Abstract
Background:
Although fibrin sealants (FSs) and fibrin glues (FGs) are predominantly utilized to strengthen repairs of cerebrospinal fluid (CSF) fistulas (deliberate/traumatic) during spinal surgery, they are also increasingly utilized to achieve hemostasis. Here, we investigated whether adding Tisseel (Baxter International Inc., Westlake Village, CA, USA), utilized to address increased bleeding during multilevel lumbar laminectomies with non-instrumented fusions, would reduce or equalize the time to drain removal and length of stay (LOS) without contributing to infections or prolonging time to fusion.
Methods:
Prospectively, 39 patients underwent multilevel laminectomies and 1-2 level non-instrumented (in situ) fusions to address stenosis/olisthesis; 22 who demonstrated increased intraoperative bleeding received Tisseel, while 17 without such bleeding did not.
Results:
The 22 receiving versus 17 not receiving Tisseel, with similar clinical parameters, underwent comparable average multilevel laminectomies (4.36 and 4.25) and 1-2 level fusions (1.4 vs. 1.29 levels). As anticipated, for those receiving Tisseel, the average intraoperative estimated blood loss (EBL), total postoperative blood loss, and total perioperative transfusion requirements [red blood cells (RBC), fresh frozen plasma (FFP), platelets] were higher. However, Tisseel had the added benefit of equalizing the time to postoperative drain removal [e.g. 3.41 days (with) vs. 3.38 days (without)] and LOS [e.g. 5.86 days (with) vs. 5.82 days (without)] without increasing the infection rates (e.g. one superficial infection per group) or average times to fusion (e.g. 5.9 vs. 5.5 months).
Conclusions:
Adding Tisseel for increased bleeding during multilevel laminectomies/in situ fusions contributed to hemostasis by equalizing the average times to drain removal/LOS compared to patients without increased bleeding and not requiring Tisseel.
Keywords: CSF fistulas: Epidural steroid injections, Fibrin sealant: Hemostasis, Lumbar laminectomy, Non-instrumented fusions, Punctate CSF Fistulas, Tisseel
INTRODUCTION
Although fibrin sealants (FSs) and fibrin glues (FGs) are predominantly utilized to strengthen repairs of cerebrospinal fluid (CSF) fistulas (e.g. deliberate/traumatic) occurring during spinal surgery, they are also increasingly applied to achieve hemostasis. Here, one FS, Tisseel (Baxter International Inc., Westlake Village, CA, USA), was utilized to control increased intraoperative bleeding encountered during 22 multilevel lumbar laminectomies with non-instrumented fusions. Results were compared with those of 17 other patients undergoing comparable procedures in the same time period, who did not demonstrate increased bleeding and, therefore, did not receive/require Tisseel. We specifically asked whether Tisseel indirectly contributed to hemostasis as measured by reducing or equalizing the time to postoperative drain removal and length of stay (LOS). Additional questions raised included whether Tisseel increased the infection rate and/or time to/or rate of fusion.
MATERIALS AND METHODS
Prospectively, 39 patients with multilevel spinal stenosis [e.g. ossification of the yellow ligament (OYL), disc disease, synovial cysts, and instability (e.g. grade I-II spondylolisthesis, spondylolisthesis/spondylolysis, or unilateral extended or full facetectomy for far lateral disc herniations) underwent multilevel laminectomies with 1-2 level non-instrumented (in situ) postero-lateral fusions utilizing lamina autograft and NanOss Bioactive (Pioneer Surgical, Marquette, MI, USA) [Tables 1 and 2].
Table 1.
