to that reported for dasatinib 100 mg/day (MCyR retained by 87%) [12] and nilotinib 800 mg/day (MCyR retained by 77%) [8] at 2 years in patients with CP CML following imatinib failure.
The results of the present study also confirm previous reports [22,23,26] indicating that bosutinib is associated with a manageable toxicity profile in patients with CP CML. The most common toxicities were transient, low-grade gastrointestinal AEs that arose early during treatment, liver function test abnormalities, and hematologic toxicity. The overall incidence of cardiac AEs considered related to bosutinib treatment was low (5%); this observation is consistent with data-reported treatment-related cardiac AEs in the phase 3 study of bosutinib (4%) versus imatinib (3%) in newly diagnosed patients with CP CML after ≥12 months follow-up [26]. The number of patients reporting a specific AE has increased only minimally from the prior report of this patient cohort [22], suggesting the toxicity profile is well-established and has not changed with this extended follow-up. Further, events were typically manageable with concomitant medication and/or bosutinib dose modification, were self-limited and reversible, and rarely resulted in treatment discontinuation. Of note, the safety profile of bosutinib remains somewhat distinct from that of imatinib, dasatinib, and nilotinib in patients with CP CML, although all TKIs are characterized by a frequent occurrence of manageable hematologic events as well as the common need for dose modification to help manage certain toxicities [7–10,12,26].
With bosutinib, 2-year PFS and OS estimates were 81% and 91%, respectively. Considering all the limitations of cross-trial comparisons, these estimates appear similar to the 2-year data for dasatinib 100 mg/day (PFS, 80%; OS, 91%) [12] and nilotinib 800 mg/day (PFS, 64%; OS, 87%) [8]. Of note, because 55% of patients in the current study had discontinued bosutinib as of the minimum 2-year follow-up, poststudy therapy may have influenced the OS estimates (evaluated on treatment and during the 2-year follow-up period); similar influences were also incorporated into the OS estimates for dasatinib (41% discontinued) [12] and nilotinib (61% discontinued) [8] as of the minimum 2-year follow-up. Longer follow-up would be required to further evaluate the effect of bosutinib on long-term survival.
A favorable benefit-to-risk profile was observed for bosutinib in older and younger patients, although specific outcomes were somewhat different between the age groups.
In summary, bosutinib demonstrated durable clinical activity and manageable toxicity as second-line therapy in patients with CP CML resistant or intolerant to imatinib, with results generally comparable to those reported for dasatinib and nilotinib as second-line therapy [8,12]. Bosutinib is also being evaluated in patients with CP CML following resistance or intolerance to imatinib plus dasatinib and/or nilotinib [23] and in patients with previously treated AP or BP CML [24].
Acknowledgments
The authors would like to thank all of the participating patients and their families as well as the global network of investigators, research nurses, study coordinators, and operations staff; a complete list of investigators who contributed to the analysis via enrolling and evaluating patients appears in the Supporting Information. This work was supported by Wyeth Research, which was acquired by Pfizer in October 2009. Data programming was provided by Gaurav Rathi of Pfizer. Medical writing support was provided by Kimberly Brooks, PhD, of SciFluent and was funded by Pfizer.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
References
- 1.Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341(3):164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 2.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™. Chronic Myelogenous Leukemia. Version 3.2013. Available at: http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf. Accessed December 5, 2013.
- 4.Diamond JM, Melo JV. Mechanisms of resistance to BCR-ABL kinase inhibitors. Leuk Lymphoma. 2011;52(Suppl. 1):12–22. doi: 10.3109/10428194.2010.546920. [DOI] [PubMed] [Google Scholar]
- 5.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22(6):1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, Pasquini R, Levy V, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R) Cancer. 2009;115(18):4136–4147. doi: 10.1002/cncr.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117(4):1141–1145. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9):841–851. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119(5):1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26(19):3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 12.Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95(2):232–240. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giles FJ, Abruzzese E, Rosti G, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia. 2010;24(7):1299–1301. doi: 10.1038/leu.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quintas-Cardama A, Kantarjian H, Jones D, et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood. 2007;109(2):497–499. doi: 10.1182/blood-2006-07-035493. [DOI] [PubMed] [Google Scholar]
- 15.Konig H, Holyoake TL, Bhatia R. Effective and selective inhibition of chronic myeloid leukemia primitive hematopoietic progenitors by the dual Src/Abl kinase inhibitor SKI-606. Blood. 2008;111(4):2329–2338. doi: 10.1182/blood-2007-05-092056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puttini M, Coluccia AM, Boschelli F, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66(23):11314–11322. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- 17.Remsing Rix LL, Rix U, Colinge J, et al. Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia. 2009;23(3):477–485. doi: 10.1038/leu.2008.334. [DOI] [PubMed] [Google Scholar]
- 18.Gambacorti-Passerini C, Tornaghi L, Cavagnini F, et al. Gynaecomastia in men with chronic myeloid leukaemia after imatinib. Lancet. 2003;361(9373):1954–1956. doi: 10.1016/S0140-6736(03)13554-4. [DOI] [PubMed] [Google Scholar]
- 19.Jabbour E, Cortes JE, Ghanem H, et al. Targeted therapy in chronic myeloid leukemia. Expert Rev Anticancer Ther. 2008;8(1):99–110. doi: 10.1586/14737140.8.1.99. [DOI] [PubMed] [Google Scholar]
- 20.Quintas-Cardama A, Kantarjian H, O'Brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25(25):3908–3914. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 21.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27(3):469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 22.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118(17):4567–4576. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119(15):3403–3412. doi: 10.1182/blood-2011-11-390120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gambacorti-Passerini C, Cortes JE, Khoury HJ, et al. Safety and efficacy of bosutinib in patients with AP and BP CML and ph+ ALL following resistance/intolerance to imatinib and other TKIs: Update from study SKI-200. J Clin Oncol. 2010;28(Suppl):Abstract 6509. [Google Scholar]
- 25.Jones D, Kamel-Reid S, Bahler D, et al. Laboratory practice guidelines for detecting and reporting BCR-ABL drug resistance mutations in chronic myelogenous leukemia and acute lymphoblastic leukemia: a report of the Association for Molecular Pathology. J Mol Diagn. 2009;11(1):4–11. doi: 10.2353/jmoldx.2009.080095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30(28):3486–3492. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


