Abstract
A 42-year-old man diagnosed with gastric non-Hodgkin's lymphoma 2 years earlier, for which he had undergone treatment, presented with expectorative cough, exertional shortness of breath and left-sided chest pain of 3 months duration. Respiratory system examination was suggestive of left-sided pneumonia with pleural effusion. Routine haemogram showed leukocytosis with high erythrocyte sedimentation rate. Chest radiograph showed blunting of left-sided cardiophrenic angle. Sputum culture grew Chryseobacterium indologenes. Diagnostic thoracocentesis was suggestive of lymphomatous metastasis. Pleural fluid culture was sterile. Contrast-enhanced CT (CECT) of the thorax showed left lower lobe consolidation with bilateral pleural effusion. The patient was treated with antibiotics, following which his cough improved and total leukocyte count normalised. Sputum culture repeated after the antibiotic course showed no growth of C. indologenes. However, the pleural effusion soon aggravated along with features suggestive of multiple metastasis. The patient finally succumbed to his underlying advanced malignancy.
Background
Gastric non-Hodgkin's lymphoma (NHL) is a rare subtype of stomach malignancy characterised by neoplastic transformation of cells that reside predominantly within the lymphoid tissues of the stomach.1 Chemotherapy administration and immunosuppressive treatment in patients with NHL make them more susceptible to infections. Moreover, complications that arise during the late stages necessitate the use of invasive procedures and indwelling catheters, which also add to the risk of infection. Chryseobacterium indologenes is a Gram-negative non-fermentative microbe found in the soil.2 It is an unusual human pathogen isolated from various sources in the hospital setting. Most infections are found in hospitalised patients with underlying immunosuppression and indwelling catheters.3 Although the microbe is not very virulent, it is, nevertheless, resistant to most antimicrobial agents.4 We present a case of C. indologenes pneumonia in a patient with gastric NHL. We aim to emphasise the possibility of infections due to rare pathogens in patients with underlying malignancy and the need to detect and to aggressively treat them early for achieving favourable outcomes.
Case presentation
A 42-year-old man presented with expectorative cough, exertional shortness of breath and left-sided, non-radiating, non-exertional, lateralised chest pain of 3 months duration. There was no history of fever or haemoptysis. The patient was diagnosed to have gastric NHL 2 years earlier, for which he had undergone total gastrectomy, following which he was on maintenance chemotherapy. He was a non-smoker and did not consume alcohol. General physical examination was unremarkable and vital signs were normal. Respiratory system examination showed a dull percussion note and reduced breath sounds with crepitations on auscultation over the left basal areas. Except for a surgical scar over the abdomen, examination of other systems did not reveal any major abnormality.
Investigations
A haemogram showed low haemoglobin level (10.3 g/dL) and leukocytosis with neutrophilia. Rest of the routine investigations were normal. Chest radiograph (posteroanterior view; figure 1) showed blunting of left-sided cardiophrenic angle, suggestive of left-sided pleural effusion. Sputum culture and identification performed using a Vitek 2 system showed growth of C. indologenes and a drug susceptibility testing showed sensitivity to amikacin, gentamicin, piperacillin, cefoperazone–sulbactam and piperacillin–tazobactam, and resistance to ceftazidime, quinolones, netilmicin, tobramycin, cefepime and imipenem. Sputum staining for fungus and Ziehl-Neelsen staining for mycobacteria were negative. Sputum culture for fungus and mycobacteria showed no growth. Pleural fluid analysis showed an exudative, lymphocyte-rich fluid with adenosine deaminase level of 17.5. Pleural fluid bacterial culture, Ziehl-Neelsen staining and fungal culture were normal. Fluid cytological analysis showed abnormal lymphocytes suggestive of malignant lymphomatous infiltration. Contrast-enhanced CT (CECT) of the thorax (figure 2) showed left lower lobe consolidation with bilateral pleural effusion. Sputum culture repeated after the antibiotic course showed no growth of C. indologenes. Abdominal sonography done later in the course of illness revealed abdominal lymphadenopathy with lymph nodal mass, suggestive of lymphomatous infiltration of the proximal ascending colon, gross ascites and lymphomatous infiltration of the right kidney with hydronephrosis.
Figure 1.
