Abstract
Burkholderia pseudomallei is a common cause of serious, difficult to treat infections in South-East Asia and Northern Australia, but is a rare imported pathogen in the USA and Europe. We report a case of a patient with a mycotic aneurysm caused by B. pseudomallei in a previously healthy returning traveller. The patient presented with 4 weeks of abdominal pain and intermittent fever after a brief vacation in Thailand. The aneurysm was excised and replaced by an autologous deep vein graft, and the patient was treated for 6 months with antibiotics adjusted according to postoperative renal impairment. Twenty-four months after surgery the patient is well and without relapse.
Background
Melioidosis is endemic in Southeast Asia and Northern Australia and is caused by Burkholderia pseudomallei, a potential bioterror agent, which is a motile, aerobic, non-spore forming Gram-negative bacillus. B. pseudomallei is an environmental saprophyte found mainly in wet soils and stagnant water and is usually acquired by inoculation through minor abrasions of the skin or by inhalation, especially in the wet season. Melioidosis is often characterised by pneumonia or multiple abscesses, but it can also present as genitourinary infections, cutaneous ulcers, septic arthritis and osteomyelitis, and parotid abscesses are characteristic in Asian children.1–3 However, mycotic aneurysm is a very rare presentation of melioidosis.4
We report a case of a previously healthy returning traveller with a mycotic aortic aneurysm caused by B. pseudomallei after a short-term visit to Thailand.
Case presentation
A 63-year-old man was admitted to the department of abdominal surgery with 4 weeks of intermittent fever, weight loss and increasing abdominal pain. He was a previously healthy non-smoking Caucasian with no family history of cardiovascular disease. He used no medication and worked as a frame worker. Six weeks prior to admission he had returned from 2 weeks of vacation in Krabi in Southern Thailand. While travelling he got an abrasion on his knee and he was subsequently exposed to stagnant water on a hiking trip.
Examination at admission showed that the lower abdomen was mildly tender at percussion, but without guarding or any palpable pulsatile enlargements. Furthermore, the patient was afebrile with a blood pressure of 142/81 mm Hg and a pulse rate of 66 bpm. Blood samples showed a C reactive protein level of 44 mg/L, haemoglobin level 8 mmol/L, white cell count (WCC) 7.4×109/L and normal creatinine level 85 µmol/L. The lipid profile included a low-density lipoprotein cholesterol level of 1.9 mmol/L, triglyceride level 2.3 mmol/L and high-density lipoprotein cholesterol level 1.1 mmol/L. A CT of the abdomen revealed a sacculate mycotic aortic aneurysm extending from the left renal artery to the aortic bifurcation with a diameter of 20×16 mm and inflammation of the peri-aortic tissue. Blood cultures were negative (one set obtained before antibiotic treatment and two sets after) and treatment with intravenous cefuroxime and ciprofloxacin was initiated, and he was transferred to the department of vascular surgery. On day 3, the antibiotic treatment was empirically changed to intravenous ceftriaxone and a 2-week course of antibiotic treatment was planned before vascular surgery.
However, due to increasing abdominal pain and an increasing diameter of the aneurysm (to a maximum of 35 mm) the patient was transferred to a national referral centre for vascular surgery on day 10 and underwent surgery the following day. During the procedure deep veins were harvested from each groin stretching from the femoral profunda vein juncture to the distal femur. The veins were prepared fashioning a ‘pantaloon anastomosis’ (figure 1). The aorta was clamped above the left renal artery and cold Ringer's lactate was infused using a 13 Fr perfusion catheter (figure 2), the aneurysm was excised and the autologous venous graft inserted end to end to the aorta (figure 3). A small partial mural thrombosis and significant inflammation in the peri-aortic tissue were found, but no pus and no other focus of infection could be identified. Tissue specimens from the excised aneurysm were cultured and analysed with broad-range gene amplification (16S rRNA), sequenced and blasted against the NCBI nucleotide database (http://blast.ncbi.nlm.nih.gov/). Both specimens were culture and gene amplification positive for B. pseudomallei. The strain was sensitive to ceftazidime, doxycycline, imipenem and trimethoprim-sulfamethoxazole (TMP-SMX), but resistant to amoxicillin/clavulanate. On day 18 the antibiotic regimen was changed to intravenous ceftazidime 2 g four time a day according to the bacterial aetiology and the antibiogram. A positron emission tomography on day 22 showed no additional or undrained focus of infection and an echocardiography revealed no vegetations or other signs of endocarditis.