Clinical data for patients undergoing multilevel laminectomies with non-instrumented fusions with or without Tisseel
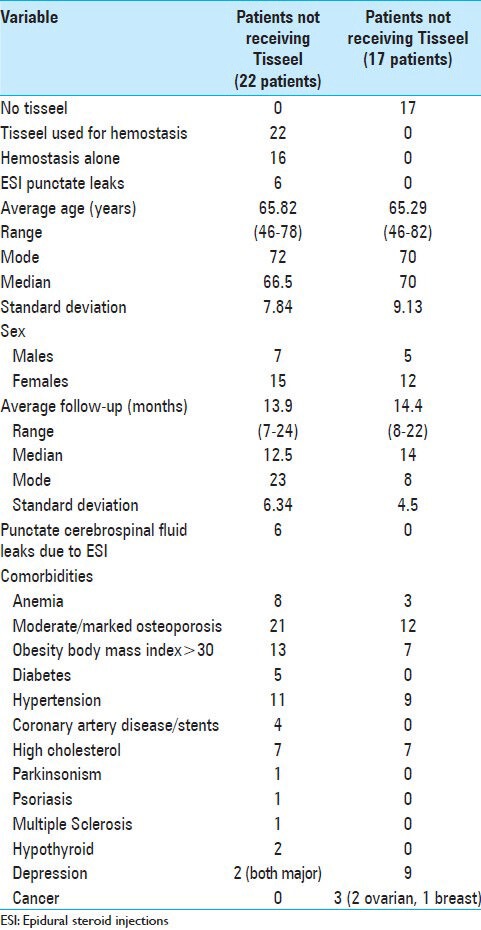
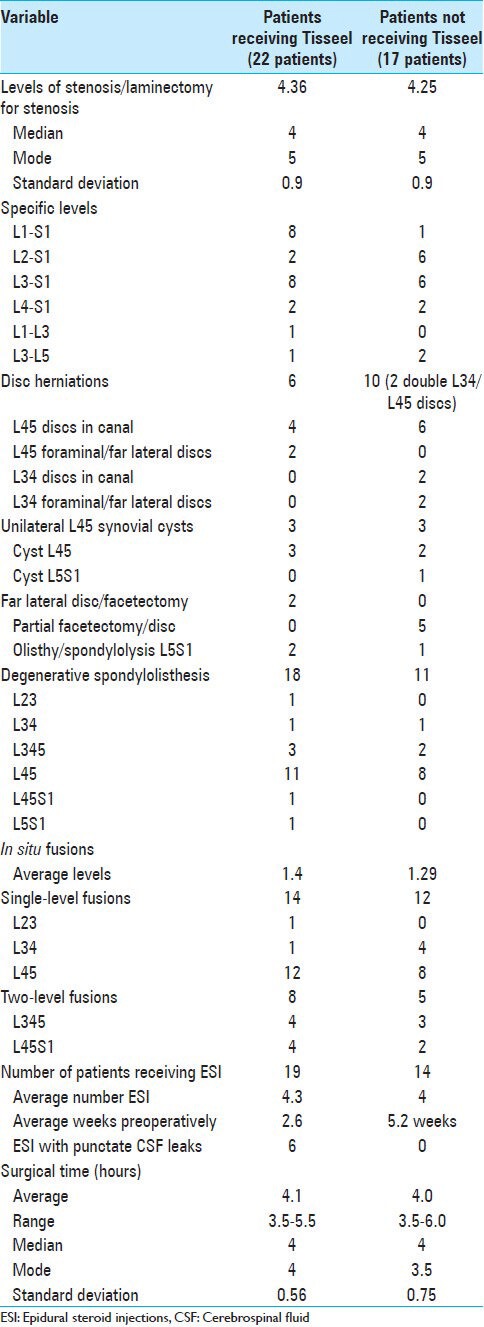
Table 2.
Surgical data for patients undergoing multilevel laminectomies with non-instrumented fusions with or without Tisseel
Preoperatively, patients stopped anti-platelet aggregants and anticoagulants
Patients were asked to stop baby Aspirin 81 mg at least 2–3 weeks prior to surgery, along with other medications/foods/vitamin and herbal supplements that are known anti-platelet aggregants or anticoagulants. Preoperatively, patients had to have normal prothrombin time (PT), partial thromboplastin time (PTT), International Normalized Ratio (INR), and platelet aggregation screening studies.
Twenty-two patients with increased intraoperative bleeding received Tisseel. These patients averaged 65.8 years of age, and were followed over an average of 13.9 months (range 7-24 months) [Table 1]. Important medical comorbidities included: moderate/marked osteoporosis (21 patients), obesity (13 patients), hypertension (11 patients), anemia (8 patients), and cardiovascular disease (4 patients). Tisseel was applied in these 22 patients who exhibited increased intraoperative bleeding during multilevel laminectomies (average 4.36 levels) and 1-2 level non-instrumented fusions (average 1.4 levels) [Tables 1 and 2]. Lumbar pathology included: OYL (22 patients), disc disease (6 patients), and synovial cysts (3 patients) [Table 2]. Spinal instability was attributed to spondylolisthesis (18 patients), spondylolisthesis/spondylolysis (2 patients), and unilateral full facetectomy for far lateral discs (2 patients). Surgery required an average of 4.1 h [Table 2].
