Abstract
Microglia constitute as much as 10–15% of all cells in the mammalian central nervous system (CNS) and are the only glial cells that do not arise from the neuroectoderm. As the principal CNS immune cells, microglial cells represent the first line of defence in response to exogenous threats. Past studies have largely been dedicated to defining the complex immune functions of microglial cells. However, our understanding of the roles of microglia has expanded radically over the past years. It is now clear that microglia are critically involved in shaping neural circuits in both the developing and adult CNS, and in modulating synaptic transmission in the adult brain. Intriguingly, microglial cells appear to use the same sets of tools, including cytokine and chemokine release as well as phagocytosis, whether modulating neural function or mediating the brain's innate immune responses. This review will discuss recent developments that have broadened our views of neuro-glial signalling to include the contribution of microglial cells.
Keywords: microglia, cerebral cortex, neurons, synapse, neurogenesis, neuroblasts
1. Introduction
The central nervous system (CNS) comprises a network of intimately associated cells—neurons and macroglia—that are pervaded by a dense vascular network and both perivascular and parenchymal macrophages. Among the latter, the largest, most extensively studied and still most controversial group are microglial cells [1–3]. Microglia serve as the innate immune effectors of the brain, in that they can eliminate antigens without triggering inflammatory responses that might otherwise interfere with homeostatic neural functions [4]. Traditionally, it was believed that the functions of microglia were limited to those attributed systematically to macrophages, i.e. the phagocytosis of pathogens and cell debris, and the mediation of local inflammatory responses [5,6]. However, the recent demonstration that microglial cells in the healthy, intact brain constantly survey their microenvironment [7] fundamentally broadened our conception of the capabilities of microglia. This new perspective has led to a series of studies that have identified a prominent role for microglia in normal homeostatic brain functions [8,9]. It now seems apparent that microglial cells function as important sources of trophic support, essential for neuronal survival and circuitry formation [10]. In addition, microglia-mediated phagocytosis of synaptic terminals and newborn neurons plays a key role in shaping neural circuitry, both at early postnatal stages [11–14] and in adult neurogenic niches [15]. This review discusses recent findings in regards to microglial physiology, which have deepened our understanding of the mechanisms by which microglia enter and establish themselves as resident cells in the CNS. We also discuss studies that have applied advanced imaging techniques to demonstrate that microglia are dynamic elements in the normal healthy brain, which play critical roles in fine-tuning of neural circuits.
2. Microglia as cellular elements in the central nervous system
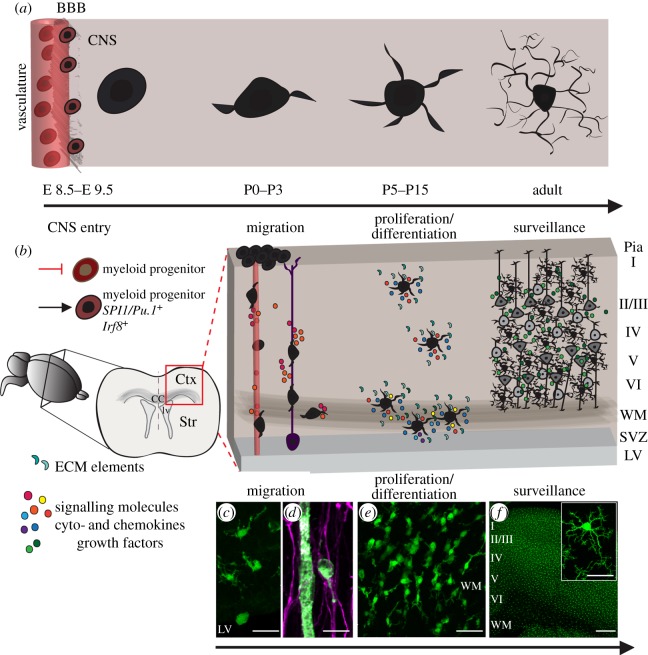