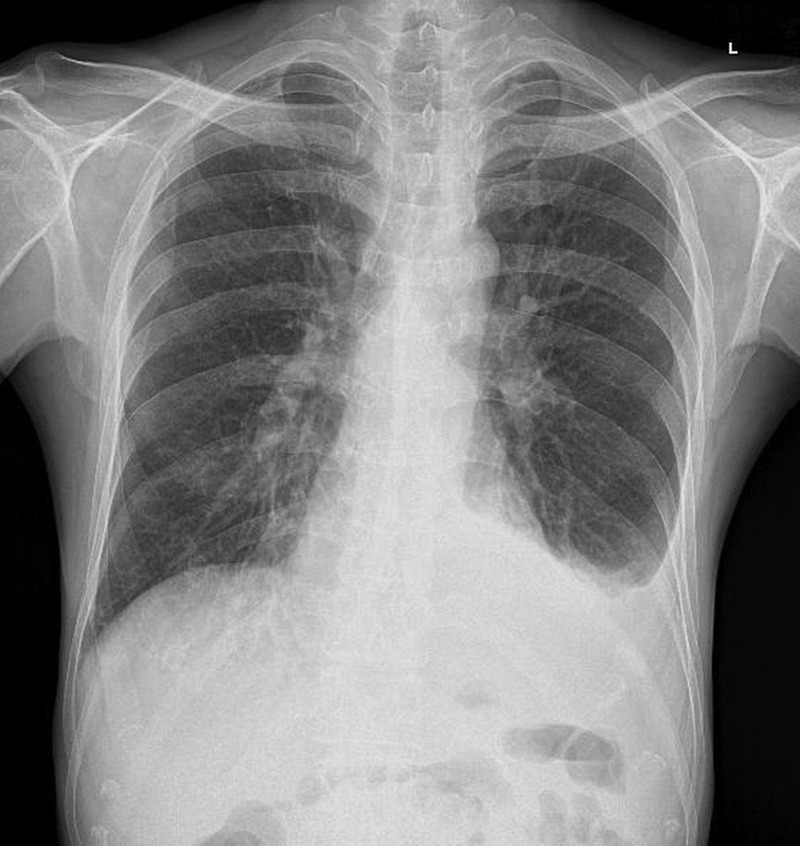
Chest roentgenogram showing blunting of left-sided costophrenic angle, suggestive of left-sided pleural effusion.
Figure 2.
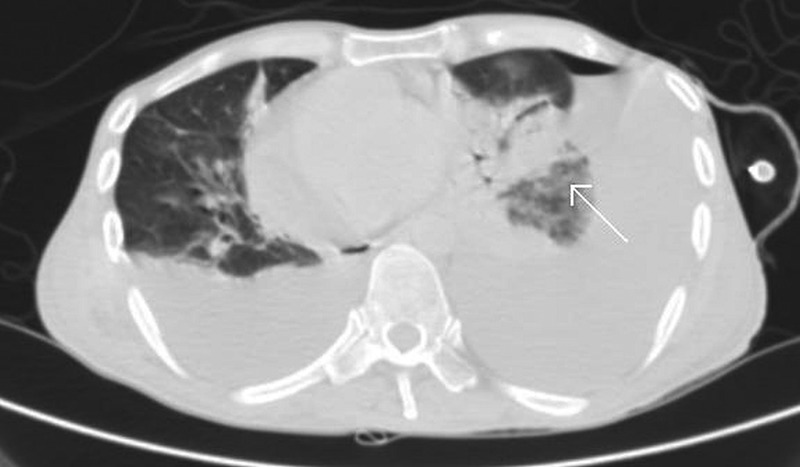
Contrast-enhanced CT (CECT) of the thorax showing left lower lobe consolidation (white arrow) with bilateral pleural effusion.
Treatment
The patient was initially treated with amikacin and ceftazidime. Ceftazidime was stopped after 4 days and cefoperazone–sulbactam was added, once the sputum culture and sensitivity reports were available, while amikacin was continued. Antibiotics were administered for a total of 14 days. Symptomatic treatment and supportive measures were given during hospital stay.
Outcome and follow-up
The patient responded slowly to antibiotics. His cough improved and leukocytosis normalised within 14 days of starting antibiotics. Follow-up sputum bacterial culture grew normal oropharyngeal flora. However, pleural effusion soon aggravated along with features of metastasis to the kidneys, ascending colon and peritoneum. The patient finally succumbed to his underlying advanced malignancy.
Discussion
Gastric NHL is a rare subtype of stomach malignancy characterised by neoplastic transformation of cells that reside predominantly within lymphoid tissues of the stomach.1 Administration of chemotherapy and immunosuppressive agents in patients with NHL make them excessively susceptible to various forms of infection. Moreover, complications that arise during the late stages of the disease necessitate the use of invasive procedures and indwelling catheters, which also add to the risk of infection. C. indologenes, formerly known as Flavobacterium indologenes, is a Gram-negative, lactose non-fermenting, oxidase-positive, rod-shaped bacillus with a distinct yellow to orange pigment, appearing in soil, plants and water sources despite chlorination, and is often recovered from wet surfaces and water sources in hospitals.2 It belongs to Centres for Disease Control group II b. It has been mostly reported to cause pneumonia or bacteraemia in immunosuppressed adults with various malignancies and severely sick hospitalised patients.3 Although not very virulent, it is, nevertheless, resistant to most antimicrobial agents.4
C. indologenes-associated infection was first reported in 1993 in a patient with ventilator-associated pneumonia.5 Since then, Chryseobacterium has been reported to cause primary bacteraemia, catheter-related bacteraemia, wound sepsis, cellulitis, pyelonephritis, peritonitis, biliary tract infection, urinary tract infection, pneumonia and keratitis of the eye. Most of these infections have been related to indwelling devices, especially intravascular catheters and mechanical ventilators. Overall, only a few cases of infection with C. indologenes have been reported. Christakis et al3 in 2005 reported a case of C. indologenes bacteraemia not related to an indwelling catheter in a patient with a solid tumour. Bayraktar et al6 in 2007 reported C. indologenes isolated from blood samples from a 5-month-old infant with septicaemia. Later on, in 2011, Calderon et al7 reported a case of ventilator-associated pneumonia caused by C. indologenes in a newborn baby boy with congenital heart disease. Chou et al8 subsequently reported 10 patients with C. indologenes bacteraemia. Also, Wang et al9 reported a case of C. indologenes peritonitis in a patient with malignant ascites. In 2013, Afshar et al10 reported a case of C. indologenes-associated peritonitis in a patient with end-stage renal disease on peritoneal dialysis. Most patients have been treated with and have responded well to piperacillin–tazobactam.