6 Patients exhibited puncate CSF fistulas attributed to prior epidural steroid injections
A subset of 6 patients also exhibited punctate CSF fistulas attributed to prior epidural steroid injections (ESI); 19 had undergone an average of 4.3 ESI, the last having been administered an average of 2.6 weeks prior to surgery [Tables 1 and 2]. All punctate CSF fistulas were identified in the midline, and were clearly the size of the Tuohy needle, tip; all were successfully treated with direct GORE-TEX suture repair (e.g. 7-0 GORE-TEX sutures; W. L. Gore and Associates, Newark, DE, USA) [Table 2].
17 Patients without increased intraoperative bleeding did not require Tisseel
Seventeen patients without increased intraoperative bleeding did not require Tisseel. They averaged 65.29 years of age, and were followed over an average of 14.4 postoperative months (range 8-22 months). Significant medical comorbidities included: moderate/marked osteoporosis (12 patients), hypertension (9 patients), obesity (7 patients), and anemia (2 due to cancer/chemotherapy) [Table 1]. They underwent average 4.25 level laminectomies with average 1.29 level non-instrumented fusions for pathology comparable to those patients receiving Tisseel [Tables 1 and 2]. Additionally, six patients had single disc herniations, two had two disc herniations, while three had accompanying synovial cysts. Spinal instability was variously attributed to: spondylolisthesis (11 patients), spondylolisthesis/lysis (1 patient), or the need for extended/full unilateral facetectomies for far lateral disc excision (5 patients). The average operative time was 4.0 h [Table 2].
Of interest, although 14 patients had received an average of 4.0 injections per patient an average of 5.2 weeks prior to surgery, none exhibited punctate CSF fistulas secondary to the ESI, [Tables 1 and 2].
Intraoperative application of Tisseel utilizing the sandwich technique
The 3-layer “sandwich technique” was utilized to apply Tisseel in 22 patients.[12] The first layer utilized 5 of the 10 cc from the combined volume of the two syringes. It was evenly applied in an initial thin layer directly over the dura [e.g. after placing Avitene (microfibrillar collagen; C. R. Bard Inc., Warwick, RI, USA) under the laminar edges]. The second layer included the application of Duragen (Integra Life Sciences Corporation, Plainsboro Township, NJ, USA) (one strip for a 3-cm wound, tw o strips for a wound up to 6 cm). The third layer required application of the remaining 5 cc of Tisseel.
RESULTS
Comparable clinical and surgical data for both patient groups (receiving vs. not receiving Tisseel)
Several clinical (e.g. average age, sex ratio, follow-up interval, duration of surgery, etc.) and surgical parameters (e.g. average extent of laminectomy/fusion) were similar for patients in both groups [Tables 1 and 2].
Greater estimated blood loss and total postoperative blood loss for those receiving versus not receiving Tisseel
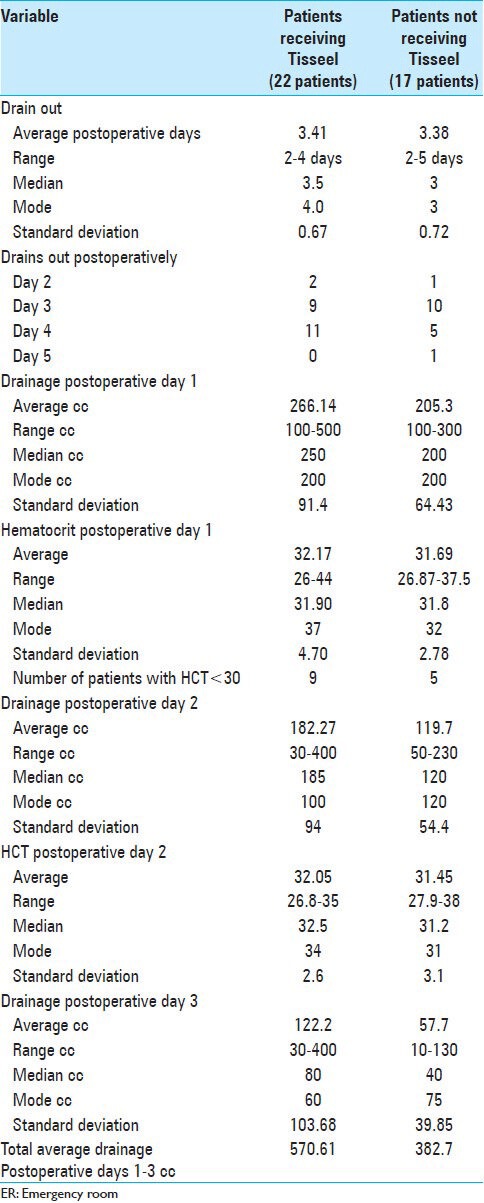
The average estimated blood loss (EBL) and range of EBL were higher for those receiving Tisseel [e.g. average EBL 209.1 cc (range 100-600 cc) (with Tisseel) vs. 157.7 cc (range 50-300) (without Tisseel)] [Tables 3 and 4]. Additionally, the total postoperative volumes of drainage were higher for those receiving Tisseel (570.61 cc) versus those not receiving Tisseel (382.7 cc), as were the average amounts of drainage observed on postoperative days 1-3 [Table 4].