Figure 1.
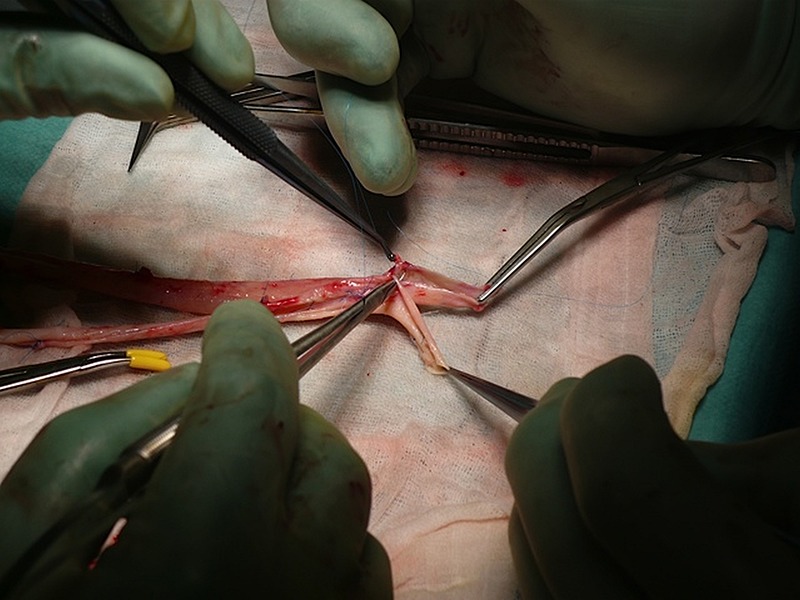
The deep veins were sewn together in a ‘pantaloon’ configuration followed by an anastomosis to the aorta.
Figure 2.
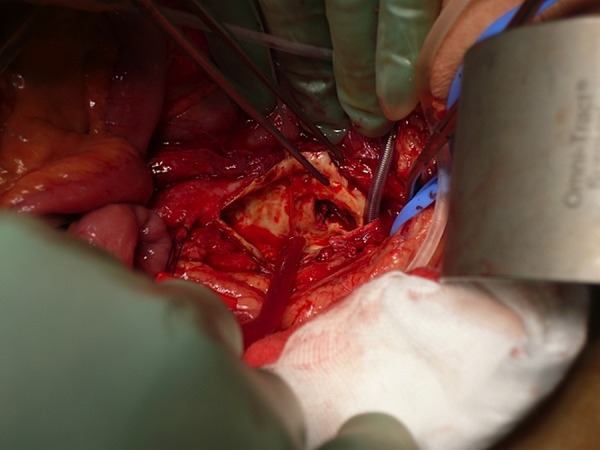
The mycotic aneurysm was resected.
Figure 3.
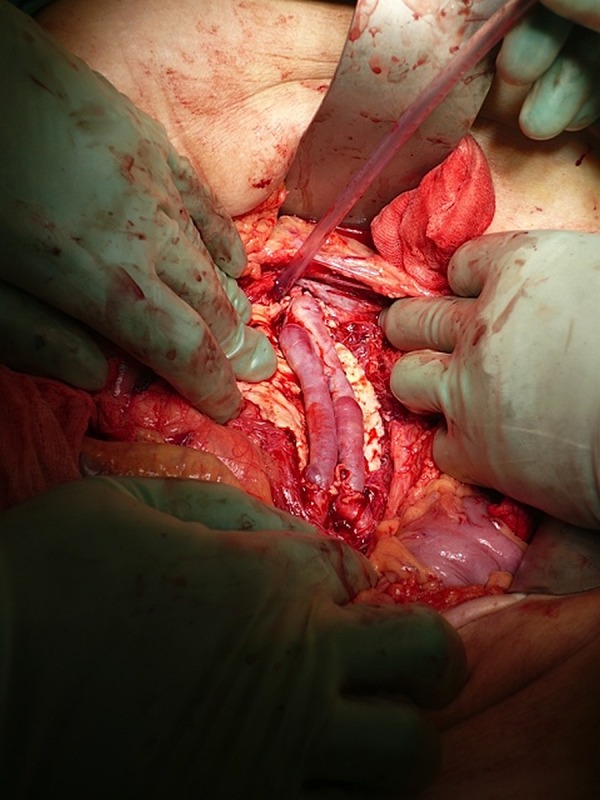
The aorta was replaced with a ‘pantaloon graft’ as a vascular conduit. The anastomoses were made proximally to the aorta below the renal arteries and distally to the bifurcation.
