Abstract
Objective
Clinical decision support has the potential to improve prevention of venous thromboembolism (VTE). The purpose of this prospective study was to analyze the effect of electronic reminders on thromboprophylaxis rates in wards to which patients were admitted and transferred. The latter was of particular interest since patient handoffs are considered to be critical safety issues.
Methods
The trial involved two study periods in the six departments of a university hospital, three of which were randomly assigned to the intervention group displaying reminders during the second period. At 6 h after admission or transfer, the algorithm checked for prophylaxis orders within 0–30 h of the patient's arrival, increasing the specificity of the displayed reminders.
Results
The significant impact of the reminders could be seen by prophylaxis orders placed 6–24 h after admission (increasing from 8.6% (223/2579) to 12% (307/2555); p<0.0001) and transfer (increasing from 2.4% (39/1616) to 3.7% (63/1682); p=0.034). In admission wards, the rate of thromboprophylaxis increased from 62.4% to 67.7% (p<0.0001), and in transfer wards it increased from 80.2% to 84.3% (p=0.0022). Overall, the rate of prophylaxis significantly increased in the intervention group from 69.2% to 74.3% (p<0.0001). No significant changes were observed in the control group. Postponing prophylaxis checks to 6 h after admissions and transfers reduced the number of reminders by 62% and thereby minimized the risk of alert fatigue.
Conclusions
The reminders improved awareness of VTE prevention in both admission and transfer wards. This approach may contribute to better quality of care and safer patient handoffs.
Keywords: Decision Support Systems, Clinical, Patient Handoff, Patient Transfer, Reminder Systems, Venous Thromboembolism
Background
Appropriate use of prophylaxis to prevent venous thromboembolism (VTE) is an important strategy for improving safety among hospitalized patients.1 2 The American College of Chest Physicians has established evidence-based guidelines for the prevention of VTE, including recommendations for the use of both pharmacological and mechanical thromboprophylaxis.3 However, insufficient guideline adherence is a recognized problem impeding appropriate prophylaxis regimens, and large clinical studies have demonstrated that many patients at risk do not receive prophylaxis.4–6
Clinical decision support (CDS) systems have the potential to improve guideline adherence and increase the rate of prophylaxis against VTE.7–10 The effect of electronic VTE alerting concepts has been shown to be sustainable.11 12 Fiumara et al13 investigated serial three-screen alerts, which improved the use of prophylaxis. However, immediately consecutive alerts increase the risk of ‘alert fatigue’.14 Despite studies that showed that CDS algorithms increase the use of thromboprophylaxis, there is still potential for patients at risk for VTE.7 11 13 15 16
Since an anticipated order of VTE prophylaxis may be forgotten after a patient's transfer to another unit, the question arises whether CDS could further improve quality of care after such a ‘handoff’. Cohen et al17 define handoff as ‘the exchange between health professionals of information about a patient accompanying either a transfer of control over, or of responsibility for, the patient’. The procedure whereby a patient is transferred from one unit to another—and a different team of providers is thereby instructed to take over the care—is referred to as ‘patient handoff’.18 Disruptions in the continuity of care are considered to be critical safety issues because individuals of different teams may inadequately communicate with each other and important information may become lost.19 20
Some researchers have demonstrated improvements in patient handoff communication using software tools.21–23 However, these tools follow a comprehensive handoff procedure and demand additional user input either into the electronic health record (EHR) or directly into the respective tool. Consequently, the stored information may be erroneous or incomplete.
The clinical information system of the University Hospital Zurich provides automated VTE reminders, previously described elsewhere.8 12 This algorithm has been upgraded to generate reminders again after transfers of patients, which is a novel approach to supporting patient handoffs. Only if no VTE prophylaxis has been ordered within the first 6 h after admission or transfer does the VTE reminder show up, in order to minimize the risk of alert fatigue.14
The purpose of this hospital-wide trial was to determine the effect of electronic reminders on the rate of thromboprophylaxis after admissions and particularly after patient handoffs. To our knowledge, no studies have so far been published on the effect of electronic reminders after admissions and transfers.
Methods
Design and site
The study was designed as a prospective single-center clinical trial. The six departments of the hospital were randomly assigned either to the intervention or to the control group. The University Hospital Zurich provides approximately 850 inpatient beds and covers all specialties. The ethics committee approved the study and patient consent was waived.