In a series of studies spanning 1919–1932, the neuroanatomist Rio-Hortega developed the modern conception of microglia, the third CNS element that was originally identified by Ramón y Cajal, along with neurons and astrocytes [3,16]. Studying brain tissue obtained from patients affected by neurological diseases, Rio-Hortega described the salient features of microglia, largely using silver impregnation techniques. His studies showed that these mesoderm-derived cells enter the brain during early stages of development, displaying amoeboid morphology, and use blood vessels and white matter (WM) tracts to guide their invasion. Once established, microglia achieve a branched ramified morphology, which is stable; at this stage, the cells are referred to as ‘resting’ microglia. Like astroglia, each microglial cell occupies a defined domain and they are evenly distributed throughout the brain (figure 1a,b). However, microglia retain migratory and proliferative capacities in pathological conditions. Activated microglia undergo morphological transformations, reacquiring the amoeboid phenotype—its ‘activated’ status—able to exert phagocytosis [16] (figure 2a). These observations by Rio-Hortega, which are detailed in the following sections, have over the years been confirmed by multiple groups. Nevertheless, microglial cells were first described as a distinct cell type in the CNS by Nissl, Alzheimer and Merzbacher [3,16]. Combined, these influential studies defined microglia as ‘pathological’ elements in the CNS, a concept that has proved to be true in a number of neurological diseases [26]. Nonetheless, recent studies have also highlighted the manifold functions of microglia in normal physiology [7,10,11,13,14,27,28].
Figure 1.
Microglial origin and establishment in the CNS. (a) Microglia cells undergo pronounced morphological changes once inside the CNS, from amoeboid immature cells to the typical ramified microglia observed throughout the brain parenchyma. (b) Remarkably, these changes in microglia morphology occur inside a tissue with an ontogenetically distinct origin, and microglia cells are exposed to several signalling molecules, including ECM proteins, cyto- and chemokines and growth factors of neural origin. Microglia derive from a restricted subpopulation of yolk sac erythromyeloid progenitors [17,18] that express the transcription factors SPI1/Pu.1+ and Irf8+ [19] and enter the CNS during embryonic (E) stages E8.5–E9.5. At neonatal stages (P0–P3), amoeboid microglia populate the neural parenchyma through the meninges and ventricular system and use vessels and RG cell processes as migratory scaffold. Once established, microglia undergo proliferation and spread in the cerebral cortex (Ctx) parenchyma (P5–P15), acquiring their mature morphology, which is observed throughout the cortical layers. (c) Laser scanning micrographic of CX3CR1-EGFP+ cells (green) shows CNS invasion by amoeboid microglia through the LV. (d) Microglial cell labelled by Isolectin B4 (green) displaying a migratory morphology attaches to RG processes (GFAP+, magenta) to populate the cortical parenchyma. (e) At neonatal stages, microglial cells (CX3CR1-EGFP+ cells, green) markedly increase in number in the WM. (f) In the adult brain, microglia (CX3CR1-EGFP+ cells, green) are widely distributed in the CNS parenchyma and exhibit a ramified morphology. BBB, blood–brain barrier; ECM, extracellular matrix; SVZ, subventricular zone. Scale bars: (c, d and inset in f) 10 µm, (e) 20 µm and (f) 500 µm.
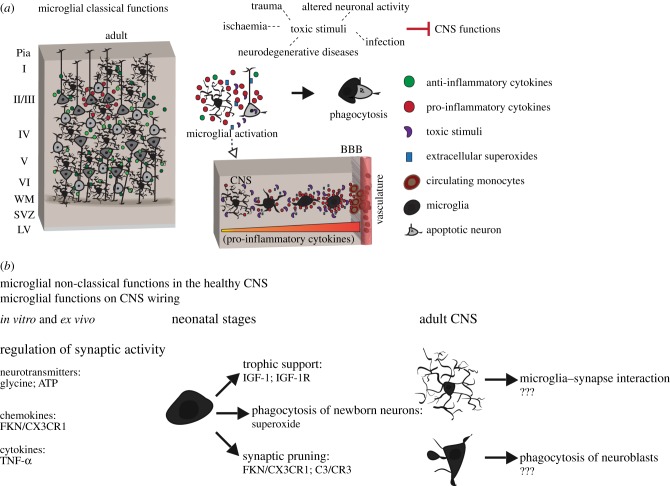
Figure 2.