The patient developed postoperative renal impairment with a creatinine level of 183 µmol/L and an estimated glomerular filtration rate (eGFR) of 42 mL/min, and accordingly the dose of ceftazidime was reduced to 2 g three times a day on day 34. After 1 month of treatment with intravenous ceftazidime, 5 months of maintenance therapy (adjusted for eGFR and a body weight >60 kg) with oral TMP/SMX 240/1200 mg twice daily and doxycycline 100 mg twice daily was planned.1 5 Seven days after initiation of the maintenance therapy the patient developed a skin rash and further renal impairment (creatinine 247 µmol/L), which progressed despite reduction of the dose of TMP/SMX (160/800 mg twice daily). The urine analysis was without proteinuria and the urine microscopy revealed no sediment. The kidney function improved when TMP-SMX was stopped, and following a few days of monotherapy with doxycycline 100 mg twice daily the patient had a peripheral intravenous central catheter (PICC-line) inserted and was continued on at-home intravenous ceftazidime 1 g twice daily throughout the maintenance period for 5 months.
Outcome and follow-up
The patient has recovered well with a stable eGFR of 40–45 mL/min and without relapse 24 months after surgery.
Discussion
Captain Alfred Whitmore first described melioidosis in Rangoon, Burma in 1911 and it has since been recognised as an important cause of infection and community-acquired bacteraemia in Southeast Asia and Northern Australia with incidence rates of up to 50/100 000 person-years. Melioidosis is particularly common in Northeast Thailand and ranks as the third most common cause of death from infectious diseases, only exceeded by HIV and tuberculosis.1 Despite increasing reports of melioidosis worldwide, the distribution and frequency of melioidosis is probably still greatly underestimated, especially in resource poor settings.2 6 7 Moreover, B. pseudomallei has a potential latency of infection and may present itself years after exposure.1
Melioidosis primarily affects indigenous people in regular contact with soil and stagnant water, and most of the patients have an underlying risk factor. In a 20-year prospective study of 540 cases of melioidosis in Northern Australia 69% of patients were male, the median age was 49 years, and 80% had risk factors including diabetes mellitus (39%), alcohol abuse (39%), chronic lung disease (26%), chronic renal disease (12%), rheumatic heart disease and/or congestive heart failure (7%), malignancies (6%) and immunosuppression (6%). In that study, 55% of the patients were bacteraemic, but mycotic aneurysm was only found in two of the cases.3
B. pseudomallei is intrinsically resistant to many antibiotics including penicillin, first-generation and second-generation cephalosporins and aminoglycosides. Quinolones and tigecycline have only weak activity against the bacterium. However, it is susceptible to third-generation cephalosporins, chloramphenicol, imipenem, meropenem, tetracycline, TMP-SMX and, often, amoxicillin/clavulunate. B. pseudomallei is difficult to eradicate and the response to treatment is often very slow with persistent fever for weeks despite adequate high-dose parenteral antibiotic. Likewise, the risks of relapse (5–20%) and death (16–44%) are high and the median time to relapse was 8 months in one study.1 3