Clinical information system
Since 2009, inpatient care has been comprehensively managed by the clinical information system (Kisim; Cistec AG, Zurich, Switzerland) including computerized physician order entry (CPOE) of all pharmacological therapies, other treatments and diagnostic procedures on all wards of the University Hospital Zurich except for intensive care units (ICUs). The system offers a number of CDS functions involving medication and laboratory data.24
Reminders
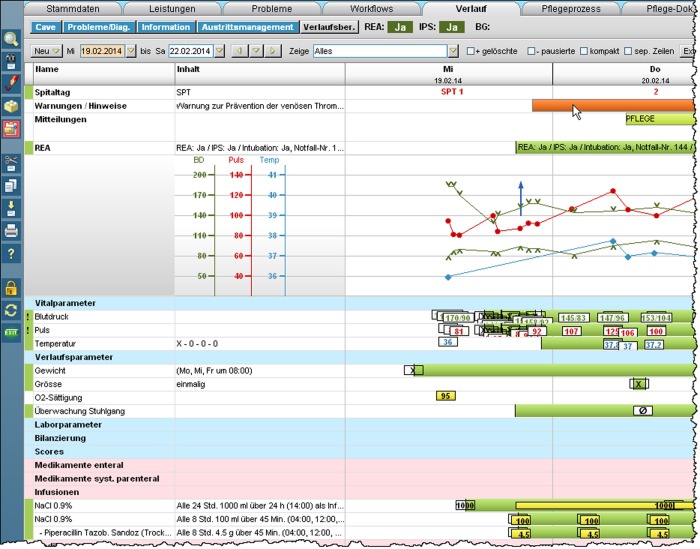
The VTE ‘reminder’25 is displayed on the graphical user interface as a non-interruptive red bar (figure 1) within the top section of the EHR.8 12 Its underlying algorithm has been upgraded to be triggered by both admissions and transfers to support patient handoffs.
Figure 1.
Synoptic view of the electronic health record (EHR). The mouse cursor displayed in the top right section points to the venous thromboembolism (VTE) reminder bar.
In the intervention group, a VTE reminder was automatically displayed in the EHR of each patient who did not receive a prophylaxis order within the first 6 h of admission or transfer. To be precise, after this 6 h delay, the algorithm checked for thromboprophylaxis orders that were active within the 0–30 h time frame after admission or transfer. Hence, orders placed before the patient's arrival or orders placed within the first 6 h—being active or becoming active during the prospective 24 h—suppressed the reminder. Further, the time frame from display of the reminder until 24 h after admission or transfer was considered to reflect the immediate effect of the intervention.
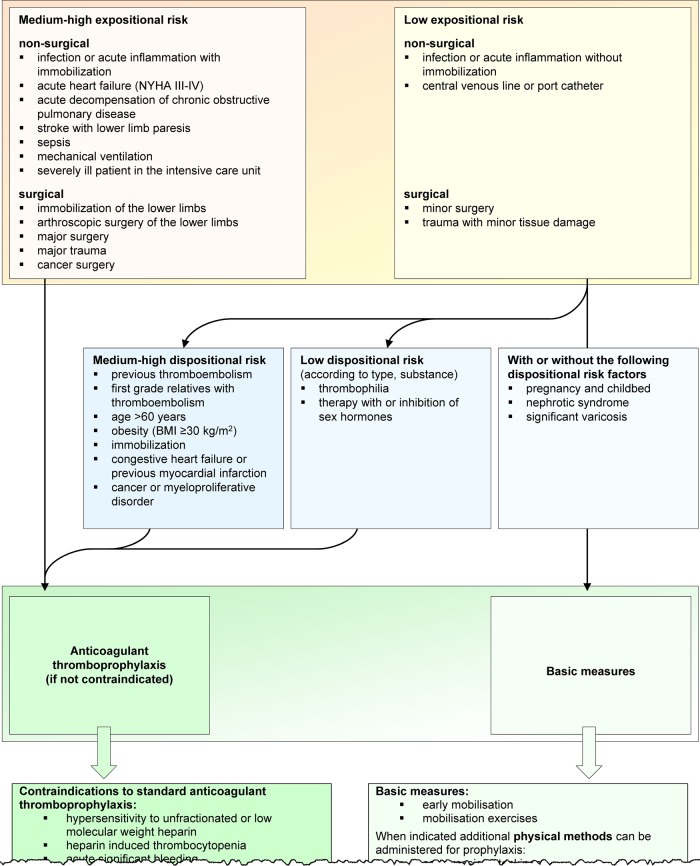
Reminders were triggered only once during the uninterrupted stay of a patient on a ward in order to minimize the number of notifications. Clicking on the reminder bar caused a flow sheet to pop up outlining the guidelines for assessing a patient's VTE risk, followed by evidence-based recommendations for appropriate prophylaxis (figure 2). This flow sheet also allowed re-evaluation of the risk of VTE after transfer. The reminder could be stopped by clicking on the ‘notification acknowledged’ button. Finally, each unacknowledged reminder was automatically stopped after 10 days.