In spite of several extensive guidelines for treating pneumonia, there are currently no specific guidelines for treating C. indologenes and, hence, antibiotic selection for treatment of this rare infection remains challenging. Studies suggest that most isolates of C. indologenes have resistance to carbapenems, aminoglycosides, chloramphenicol, tetracyclines, macrolides, linezolid and vancomycin. According to the SENTRY Antimicrobial Surveillance Program, the most effective drugs against C. indologenes are the quinolones (levofloxacin, gatifloxacin, garenoxacin, around 95% susceptibility), trimethoprim–sulfamethoxazole (95% susceptibility) and piperacillin–tazobactam (90% susceptibility). Alternative effective agents are ciprofloxacin, piperacillin, cefepime, ceftazidime and rifampicin (85% susceptibility).4 Hence, clinicians should use antimicrobial susceptibility testing in order to ensure definitive and appropriate treatment of C. indologenes-associated infections.
Despite their rare prevalence, the incidence of C. indologenes-associated infections has been increasing around the world. Increased prevalence of healthcare-associated C. indologenes infections may be related to frequent exposure to broad-spectrum antibiotics, such as colistin and tigecycline.11 We report a case of C. indologenes pneumonia in a patient with gastric NHL. Our case demonstrates the importance of considering rare causative agents of pneumonia such as C. indologenes in immunosuppressed and seriously ill patients. C. indologenes may be resistant to commonly used broad-spectrum antibiotics and prompt antimicrobial susceptibility testing ensures successful treatment.
Learning points.
Patients who are immunosuppressed and severely sick hospitalised patients are prone to infections by rare organisms including Chryseobacterium indologenes.
It is mandatory for clinicians to use antimicrobial drug susceptibility testing in suspected cases and modify antibiotic therapy based on the results.
Infections due to C. indologenes are on the rise.
C. indologenes may be resistant to commonly used broad-spectrum antibiotics. Hence prompt identification and targeted therapy is essential.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Aisenberg AC Coherent view of non-Hodgkin's lymphoma. J Clin Oncol 1995;13:2656–75 [DOI] [PubMed] [Google Scholar]
- 2.Murray PR, Pfaller MA, Tenover FC, et al. Manual of clinical microbiology. 6th edn Washington, DC: ASM Press, 1995:528–30 [Google Scholar]
- 3.Christakis GB, Perlorentou SP, Chalkiopoulou I, et al. Chryseobacterium indologenes non-catheter-related bacteremia in a patient with a solid tumor. J Clin Microbiol 2005;43:2021–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirby JT, Sader HS, Walsh TR, et al. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp.: report from the SENTRY Antimicrobial Surveillance Program (1997–2001). J Clin Microbiol 2004;42:445–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonten MJ, Van Tiel FH, Van Der Geest S, et al. Topical antimicrobial prophylaxis of nosocomial pneumonia in mechanically ventilated patients: microbiological observations. J Infect 1993;21:137–9 [DOI] [PubMed] [Google Scholar]
- 6.Bayraktar MR, Aktas E, Ersay Y, et al. Postoperative Chryseobacterium indologenes bloodstream infection caused by contamination of distillate water. Infect Control Hosp Epidemiol 2007;28:368–9 [DOI] [PubMed] [Google Scholar]
- 7.Calderon G, Garcia E, Rojas P, et al. Chryseobacterium indologenes infection in a newborn: a case report. J Med Case Rep 2010;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou DW, Wu SL, Lee CT, et al. Clinical characteristics, antimicrobial susceptibilities, and outcomes of patients with Chryseobacterium indologenes bacteremia in an intensive care unit. Jpn J Infect Dis 2011;64:520–4 [PubMed] [Google Scholar]
- 9.Wang YC, Yeh KM, Chiu SK, et al. Chryseobacterium indologenes peritonitis in a patient with malignant ascites. Int Med Case Rep J 2011;4:13–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afshar M, Nobakht E, Lew SQ. Chryseobacterium indologenes peritonitis in peritoneal dialysis. BMJ Case Rep 2013;2013:pii: bcr2013009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsueh PR, Teng LJ, Yang PC, et al. Increasing incidence of nosocomial Chryseobacterium indologenes infections in Taiwan. Eur J Clin Microbiol Infect Dis 1997;16:568–74 [DOI] [PubMed] [Google Scholar]