Some uncertainty still remains regarding the choice and duration of antibiotic treatment of melioidosis.1 2 8–11 The currently recommended antibiotic regimen for mycotic aneurysm in melioidosis consists of 4–6 weeks of high-dose intravenous ceftazidime or imipenem/meropenem followed by oral maintenance therapy usually with TMP-SMX as a backbone for 3–6 months.1 12 With regard to surgery, replacement with deep vein as a vascular conduit, also known as neoaortoiliac system (NAIS) bypass, has proven to be robust and, importantly, is the only procedure definitively eliminating the focus of infection.13 In a study from Northeast Thailand, the main complications of mycotic aneurysm in melioidosis were postoperative wound infections (29%), postoperative sepsis (18%), renal impairment (18%) and vascular graft infection or leakage (18%). Even with a combination of surgery and adequate antibiotic therapy the rates of postoperative complications were significantly higher in mycotic aneurysms caused by B. pseudomallei than in those caused by other bacterial pathogens (65% vs 24%, p=0.01). However, case fatalities were similar (24% vs 18%, p=0.71).8 Another smaller case series of mycotic aneurysm by B. pseudomallei from Singapore revealed a relapse rate of 33% and case fatality of 17%.4
To the best of our knowledge, only very few reports exist of mycotic aneurysms by B. pseudomallei in returning travellers without any established risk factors,14 15 but mycotic aneurysms have been described in travellers with risk factors16 and in endemic areas such as Australia,12 Southeast Asia4 8 17 and Brazil.7
The patient in our case was noteworthy in several ways. He was without risk factors for melioidosis, he had an unusual focus of infection and had not visited the highly endemic Northeastern part of Thailand. Moreover, he was afebrile with a normal WCC at admission, which is rare in mycotic aneurysms, and blood cultures obtained at admission before antibiotic therapy were negative, as seen in 50–75% of cases.18 19 In terms of treatment, empirical antibiotic therapy with cefuroxime and ciprofloxacin was changed to ceftriaxone to ensure antimicrobial coverage of Enterobacteriaceae including ciprofloxacin resistant Salmonella spp. as well as for Staphylococcus and Streptococcus spp.18 According to the results of tissue cultures, antibiogram and postoperative renal impairment the antibiotic regimen was altered to dose-adjusted ceftazidime for 4 weeks. Unfortunately, the patient developed increasing renal impairment after initiation of maintenance therapy with TMP-SMX in spite of further reduced dosages of TMP-SMX. Doxycycline monotherapy for the maintenance phase was not considered an option, because it has been associated with more relapses in melioidosis (20% vs 5%) than a standard four-drug regimen (chloramphenicol, doxycycline and TMP-SMX).4 20 Furthermore, this particular strain of B. pseudomallei was resistant to amoxicillin/clavulanate. Therefore, we chose to continue treatment with ceftazidime and showed that at-home intravenous treatment was successful and feasible in a resourceful Western setting.
In conclusion, we report a case of mycotic aneurysm caused by B. pseudomallei in a returning traveller with no established risk factors of melioidosis, who was successfully treated with NAIS bypass and 6 months of intensive antibiotic treatment. The possibility of melioidosis should be considered in the febrile traveller returning from an endemic area, especially if the patient is immunosuppressed or has other melioidosis risk factors.
Learning points.
Melioidosis should be included as a differential diagnosis in travellers returning from endemic countries, even in previously healthy returning travellers.
Mycotic aneurysm by Burkholderia pseudomallei requires surgery and long-term antibiotic treatment.
At-home treatment with intravenous antibiotics for the maintenance phase is feasible in a high-resource country.
Footnotes
Acknowledgements: The authors thank Professor Bart Currie, Northern Territory Clinical School and Infectious Diseases Department, Royal Darwin Hospital, for advice in treating the patient.