Figure 2.
Pop-up window showing guidelines for assessing a patient's venous thromboembolism (VTE) risk.26 27
The patients were blinded, since they had no access to their EHR. Health professionals on wards assigned to the control group did not see any reminders. However, all professionals were informed about the study at the beginning, independent of their study group. Professionals working on wards assigned to the intervention group could see reminders within the EHRs.
Definitions
The ‘admission ward’ is defined as the ward to which a patient is admitted, either directly or via the emergency unit. However, the emergency unit is not considered an admission ward, since most inpatients are transferred within a few hours.
The ‘transfer ward’ is defined as the ward to which a patient is transferred, either from the admission ward or a preceding transfer ward. To be precise, neither a patient's change of room nor a health professional’s shift change is considered to be a transfer or a patient handoff.
‘Stay’ is defined as the continuation of an inpatient on the same ward, from admission or transfer until transfer or discharge. One hospitalization includes one or more stays. Only stays with durations of at least 24 h were considered. Stays overlapping the study periods and stays in ICUs were excluded.
The ‘rate of prophylaxis’ is defined as the percentage of stays that included at least one treatment with pharmacological or mechanical VTE prophylaxis compared with the total number.
‘Adequacy’ is defined as the number of stays with correctly ordered or withheld prophylaxes according to evidence-based guidelines divided by the total number of stays.
Study periods
After the ‘baseline period’ (2 June 2011 to 31 August 2011; 13 weeks), the VTE reminders were activated in the intervention group for the following 13 weeks (‘reminder period’ until 30 November 2011).
Clinical outcome
ICD-10 diagnosis codes (International Classification of Diseases, WHO, Geneva, Switzerland) were analyzed to determine differences in the frequencies of bleeding due to anticoagulants, other bleeding events, heparin-induced thrombocytopenia and VTE events (table 1). All cases were included in this analysis except for hospitalizations overlapping the two study periods and where patients switched study group (158 excluded patients; 1%). The EHRs had been reviewed in order to eliminate diagnoses prevalent at admission.
Table 1.
ICD-10 diagnosis codes used to determine differences in the frequencies of bleeding due to anticoagulants, other bleeding events, heparin-induced thrombocytopenia and VTE events (adapted from Huo et al28 and Casez et al29 and updated)
| Event | ICD-10 diagnosis codes |
|---|---|
| Bleeding due to anticoagulants | D68.3* |
| Other bleeding events | D69.9*, H11.3*, H31.3*, H35.6*, H43.1*, H45.0*, I60.*, I61.*, I62.*, K22.8*, K62.5*, K66.1*, K92.0*, K92.1*, K92.2*, M25.0*, R04.*, R23.3*, R31, R58 |
| Heparin-induced thrombocytopenia | D69.53 |
| VTE | I26.*, I80.1*, I80.2*, I80.3*, I80.8*, I80.9*, I82.1*, I82.2*, I82.3*, I82.8*, I82.9*, O22.3*, O87.1*, O88.2* |
The * character is used as a wildcard that matches zero or more numeric digits.
ICD, International Classification of Diseases; VTE, venous thromboembolism.
Quality assessment
Adequacy was assessed in computer-generated random samples of 40 inpatients determined for each week during the reminder period (resulting in a total of 520 patients). Each sample consisted of 20 patients from the intervention group (10 receiving a VTE prophylaxis, 10 without prophylaxis) and 20 patients from the control group (10 receiving a VTE prophylaxis, 10 without prophylaxis).
Two angiologists adjudicated the adequacy of the decisions to order or withhold VTE prophylaxis in each of these hospitalizations. The same patients were contacted by phone 3 months after discharge and interviewed using a questionnaire to determine whether a new VTE was diagnosed. 30
Assignment of the departments to the study groups
The hospital's departments are organizational structures that were defined by the management in order to simplify the handling of jointly used resources in terms of both infrastructure and professional staff. All divisions hosting and caring for inpatients are allocated to the six considered departments.
The decision to randomize departments instead of individual patients, teams, or divisions was made to minimize potential contamination due to physician rotations across intervention and control groups. Randomization included ranking and pairing of the departments: first, the two departments with the highest prophylaxis rate were randomized, second, the two intermediate departments, and third, the two departments with the lowest prophylaxis rate.