Table 3.
Estimated blood loss, transfusion requirements, complications, and time to fusion for patients undergoing multilevel laminectomies with non-instrumented fusions with or without Tisseel
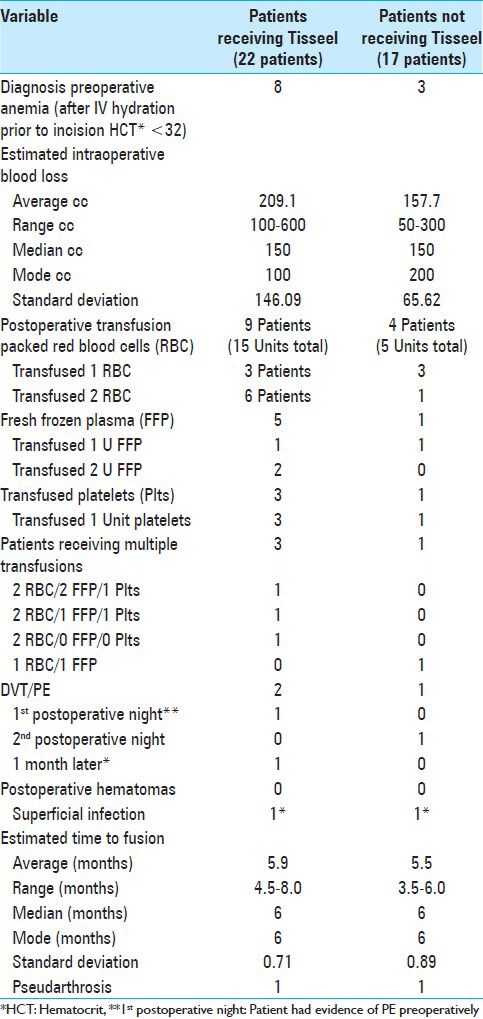
Table 4.
Assessment of postoperative drainage, volume/time to drain removal, and postoperative hematocrit for patients undergoing multilevel laminectomies with non-instrumented fusions with/without Tisseel
Greater transfusion requirements for those receiving versus not receiving Tisseel
Perioperative transfusion requirements were higher for patients receiving Tisseel for increased intraoperative bleeding versus for those without such bleeding and not receiving Tisseel. Those receiving Tisseel required 15 Units (U) of packed red blood cells (RBC), 5 U of fresh frozen plasma (FFP), and 3 U of platelets vs. for those not receiving Tisseel who had lower transfusion requriementes (e.g. 5 U of RBC, 1 U of FFP, and 1 U of platelets) [Table 3].
Comparable morbidity for patients receiving versus not receiving Tisseel
The incidence of major perioperative morbidities [e.g. including deep venous thrombosis (DVT)/pulmonary embolism (PE), postoperative hematomas/seromas, and postoperative infections] were comparable for both groups. Although two patients receiving Tisseel had DVT/PE, one with a PE on the first postoperative night clearly had had a PE prior to admission. Notably, no patients from either group experienced postoperative hematomas or seromas. One patient from each group developed a superficial wound infection 1 month postoperatively; both were successfully managed with 10 days of antibiotic therapy. Notably, both patients had high body mass indexes (BMI: >35), and the one receiving Tisseel was also a diabetic.