Microglia classical and non-classical functions. (a) Toxic stimuli trigger microglial activation, which reacquire their migratory capacity and reassume the amoeboid morphology. Activated microglia release proinflammatory cytokines and superoxides that mediate phagocytosis of damaged cells. Under severe conditions, microglia-released proinflammatory cytokines recruit circulating monocytes, which can enter the CNS through disrupted blood–brain barrier. (b) Alternative functions have been proposed for microglial cells and microglia-released molecules in the physiological conditions. Microglia act as pivotal synapse regulatory elements through the release of neurotransmitters, such as glycine [20] and ATP [21], chemokine signalling (FKN/CX3CR1 [22]) and cytokines (TNF-α [23,24]). In vivo, microglial cells have been shown to be an important trophic source for neurons (IGF-1/IGF-1R [10]) and phagocyte newborn neurons in the cerebellum [11], and act on synaptic pruning via FKN/CX3CR1 signalling pathway [13] and through C3/CR3 cascade [14]. In the adult CNS, although the molecules mediating non-classical microglia functions remain elusive, microglia establish critical synapse contact [25] and phagocytose a great part of newly generated neuronal precursors in the hippocampus [15].
(a). The origins of microglia
Rio-Hortega believed that microglia derived from mononuclear cells present in the circulating blood [16]. However, some studies claimed that microglia could be obtained from embryonic neuroepithelium [29], and that microglia share a common progenitor cell with the astroglial lineage [30,31]. The hypothesis of a neuroectodermal origin was contradicted by later findings showing that microglia derive from the haematopoietic system [32–34], and specifically from progenitors originated in the yolk sac [17]. In that regard, a recent study confirmed that microglia derive from primitive myeloid progenitors that arise in early embryonic stages (E). Using in vivo lineage tracing, Ginhoux et al. [18] elegantly showed that yolk sac progenitors seed the brain between E8.5 and E9.5, and these brain-infiltrating macrophages appear only when blood circulation develops. Accordingly, subsequent studies supported a mesodermal origin for microglia, showing that they correspond to a restricted macrophage population, which derives from myeloid precursors responsive to SPI1/Pu.1 and Irf8 [19] (figure 1b), transcription factors that are critical for the myeloid lineage commitment [35,36].
Yet their distinct ontogenetic origin notwithstanding, microglia clearly comprise a distinct subset of cells that is highly integrated with neuroepithelial-derived neurons and macroglia, and whose functions we now realize to be critical for the homeostasis of the normal brain and its constituent cells.
(b). Microglia entry into the central nervous system: routes and mechanisms
Rio-Hortega proposed the meninges as a route of entry of microglial precursors [16]. This hypothesis was later confirmed in studies that have shown microglial cells invading the cerebral cortex (Ctx) through the pial surface during development [37–41]. However, subsequent studies have shown that alternative pathways are also used by microglia cells to enter the CNS, including the vasculature and ventricular layers as well as the meninges [42]. Consistent with their myeloid origin, the development of functional blood vessels is a prerequisite for microglial precursors' recruitment into the embryonic brain [18], but microglial cells also enter regions of the developing CNS devoid of vascularization. Instead, microglia reach the parenchyma from the ventricles, as monocyte/macrophage lineage cells, which are distributed in the ventricular lumen [1], were shown to transverse its surface [39,40,43] (figure 1c).