The resulting intervention group consisted of the following three interdisciplinary departments:
‘traumatology/reconstructive surgery/dermatology/rheumatology’ (highest prophylaxis rate)
‘internal medicine/oncology/radiation oncology/hematology/infectious diseases’ (intermediate prophylaxis rate)
‘cardiac surgery/vascular surgery/thoracic surgery/angiology/cardiology/pulmonology’ (lowest prophylaxis rate).
The control group consisted of the following three interdisciplinary departments:
‘endocrinology/diabetology/gastroenterology/nephrology/urology/abdominal surgery’ (highest prophylaxis rate)
‘ophthalmology/psychiatry/neurology/neuroradiology/otolaryngology/oral and maxillofacial surgery/neurosurgery’ (intermediate prophylaxis rate)
‘gynecology/obstetrics/neonatology’ (lowest prophylaxis rate).
Statistical analysis
Levels of p≤0.05 were considered significant. Fisher's exact tests were used for 2×2 contingency tables. Comparisons of continuous variables were performed using the Wilcoxon rank-sum test.
The primary end point was the change in the rate of prophylaxis. Proportions of orders placed 6–24 h after admissions and transfers represented the immediate effect of the reminders.
Duration of the study periods was defined to obtain sample sizes above the minimum, assuming an increase in the prophylaxis rate from 70% to 75%, a significance level of 0.01, and a power of 90%.
Calculations were performed using the software R, V.2.15.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 15 736 patients were included. These patients stayed 11 770 times in admission wards and 7780 times in transfer wards. Stays of 106 patients (0.7%) transferred from the intervention group to the control group and vice versa were included in the analyses.
In the intervention group and control group, the mean duration of hospitalization was 8.4 and 6.4 days, the mean age was 59 and 43 years, and the percentage of female patients was 40.2% and 59.7%, respectively (each p<0.0001). None of these demographics differed significantly in the intervention group between the baseline and reminder period (each p>0.16). In the control group, the duration of hospitalization (p=0.54) and the age of the patients (p=0.67) did not differ significantly between the periods, but the proportion of male and female patients did (61.1% female during the baseline period; 58.4% female during the reminder period; p=0.0083).
The prophylaxis rate increased significantly in the intervention group by 5.1% from 69.2% to 74.3% (p<0.0001). In the control group, the change in the prophylaxis rate from 68.1% to 69.7% was not significant (p=0.070).
Stays in admission wards
The prophylaxis rate increased in the admission wards of the intervention group by 5.3% (p<0.0001; table 2). No significant change was observed in the control group (p=0.21). The proportions of prophylaxes ordered within the 6–24 h time frame after admission increased significantly in the intervention group, reflecting the immediate effect of the VTE reminders (p<0.0001). No significant change in these proportions was observed in the control group (p=0.25).
Table 2.
Prophylaxis rates in the two study groups in the admission wards according to the timing of order entry
| Intervention group | p Value | Control group | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline period | Reminder period | Baseline period | Reminder period | |||||||
| Number of stays | Per cent | Number of stays | Per cent | Number of stays | Per cent | Number of stays | Per cent | |||
| Stays with prophylaxis orders placed before admission or in the time frame 0–6 h | 1248 | 48.4 | 1296 | 50.7 | 0.099 | 1623 | 51.1 | 1766 | 51 | 0.92 |
| Stays with prophylaxis orders placed in the time frame 6–24 h after admission | 223 | 8.6 | 307 | 12 | <0.0001 | 242 | 7.6 | 291 | 8.4 | 0.25 |
| Stays with prophylaxis orders placed >24 h | 138 | 5.4 | 127 | 5 | 0.57 | 114 | 3.6 | 153 | 4.4 | 0.091 |
| Stays without prophylaxis orders | 970 | 37.6 | 825 | 32.3 | <0.0001 | 1195 | 37.6 | 1252 | 36.2 | 0.21 |
| Total number of stays | 2579 | 100 | 2555 | 100 | 3174 | 100 | 3462 | 100 | ||
Compared with a hypothetical algorithm that would alert immediately at the time of admission (without considering orders placed for the period 0–30 h), the 6 h postponed prophylaxis check reduced the number of displayed reminders by 51% in the intervention group.
Stays in transfer wards
This analysis included 6352 patients with 7780 stays. In the intervention group, the prophylaxis rate increased significantly by 4.1% (p=0.0022; table 3), whereas no significant change was observed in the control group (p=0.17). The proportions of prophylaxes ordered within the 6–24 h time frame after transfer increased significantly in the intervention group (p=0.034) and not in the control group (p=0.25).
Table 3.