Comparable time to fusion for those receiving versus not receiving Tisseel
For patients undergoing multilevel laminectomy with non-instrumented fusion with versus without Tisseel, the average times to fusion were nearly the same (5.9 vs. 5.5 months, respectively), while one from each group developed an asymptomatic pseudarthrosis that did not require further surgery [Table 3].
Comparable times until drain removal with/without Tisseel
The protocol was to remove drains when the drainage for the prior 12-h shift was <30 cc. Despite a total higher average amount of drainage for patients receiving versus not receiving Tisseel, patients exhibited nearly identical average time (days) to postoperative drain removal [e.g. 3.41 days (with Tisseel) vs. 3.38 days (without Tisseel)] [Table 4].
Comparable LOS with/without Tisseel
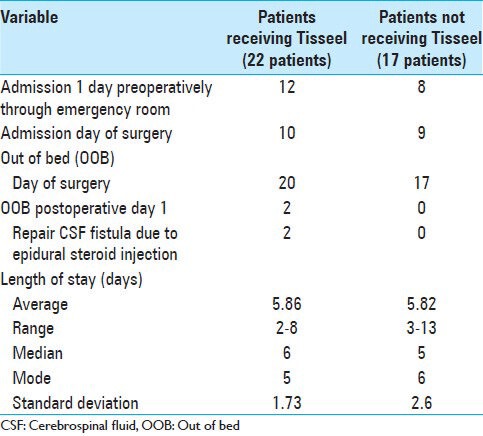
The average LOS for those receiving versus not receiving Tisseel were also comparable [5.86 days (with Tisseel) vs. 5.82 days (without Tisseel)]. The average LOS for both groups should be reduced by 0.5 days as approximately half of each group had been admitted one the day prior to surgery [Table 5]. Of added interest, almost all patients in both groups were out of bed on the day of surgery (OOB) rather than the more routine first postoperative day; the two exceptions were those requiring repair of ESI receiving Tisseel who were kept on bed rest for 24 h.
Table 5.
Most patients out of bed on the day of surgery and comparable lengths of stay for patients undergoing multilevel laminectomies with non-instrumented fusions with/without Tisseel
Preoperative anemia correlated with greater transfusion requirements in both groups
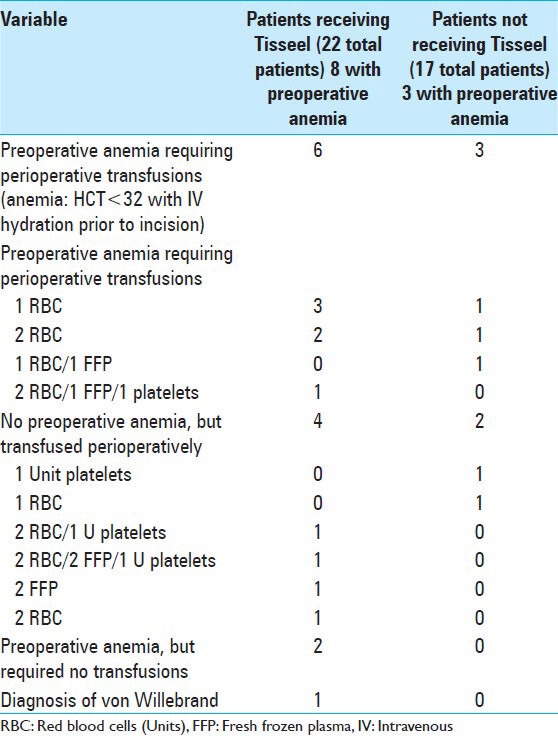
Preoperative anemia in either group, defined as a preoperative hematocrit (HCT) of 32 in the operating room following intravenous hydration (e.g. typically consisting of Normal Saline 500 cc) prior to making an incision, correlated with greater perioperative transfusion requirements [Table 6]. Six of eight anemic patients receiving Tisseel required 12 perioperative transfusions, consisting mostly of RBC (e.g. 9 RBC, 2 U FFP, 1 U platelets). Alternatively, the 4 patients without anemia in the Tisseel group with increased intraoperative bleeding required 12 transfusions that included fewer RBC (e.g. 6 U RBC, 4 U FFP, 2 U platelets). Of the 17 patients not receiving Tisseel due to the lack of increased intraoperative bleeding, 3 with preoperative anemia required five perioperative transfusions, consisting mostly of RBC (4 U RBC and 1 U FFP), versus 2 of 14 non-anemic patients who required few transfusions and fewer RBC units (1 U RBC and 1 U platelets). Of interest, one patient in the Tisseel group later tested positive for von Willebrand disease [Table 6].