Contributors: JB wrote the first draft of the manuscript. SV performed the surgery, KF cultured the blood and tissue samples, and UH treated the patient at both admission and during out-patient follow-up. All authors participated in writing and proof reading of subsequent drafts and analyses of other articles related to the subject (melioidosis) until the final version was complete. All authors agreed to submit the manuscript in its current form.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med 2012;367:1035–44 [DOI] [PubMed] [Google Scholar]
- 2.White NJ. Melioidosis. Lancet 2003;361:1715–22 [DOI] [PubMed] [Google Scholar]
- 3.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 2010;4:e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Low JGH, Quek AML, Sin YK, et al. Mycotic aneurysm due to Burkholderia pseudomallei infection: case reports and literature review. Clin Infect Dis 2005;40:193–8 [DOI] [PubMed] [Google Scholar]
- 5.Grayson ML, ed. Kucers’ the use of antibiotics: a clinical review of antibacterial, antifungal, antiparasitic and antiviral drugs. 6th Edition. London (UK): Hodder Arnold; 2010
- 6.Currie BJ, Dance DAB, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg 2008;102:S1–4 [DOI] [PubMed] [Google Scholar]
- 7.Sidrim JJC, Rocha MFG, Bandeira TJPG, et al. Mycotic aneurysm caused by Burkholderia pseudomallei: report of a Brazilian strain genetically related to Thai strains. Clin Microbiol Infect 2010;17:719–21 [DOI] [PubMed] [Google Scholar]
- 8.Anunnatsiri S, Chetchotisakd P, Kularbkaew C. Mycotic aneurysm in Northeast Thailand: the importance of Burkholderia pseudomalleias a causative pathogen. Clin Infect Dis 2008;47:1436–9 [DOI] [PubMed] [Google Scholar]
- 9.Elliott JH, Currie BJ. Diagnosis and treatment of mycotic aneurysm due to Burkholderia pseudomallei. Clin Infect Dis 2005;41:572–3 [DOI] [PubMed] [Google Scholar]
- 10.Chierakul W, Anunnatsiri S, Short JM, et al. Two randomized controlled trials of ceftazidime alone versus ceftazidime in combination with trimethoprim- sulfamethoxazole for the treatment of severe melioidosis. Clin Infect Dis 2005;41:1105–13 [DOI] [PubMed] [Google Scholar]
- 11.Chierakul W, Anunnatsiri S, Chaowagul W, et al. Addition of trimethoprim-sulfamethoxazole to ceftazidime during parenteral treatment of melioidosis is not associated with a long-term outcome benefit. Clin Infect Dis 2007;45:521–3 [DOI] [PubMed] [Google Scholar]
- 12.Elliott JH, Carson P, Currie BJ. Burkholderia pseudomallei mycotic aneurysm. Intern Med J 2003;33:323–4 [DOI] [PubMed] [Google Scholar]
- 13.Ali AT, Modrall JG, Hocking J, et al. Long-term results of the treatment of aortic graft infection by in situ replacement with femoral popliteal vein grafts. J Vasc Surg 2009;50:30–9 [DOI] [PubMed] [Google Scholar]
- 14.Amezyane T, Lecoules S, Algayres J-P. Mycotic iliac aneurysm associated with Burkholderia pseudomallei. Int J Infect Dis 2010;14:e381–2 [DOI] [PubMed] [Google Scholar]
- 15.Steinmetz I, Stosiek P, Hergenröther D, et al. Melioidosis causing a mycotic aneurysm. Lancet 1996;347:1564–5 [DOI] [PubMed] [Google Scholar]
- 16.Tan Boun K, Biron F, Chidiac C, et al. Imported melioidosis in France revealed by a cracking abdominal mycotic aortic aneurysm in a 61-year-old man. BMJ Case Rep 2012;2012:pii: bcr2012006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanyaowalak W, Sunthornyothin S, Luengtaviboon K, et al. Mycotic aneurysm caused by Burkholderia pseudomallei with negative blood cultures. Scand J Infect Dis 2004;36:68–70 [DOI] [PubMed] [Google Scholar]
- 18.Fisk M, Peck LF, Miyagi K, et al. Mycotic aneurysms: a case report, clinical review and novel imaging strategy. QJM 2012;105:181–8 [DOI] [PubMed] [Google Scholar]
- 19.Jaffer U, Gibbs R. Mycotic thoracoabdominal aneurysms. Ann Cardiothorac Surg 2012;1:417–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaowagul W, Simpson AJ, Suputtamongkol Y, et al. A comparison of chloramphenicol, trimethoprim-sulfamethoxazole, and doxycycline with doxycycline alone as maintenance therapy for melioidosis. Clin Infect Dis 1999;29: 375–80 [DOI] [PubMed] [Google Scholar]