Prophylaxis rates in the two study groups in the transfer wards according to the timing of order entry
| Intervention group | p Value | Control group | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline period | Reminder period | Baseline period | Reminder period | |||||||
| Number of stays | Per cent | Number of stays | Per cent | Number of stays | Per cent | Number of stays | Per cent | |||
| Stays with prophylaxis orders placed before transfer or in the time frame 0–6 h | 1214 | 75.1 | 1311 | 77.9 | 0.058 | 1512 | 70.6 | 1666 | 71.2 | 0.69 |
| Stays with prophylaxis orders placed in the time frame 6–24 h after transfer | 39 | 2.4 | 63 | 3.7 | 0.034 | 77 | 3.6 | 100 | 4.3 | 0.25 |
| Stays with prophylaxis orders placed >24 h | 43 | 2.7 | 44 | 2.6 | 1 | 54 | 2.5 | 71 | 3 | 0.31 |
| Stays without prophylaxis orders | 320 | 19.8 | 264 | 15.7 | 0.0022 | 498 | 23.3 | 504 | 21.5 | 0.17 |
| Total number of stays | 1616 | 100 | 1682 | 100 | 2141 | 100 | 2341 | 100 | ||
Compared with a hypothetical algorithm that would alert immediately at the time of a patient's transfer (without considering orders placed for the period 0–30 h), the 6 h postponed prophylaxis check reduced the number of displayed reminders by 78% in the intervention group.
Clinical outcome
On analyzing the frequencies of the ICD-10 diagnosis codes, no significant change was observed for the incidence of bleeding due to anticoagulants, other bleeding events, heparin-induced thrombocytopenia and VTE events (table 4).
Table 4.
Number of patients with bleeding due to anticoagulants, other bleeding events, heparin-induced thrombocytopenia or VTE events
| Intervention group | p Value | Control group | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline period | Reminder period | Baseline period | Reminder period | |||||||
| Number of patients | Per cent | Number of patients | Per cent | Number of patients | Per cent | Number of patients | Per cent | |||
| Bleeding due to anticoagulants | 6 | 0.18 | 4 | 0.12 | 0.54 | 1 | 0.02 | 1 | 0.02 | 1 |
| Other bleeding event | 29 | 0.87 | 31 | 0.93 | 0.89 | 11 | 0.26 | 14 | 0.3 | 0.84 |
| Heparin-induced thrombocytopenia | 1 | 0.03 | 3 | 0.09 | 0.62 | 0 | 0 | 0 | 0 | 1 |
| VTE event | 19 | 0.57 | 13 | 0.39 | 0.29 | 12 | 0.28 | 10 | 0.21 | 0.53 |
| Total number of patients | 3321 | 100 | 3332 | 100 | 4256 | 100 | 4669 | 100 | ||
Multiple ICD-10 diagnosis codes per patient and category are counted as one—for example, two different VTE codes in one patient are considered as one patient with VTE.
ICD, International Classification of Diseases; VTE, venous thromboembolism.
Quality assessment
Regarding the adequacy of thromboprophylaxis, no significant differences were observed in a sample of 520 patients analyzed in detail (table 5). In the intervention group, 88.1% of prophylaxis regimens were adequate, and in the control group 89.2% of the prophylaxis regimens were adequate (p=0.78).
Table 5.
Assessment of adequacy of the prophylaxis regimens
| Prophylaxis | p Value | ||||
|---|---|---|---|---|---|
| Ordered | Per cent | Withheld | Per cent | ||
| Intervention group | |||||
| Decision | |||||
| Adequate | 111 | 85.4 | 118 | 90.8 | 0.25 |
| Inadequate | 19 | 14.6 | 12 | 9.2 | |
| Total | 130 | 100 | 130 | 100 | |
| Control group | |||||
| Decision | |||||
| Adequate | 108 | 83.1 | 124 | 95.4 | 0.0021 |
| Inadequate | 22 | 16.9 | 6 | 4.6 | |
| Total | 130 | 100 | 130 | 100 | |
Of these 520 patients, 453 were contacted by phone 3 months after discharge (lost to follow-up: intervention 45, control 22). No difference in the frequency of post-discharge VTE was observed between the intervention group (three VTE events) and the control group (three VTE events).
Discussion
We implemented an algorithm displaying non-interruptive reminders on thromboprophylaxis. Only if no VTE prophylaxis had been ordered within the first 6 h after admission or transfer including patient handoff was a reminder displayed in the EHR. The VTE reminders had a significant effect on the prophylaxis rate in the admission wards and transfer wards of the intervention group. The immediate effect of the reminders was demonstrated by increased numbers of VTE prophylaxes ordered 6–24 h after admissions and even after transfers. None of these end points were significantly affected in the control group where the notifications were suppressed. To our knowledge, this study shows for the first time the significant effect of VTE prophylaxis reminders after both admissions and patient transfers.