Table 6.
Preoperative anemia led to increased perioperative transfusion requirements in patients undergoing multilevel laminectomies with non-instrumented fusions with/without Tisseel
DISCUSSION
Anticipated greater EBL, volume of postoperative drainage, and transfusion requirements for those receiving versus not receiving Tisseel
During multilevel laminectomies and non-instrumented fusions, those exhibiting increased intraoperative bleeding received Tisseel to promote hemostasis while those without such increased bleeding did not. As anticipated, therefore, those receiving Tisseel exhibited greater EBL, postoperative drainage, and perioperative transfusion requirements (especially if they were anemic preoperatively) [Tables 1–6].
Tisseel indirectly contributed to hemostasis allowing for comparable times to drain removal and LOS versus in those not requiring Tisseel
Tisseel indirectly contributed to postoperative hemostasis as both groups demonstrated nearly equivalent times to postoperative drain removal [3.41 (with Tisseel) vs. 3.38 (without Tisseel) days] and LOS (e.g. 5.86 vs. 5.82 days) [Tables 4 and 5].
Preoperative anemia correlated with greater perioperative transfusion requirements for patients receiving versus not receiving Tisseel
Preoperative anemia in either group correlated with greater perioperative transfusion requirements [Table 6]. Six of eight anemic versus four non-anemic patients in the Tisseel group were transfused, and their transfusion requirements were greater when compared with those warranted for the three anemic and two non-anemic patients in the non-Tisseel group. Notably, the anemic patients required more RBC transfusions compared to other products.
A higher incidence of comorbid factors likely contributed to increased intraoperative bleeding in patients receiving Tisseel
Multiple comorbid factors likely contributed to increased intraoperative bleeding for those receiving Tisseel versus those not receiving Tisseel. These included: A higher incidence of moderate/severe osteoporosis (21 of 22 vs. 12 of 17), more obesity [BMI >30: 13 patients with Tisseel (7 with >35 BMI) vs. 7 without Tisseel], anemia (8 vs. 3), and coronary artery disease (CAD)/stents (4 vs. 0) [Table 1]. It is well understood that osteoporosis increases intraoperative back bleeding from both the laminectomy site and the decorticated transverse processes; however, when Tisseel is added, it decreases the back bleeding from the laminectomy edges as it is applied centrally in the spinal canal. The increased BMI certainly contributes to intraoperative back bleeding from all sites, including Batson's plexus (e.g. Tisseel helps control this bleeding). Anemic patients exhibit increased blood flow dynamics that contribute to increased perioperative bleeding. Finally, those with CAD/stents, previously on anti-platelet aggregants, may not regenerate adequate platelet function prior to surgery even when they are stopped for several preoperative weeks.