The mechanisms underlying microglia recruitment into the CNS have also been assessed. Colony-stimulating factor 1 (CSF-1) was originally shown to act in vitro as a microglia chemoattractant [44]. This work was extended in vivo by the finding that CSF-1, and its receptor CSF-1R, as well as interleukin (IL)-34 (a second CSF-1R ligand) are important elements for microglia progenitors entry into the CNS [18]. Furthermore, microglia chemotaxis mediated by CSF-1R has been shown to act in synergism with vascular endothelial growth factor receptor 1 (VEGFR1) in the neonatal subventricular zone (SVZ) that lines the lateral ventricle (LV) walls. The signalling mechanisms triggered by these receptors are in turn regulated by superoxide ions generated by Nox2, an NADPH oxidase isoform that is activated in microglial cells present in the SVZ [43]. Other, metalloproteinases (MMPs), namely MMP-8 and MMP-9, were also found to be key modulators of microglial recruitment during embryogenesis [19].
Once within the CNS, microglial precursors proliferate and migrate, probably using the same mechanisms as neural progenitor cells, which migrate from the germinal zones to the layers into which they are functionally integrated. Such migration guidance cues include not only cytokines and chemokines, but also developmental morphogens and growth factors, as well as the use of axons, radial glial (RG) cell processes and perivascular sheaths as migratory scaffolds [45]. For instance, in the quail brain and neural retina alike, amoeboid microglia migrate along radial processes of glial cells [39,46,47]. The observation of amoeboid microglia in the periventricular WM before the grey matter suggests that radial migration similarly occurs in mammals [32]. In this regard, confocal microscopy has revealed the attachment of microglia to RG cell processes in cortical parenchyma of neonatal mice (figure 1d). Such migration along the RG processes also occurs in the embryonic spinal cord, the parenchyma of which is invaded by microglia at E11.5, concomitantly with the establishment of a functional neuronal circuitry [48]. In humans, microglia invade the CNS attached to blood vessels present in the cortical parenchyma, or associated with RG fibres in the spinal cord [49,50].
During the embryonic and neonatal stages, the density of microglia continues to increase with the continued invasion of myeloid precursors, as well as from active microglial proliferation during brain development (figure 1e). In particular, microglia present in the WM of neonatal rodents are highly proliferative [51–53], and in the spinal cord, microglia proliferate concomitantly with spinal cord colonization [48]. However, the signalling pathways controlling developmental microglial expansion in these distinct CNS regions remain elusive.
(c). Microglia morphological plasticity
Microglia undergo cellular differentiation within the neural parenchyma during the first postnatal weeks (figure 1a). In the mature cerebral cortex (figure 1f), microglia can be easily distinguished by their oval cell bodies, which are the smallest of the glial cell populations. Instead of a visible cytoplasm, microglia have abutting dense or inclusion bodies (lysosomes or phagosomes) and a variable number of highly ramified branching processes, quite unlike the macrophages observed in other tissues [1,33,54–58]. Indeed, this ramified structure is generally considered the morphological prototype of mature microglia. However, microglial cells are heterogeneous and dynamically pleomorphic, such that their density, morphology and activation status are determined by their local environment [6,33,59,60]. Indeed, the physiological relevance of such CNS microglial morphological diversity remains largely unknown [60]. Regional differences in extracellular matrix (ECM) elements are probably significant contributors to microglial phenotype, so that the modulation of the ECM by perivascular and glial cells may have significant paracrine effects on microglial state and function [61,62]. Furthermore, the permeability in certain areas of the CNS of the blood–brain barrier (BBB), which may admit circulating macrophages and serum molecules, may also influence local microglial phenotype [63,64]. Of note, microglial phenotype may also be dictated by signals released by surrounding neural cells, both neuronal and glial [16,60] (figure 1b).
(d). Classical microglia functions: the central nervous system immune effectors
The classical role of microglia as immune effectors has been extensively studied. Microglial cells continuously monitor the neural parenchyma and any disturbance or loss of CNS homeostasis (e.g. infection, trauma, ischaemia, neurodegenerative diseases or altered neuronal activity) evokes morphological changes and alterations in the gene expression pattern of microglia, a process called microglial activation [16] (figure 2a). This surveillance system relies on several pattern recognition receptors expressed by microglia (reviewed in [65,66]), which recognize pathogen-associated molecular patterns (PAMPs) and detect altered features of soluble and insoluble factors released by damaged cells (damage-associated molecular patterns, DAMPs) [67–71]. Under these circumstances, microglia trigger innate defence mechanisms, which promote the internalization and phagocytosis of toxic stimuli, mediated by the production of extracellular superoxide [72] and the release of proinflammatory compounds [73–75]. If microglia cannot eliminate PAMPs or DAMPs they will phagocytose the damaged cells, and under extreme conditions circulating monocytes are recruited into the CNS through a disrupted BBB [4,16,76–80] (figure 2a).