The aim of the reminders was to increase awareness of VTE prevention and to foster guideline adherence. When the user clicks on the reminder bar, a pop-up window displays evidence-based prophylaxis guidelines. Notifications featuring improved acceptance are characterized by a high quality of knowledge and presentation of detailed advice in a user-friendly manner.31
It was the purpose of the study to document the improvement in the process of VTE prevention. Yet, the observed trend towards better clinical outcome in the intervention group did not reach significance (from 0.57% to 0.39%; p=0.29). To show a statistically significant reduction in VTE events due to the reminders, a much larger sample size would be required. A power calculation using data from table 4 (a significance level of 0.01, two-sided, with a power of 90%) results in more than 2×43 000 patients required to be enrolled in the intervention group. However, the clinical and economic benefit of improved adherence to evidence-based guidelines has been recognized,15 32 and the effect of computer-based decision support on reduction of symptomatic and asymptomatic deep-vein thrombosis was shown in a landmark publication.7
Increasing the thromboprophylaxis rate by electronic reminders might induce overuse of prophylaxis. However, the assessment of the clinical outcome in the intervention group did not show a trend toward increased bleeding events due to anticoagulants (from 0.18% to 0.12%), and the percentage of inadequately ordered prophylaxes was not high in the intervention group compared with the control group (14.6% vs 16.9%). However, the 9.2% inadequately withheld prophylaxes despite the display of reminders in the intervention group's sample indicate that there is still room for improvement. A further increase in the adequacy of the prophylaxis regimens might be achieved by a specialist service reviewing EHRs with unacknowledged VTE reminders.
The higher prophylaxis rate observed in the transfer wards compared with the admission wards is probably a result of both the carry over of prophylaxis orders from preceding wards and the more complex illness of transferred patients. The latter is supported by two findings: (1) the patients included in the transfer-ward analysis were hospitalized for on average 11.2 days, whereas those included in the admission wards were hospitalized for on average 6.2 days, whether or not they were transferred later (p<0.0001); (2) the patients analyzed in the transfer wards were transferred from or to an ICU in 12.3% of cases during their hospitalization, whereas the patients analyzed in the admission wards were transferred to an ICU in only 6.6% of cases during their hospitalization (p<0.0001).
Reminders were displayed after a delay of 6 h, thereby allowing physicians to order VTE prophylaxes proactively. After this 6 h delay, the algorithm checked for thromboprophylaxis orders that were active within the 0–30 h time frame after admission or transfer. Hence, orders being active or becoming active during the subsequent 24 h suppressed the reminder. Reminders were triggered only once during the uninterrupted stay of a patient on a ward in order to minimize the number of notifications. These features helped to improve the specificity: compared with a hypothetical algorithm immediately alerting at each admission and transfer of a patient, the 6 h postponed prophylaxis check reduced the number of reminder bars in the admission and transfer wards by 51% and 78%, respectively. This corresponds to an average reduction of 62%, minimizing the risk of alert fatigue.14
A patient's need for prophylaxis varies during hospitalization, therefore VTE risk should be re-evaluated after transfers. This is of particular interest, since, in this study, stays in transfer wards represented 40% of the total number of stays. Triggering a single reminder after both admission and patient handoff may be a compromise between excessive alerting and maximum impact. This approach could help to improve the transfer of important information through the change of care team in patient handoffs.18–20
The algorithm triggering the reminder does not identify high-risk patients based on VTE risk score calculation, since the identification of individual risk factors by computers may be unreliable, particularly if important information is lacking or not interpretable. An algorithm should pre-empt neither the decision to order prophylaxis nor the risk assessment by the responsible physician,33 since both underuse and overuse of thromboprophylaxis are known problems.34 35 However, the described pop-up window showing evidence-based prophylaxis recommendations offers user-friendly guidance (figure 2).
On the one hand, learning effects might have contributed to the increased proportions of prophylaxes ordered up until 6 h after the patient’s arrival, particularly in the admission wards of the intervention group (table 2). On the other hand, the decision to order thromboprophylaxis may directly be influenced by the algorithm as soon as the reminder bar is displayed. Thus, without considering prophylaxis orders placed before the appearance of the reminders, three categories of order entry timing could be distinguished: (1) patients receiving their first order for VTE prophylaxis immediately after the notification —that is, within the 6–24 h time frame; (2) patients receiving their first order >24 h after (until transfer or discharge); (3) patients receiving no prophylaxis at all. Regarding only these ‘reminder-influenced categories’ in the intervention group, the proportion of orders placed within the 6–24 h time frame increased by 7.6% ((307/1259)−(223/1331)) and 7.3% ((63/371)−(39/402)) in the admission and transfer wards, respectively.