Clinical evidence that Tisseel promotes hemostasis
When used clinically, Tisseel successfully facilitatess cranial and spinal hemostasis and limits bone morphogenetic protein (BMP) diffusion (a benefit), but may inhibit spinal fusion.[1,8,9,13] In one study, Tisseel achieved hemostasis without complications in the cranial epidural space (n = 200 patients), anterior cavernous sinus (n = 46 patients), and vertebral venous plexus (n = 20 patients); however, in the superior petrosal sinus, it contributed to brain stem stroke.[8] In another study, Tisseel reduced postoperative drainage and LOS without increasing the complication rate following spinal surgery.[1,9,13,14] In a third study, when utilized to perform three or more level anterior cervical fusions, Tisseel successfully reduced the average amount/duration of postoperative drainage and LOS (1.2 s vs. 2.1 days) without increasing the complication rate.[14] Specifically, patients undergoing anterior cervical diskectomy/fusion (ACDF) with Tisseel (30 patients) versus without Tisseel (30 patients), respectively, demonstrated the following: average drainage (47 ml vs. 98 ml), average time of drainage/removal (≤20 ml per shift) [17 h (range 8-29 h) vs. 24 h (range 7-43 h)], and average LOS [1.2 days (range 1-4 days) vs. 2.1 days (range 1-5 days)].[14] In another study, an absorbable gelatin sponge applied for multilevel posterolateral lumbar fusions (PLF) or posterior lumbar interbody fusions (PLIF) also significantly reduced postoperative drainage (173 cc gelatin sponge vs. 392 cc control patients), transfusion requirements (34.15% vs. 58.5%), and average LOS (12.58 vs. 14.46 days) without increasing short- or long-term sequelae.[13]
Other clinical evidence showing that Tisseel increased fusion rates and reduced the incidence of infections when combined with antibiotics
Clinically, the addition of FS/FG contributed to the spinal fusion rate when combined with bone graft products, or reduced infection rates when combined with antibiotics. Fibrin glue, in this case consisting of a unique platelet-rich gel plus autograft/allograft, accelerated bony deposition and tissue healing, while also increasing bone density 3 months following 14-instrumented PLF.[4] In a separate clinical series, utilization of an antibiotic-impregnated fibrin sealant (AFS) applied to 196-instrumented spinal fusions led to no deep surgical site infections (SSI) versus a 5.8% (11 patients) incidence of SSI occurring for 188 comparable procedures performed without AFS impregnated with antibiotics.[10]
Other pros and cons for FS/FG or Tisseel demonstrated in animal models
Several other pros and cons were demonstrated for FS/FG tested in animal models.[2,3,4,5,6,7,11] When Tisseel was applied prior to placing BMP in a rat fusion model, it markedly limited BMP/INFUSE diffusion into “unwanted” areas, but reduced or nearly completely eliminated fusions.[5] Tisseel similarly reduced, fusion rates in an ACDF feline (cat) model.[11] In a rat model mimicking instrumented spinal fusions, Cashman et al. demonstrated the utility of antibiotic-impregnated Vitagel/FS to reduce infections and infection-related mortality.[2]
FS/FG fails or succeeds in reducing epidural fibrosis
In two animal models, FS/FG either failed or succeeded in the reducton of postoperative epidural fibrosis. In a rat spinal surgery model, FG alone or mixed with steroids failed to reduce postoperative epidural fibrosis. Alternatively, in a sheep model, Adcon-Gel (Tributyrin; Gliatech, Cleveland, OH, USA) effectively reduced posterior spinal epidural adhesions following laminectomies.[3,7]
Animal model demonstrating efficacy of FS Impregnated with Injectable Calcium Phosphate and rh-BMP-2 for vertebroplasty
Supplementing injectable calcium phosphate cement (ICPC) with FS impregnated with recombinant human bone morphogenetic protein-2 (rh-BMP-2) proved to be an effective alternative to poly methylmethacrylate (PMMA) for performing vertebroplasties in New Zealand rabbits.[6]
Limitations
In this study, multiple variables may have contributed to the reduction in the time to drain removal and LOS for those receiving versus not receiving Tisseel. We were unable to differentiate the volume of postoperative drainage from the epidural compartment (should be reduced with Tisseel) versus drainage from the decorticated transverse processes (should be comparable in both groups) versus overall soft tissue of the wound. Among the patients receiving Tisseel for increased intraoperative bleeding, hematology diagnosed von Willebrand disease in one patient, but may have failed to pick up other factor deficiencies in the remainder. Additionally, there were only a small number of patients in both groups as the study only started after we elected to prospectively apply Tisseel (1) for hemostasis alone and (2) only in the most recent patients fused with lamina autograft and NanOss Bioactive. Future studies warrant more patients in both populations to achieve greater validity.