Some limitations are noteworthy in interpreting this study. On the one hand, the randomization of the hospital's departments has the advantage of minimized staff exchange across the study groups allowing more precise measurement of the effect of the reminders in the admission and transfer wards. On the other hand, this approach limits conclusions drawn with respect to prophylaxis regimens and clinical outcomes, since different specialties with different views, knowledge, and experience in VTE are assembled within the departments. We considered this issue by comparing not only the intervention group versus the control group during the intervention period but also the changes within each study group before and after the implementation of the reminders. The slight increase in the prophylaxis rate in the analysis of the control group might indicate that limited contamination occurred between the study groups —for example, due to carry-over effects of patients from the intervention group transferred to wards in the control group or due to physician rotations across the hospital departments. If a Hawthorne effect had influenced the health professionals, its contribution would have been minimal, since no significant change was observed in the control group.36
Numbers of patients with specific diagnoses according to ICD-10 codes need to be carefully interpreted because most codes are generated by medical coding staff after discharge of the patients. It is noteworthy with regard to the reviews of the EHRs that only 39% of the patients with ICD-10 codes related to VTE had hospital-acquired VTE (hospital-acquired conditions: see table 4).
The in-depth assessment of adequacy of the prophylaxis regimens revealing no significant differences between the study groups was based on a limited sample of 520 patients. Three months after discharge, 453 of these patients were available for the follow-up interviews (87%). This sample might not be large enough to detect minor—but potentially relevant—differences in the adequacy of prophylaxis regimens or the incidence of events after discharge.
In conclusion, the electronic reminders improved awareness of VTE prevention in both admission and transfer wards. This approach may contribute to better quality of care and safer patient handoffs.
Acknowledgments
We thank Gabriela Gitzelmann (University Hospital Zurich) for her relentless efforts in calling discharged patients, and we also thank Peter Amberg (Cistec AG, Zurich) for the programming of the upgraded algorithm.
Footnotes
Contributors: PEB designed and performed the research, analyzed and interpreted data, and wrote the manuscript. EE performed research, analyzed data, and reviewed the manuscript. AS and JDS performed research, and reviewed the manuscript. BA-V and JB designed the research, analyzed and interpreted data, and reviewed the manuscript. All authors approved the final submitted version of the manuscript.
Competing interests: None.
Ethics approval: Kantonale Ethikkommission Zurich.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001(43):i–x, pp 1–668 [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn SL. Prophylaxis of venous thromboembolism in the US: improving hospital performance. J Thromb Haemost 2009;7:1437–45 [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Akl EA, Crowther M, et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):7S–47S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008;371:387–94 [DOI] [PubMed] [Google Scholar]
- 5.Goldhaber SZ, Tapson VF. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol 2004;93:259–62 [DOI] [PubMed] [Google Scholar]
- 6.Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007;132:936–45 [DOI] [PubMed] [Google Scholar]
- 7.Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005;352:969–77 [DOI] [PubMed] [Google Scholar]
- 8.Kucher N, Puck M, Blaser J, et al. Physician compliance with advanced electronic alerts for preventing venous thromboembolism among hospitalized medical patients. J Thromb Haemost 2009;7:1291–6 [DOI] [PubMed] [Google Scholar]
- 9.Dexter PR, Perkins S, Overhage JM, et al. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med 2001;345:965–70 [DOI] [PubMed] [Google Scholar]
- 10.Durieux P, Nizard R, Ravaud P, et al. A clinical decision support system for prevention of venous thromboembolism: effect on physician behavior. JAMA 2000;283:2816–21 [DOI] [PubMed] [Google Scholar]
- 11.Lecumberri R, Marques M, Diaz-Navarlaz MT, et al. Maintained effectiveness of an electronic alert system to prevent venous thromboembolism among hospitalized patients. Thromb Haemost 2008;100:699–704 [DOI] [PubMed] [Google Scholar]