CONCLUSION
Twenty-two patients received Tisseel for increased intraoperative bleeding encountered during multilevel laminectomies and non-instrumented fusions performed for stenosis/instability. In comparision, 17 comparable patients without increased bleeding received no Tisseel. As anticipated, the former 22 patients receiving Tisseel exhibited increased postoperative EBL, total drainage, and higher transfusion requirements versus the 17 patients not receiving Tisseel. We found, however, that the addition of Tisseel equalized the average time to postoperative drain removal (e.g. 3.4 days) and average LOS (5.8 days). for the two groups.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/8/354/139668
REFERENCES
- 1.Bou Monsef J, Buckup J, Waldstein W, Cornell C, Boettner F. Fibrin sealants or cell saver eliminate the need for autologous blood donation in anemic patients undergoing primary total knee arthroplasty. Arch Orthop Trauma Surg. 2014;134:53–8. doi: 10.1007/s00402-013-1876-5. [DOI] [PubMed] [Google Scholar]
- 2.Cashman JD, Jackson JK, Mugabe C, Gilchrist S, Ball K, Tredwell S, et al. The use of tissue sealants to deliver antibiotics to an orthopaedic surgical site with a titanium implant. J Orthop Sci. 2013;18:165–74. doi: 10.1007/s00776-012-0325-6. [DOI] [PubMed] [Google Scholar]
- 3.Cekinmez M, Sen O, Atalay B, Erdogan B, Bavbek M, Caner H, et al. Effects of glue and combination of methyl prednisolone acetate and fibrin glue in prevention of epidural fibrosis in a rat model. Neurol Res. 2010;32:700–5. doi: 10.1179/016164110X12556180206239. [DOI] [PubMed] [Google Scholar]
- 4.Landi A, Tarantino R, Marotta N, Ruggeri AG, Domenicucci M, Giudice L, et al. The use of platelet gel in postero-lateral fusion: Preliminary results in a series of 14 cases. Eur Spine J. 2011;(20 Suppl 1):S61–7. doi: 10.1007/s00586-011-1760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel VV, Zhao L, Wong P, Pradhan BB, Bae HW, Kanim L, et al. An in vitro and in vivo analysis of fibrin glue use to control bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth. Spine J. 2006;6:397–403. doi: 10.1016/j.spinee.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Qian G, Dong Y, Yang W, Wang M. Injectable calcium phosphate cement and fibrin sealant recombined human bone morphogenetic protein-2 composite in vertebroplasty: An animal study. Bosn J Basic Med Sci. 2012;12:231–5. doi: 10.17305/bjbms.2012.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards PJ, Turner AS, Gisler SM, Kraft S, Nuss K, Mark S, et al. Reduction in post laminectomy epidural adhesions in sheep using a fibrin sealant-based medicated adhesion barrier. J Biomed Mater Res B Appl Biomater. 2010;92:439–46. doi: 10.1002/jbm.b.31533. [DOI] [PubMed] [Google Scholar]
- 8.Sekhar LN, Natarajan SK, Manning T, Bhagawati D. The use of fibrin glue to stop venous bleeding in the epidural space, vertebral venous plexus, and anterior cavernous sinus: Technical note. Neurosurgery. 2007;(61 3 Suppl):E51. doi: 10.1227/01.neu.0000289711.95426.50. [DOI] [PubMed] [Google Scholar]
- 9.Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009;17:727–36. doi: 10.5435/00124635-200912000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Tofuku K, Koga H, Yanase M, Komiya S. The use of antibiotic-impregnated fibrin sealant for the prevention of surgical site infection associated with spinal instrumentation. Eur Spine J. 2012;21:2027–33. doi: 10.1007/s00586-012-2435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turgut M, Erkuş M, Tavus N. The effect of fibrin adhesive (Tisseel) on interbody allograft fusion: An experimental study with cats. Acta Neurochir (Wien) 1999;141:273–8. doi: 10.1007/s007010050298. [DOI] [PubMed] [Google Scholar]
- 12.Wang HR, Cao SS, Jiang YQ, Li JN, Li XL, Fu YG, et al. A comparison between “sandwich” and conventional methods of repairing spinal dura rupture. Orthop Surg. 2012;4:233–40. doi: 10.1111/os.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Jin Y, Zhang J, Shao H, Yang D, Chen J. Hemostatic Techniques Following Multilevel Posterior Lumbar Spine Surgery: A randomized control trial. J Spinal Disord Tech. 2013 Dec 11; doi: 10.1097/BSD.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 14.Yeom JS, Buchowski JM, Shen HX, Liu G, Bunmaprasert T, Riew KD. Effect of fibrin sealant on drain output and duration of hospitalization after multilevel anterior cervical fusion: A retrospective matched pair analysis. Spine (Phila Pa 1976) 2008;33:E543–7. doi: 10.1097/BRS.0b013e31817c6c9b. [DOI] [PubMed] [Google Scholar]