- 12.Beeler PE, Kucher N, Blaser J. Sustained impact of electronic alerts on rate of prophylaxis against venous thromboembolism. Thromb Haemost 2011;106:734–8 [DOI] [PubMed] [Google Scholar]
- 13.Fiumara K, Piovella C, Hurwitz S, et al. Multi-screen electronic alerts to augment venous thromboembolism prophylaxis. Thromb Haemostasis 2010;103:312–17 [DOI] [PubMed] [Google Scholar]
- 14.van der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006;13:138–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecumberri R, Panizo E, Gomez-Guiu A, et al. Economic impact of an electronic alert system to prevent venous thromboembolism in hospitalised patients. J Thromb Haemost 2011;9:1108–15 [DOI] [PubMed] [Google Scholar]
- 16.Piazza G, Rosenbaum EJ, Pendergast W, et al. Physician alerts to prevent symptomatic venous thromboembolism in hospitalized patients. Circulation 2009;119:2196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MD, Hilligoss B, Kajdacsy-Balla Amaral AC. A handoff is not a telegram: an understanding of the patient is co-constructed. Crit Care 2012;16:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham JC, Aswani MS, Rosen M, et al. Reducing medical errors and adverse events. Annu Rev Med 2012;63:447–63 [DOI] [PubMed] [Google Scholar]
- 19.Cook RI, Render M, Woods DD. Gaps in the continuity of care and progress on patient safety. BMJ 2000;320:791–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg CC, Regenbogen SE, Studdert DM, et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg 2007;204:533–40 [DOI] [PubMed] [Google Scholar]
- 21.Petersen LA, Orav EJ, Teich JM, et al. Using a computerized sign-out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv 1998;24:77–87 [DOI] [PubMed] [Google Scholar]
- 22.Van Eaton EG, Horvath KD, Lober WB, et al. A randomized, controlled trial evaluating the impact of a computerized rounding and sign-out system on continuity of care and resident work hours. J Am Coll Surg 2005;200:538–45 [DOI] [PubMed] [Google Scholar]
- 23.Anderson J, Shroff D, Curtis A, et al. The Veterans Affairs shift change physician-to-physician handoff project. Jt Comm J Qual Patient Saf 2010;36:62–71 [DOI] [PubMed] [Google Scholar]
- 24.Beeler PE, Eschmann E, Rosen C, et al. Use of an on-demand drug-drug interaction checker by prescribers and consultants: a retrospective analysis in a Swiss Teaching Hospital. Drug Saf 2013;36:427–34 [DOI] [PubMed] [Google Scholar]
- 25.Randolph AG, Haynes RB, Wyatt JC, et al. Users’ guides to the medical literature: XVIII. How to use an article evaluating the clinical impact of a computer-based clinical decision support system. JAMA 1999;282:67–74 [DOI] [PubMed] [Google Scholar]
- 26.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(6 Suppl):381S–453S [DOI] [PubMed] [Google Scholar]
- 27.AWMF. S3-Leitlinie Prophylaxe der venösen Thromboembolie, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, version 18 March 2009 with addendum 8 May 2010. 2009, 2010. http://www.awmf-leitlinien.de
- 28.Huo MH, Spencer DL, Borah BJ, et al. Post-discharge venous thromboembolism and bleeding in a large cohort of patients undergoing total hip or total knee arthroplasty. J Clin Outcomes Manage 2012;19:355–63 [Google Scholar]
- 29.Casez P, Labarere J, Sevestre MA, et al. ICD-10 hospital discharge diagnosis codes were sensitive for identifying pulmonary embolism but not deep vein thrombosis. J Clin Epidemiol 2010;63:790–7 [DOI] [PubMed] [Google Scholar]
- 30.Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res 1996;5:539–54 [DOI] [PubMed] [Google Scholar]
- 31.Seidling HM, Phansalkar S, Seger DL, et al. Factors influencing alert acceptance: a novel approach for predicting the success of clinical decision support. J Am Med Inform Assoc 2011;18:479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amin AN, Lin J, Johnson BH, et al. Clinical and economic outcomes with appropriate or partial prophylaxis. Thromb Res 2010;125:513–17 [DOI] [PubMed] [Google Scholar]
- 33.Marco P, Lopez-Abadia E, Lucas J. More on thromboprophylaxis: electronic alerts in hospitalized patients at risk of venous thromboembolism. Thromb Haemost 2008;100:525–6 [PubMed] [Google Scholar]
- 34.Chopard P, Dorffler-Melly J, Hess U, et al. Venous thromboembolism prophylaxis in acutely ill medical patients: definite need for improvement. J Intern Med 2005;257:352–7 [DOI] [PubMed] [Google Scholar]
- 35.Kakkar AK, Mueller I, Bassand JP, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE 2013;8:e63479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarney R, Warner J, Iliffe S, et al. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]