3. Cytokines and chemokines: microglial immunomediators or more?
Microglial immune effector functions are dependent upon the release of cytokines, small proteins that act on membrane receptors to trigger recipient cell responses, both autocrine and paracrine in nature; though local effectors, they may act distantly as well, carrying biological information through body fluids [81]. Among these molecules, a group of chemoattractive cytokines—the chemokines—promotes cell migration or arrest, modulating the spatio-temporal positioning of target cells under both physiological and pathological conditions [71]. Importantly, chemokines are key regulators of both apoptosis and microglia-mediated phagocytosis [82]. Despite their historical association with haematopoietic cells and immune physiology, these microglial signalling proteins act upon many cell types and within the CNS. Several cytokines and their respective receptors have been described in neural cells, including tumour necrosis factor α (TNF-α), interferons (IFNs), interleukins (ILs), transforming growth factor β (TGF-β), macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) [81,83,84]. Of note, chemokines are not only expressed by microglia but are also secreted by neurons and macroglial cells. They comprise 50 distinct peptides, which are divided into four families: CXC, CC, XC and CXC3, according to the sequence motif of conserved N-terminal cysteine residues [71,84,85]. Unlike cytokines, each of which has its own receptor, only 20 chemokine receptors have been identified, reflecting the complexity of chemokine ligand–receptor interactions. Some chemokine/receptor pairs are exclusive, whereas for other chemokine receptors responses can be elicited by as many as 10 distinct ligands. Conversely, some chemokines can trigger signalling via as many as three different receptors [16,71].
Notwithstanding the complexity of cyto- and chemokine signalling pathways, upon microglial activation a characteristic array of proinflammatory cytokines, including TNF-α, IFN-γ and IL-1 [73–75], are commonly released in the CNS, presumably in order to eliminate toxic stimuli. Regardless of their benefit to the organism, cytokines along with chemokines, proteases and superoxides [70] can cause damage in the surrounding tissue. To counterbalance this detrimental effect, microglial cell-derived anti-inflammatory cytokines, namely IL-4, IL-10, IL-13 and TGF-β [70,86–88] act on homeostasis restoration, and promote cell replacement and tissue repair mechanisms. It is not uncommon, however, for the same cytokine to be assigned antagonistic roles as pro- and anti-inflammatory. Such ambiguity arises from cytokine interactions with other molecules and ECM elements present in the injured area [68,76,89,90], which are critical factors for determining whether the beneficial effects of their actions will override their toxicity, leading to either neural protection and homeostasis resumption or neural damage.
Cytokines have also been shown to play neurodevelopmental roles. In fact, the classification of growth factors, including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), insulin-like growth factors (IGF), nerve growth factor (NGF), neurotrophins (NT-3 and -4) and brain-derived neurotrophic factor (BDNF) as cytokines [81] renders these molecules as the putative effectors of several neurodevelopmental functions. For example, Ueno and colleagues have shown that microglial cells accumulate along callosal and subcerebral tracts, which project from layer V cortical neurons (CTIP2+/SATB2+) at early postnatal stages. Microglia-derived IGF1 acts as a specific trophic source for layer V neurons, which express the receptor IGF1Ra. Remarkably, locally induced microglia depletion triggers neuronal death in layer V [10], and these results support the concept that microglia influence wiring of neural circuitries in the developing brain.
4. Microglial cells as a dynamic element in the central nervous system
For decades, microglia were thought to rest in a dormant state. Structural modifications, such as motile branches or cell soma migration, were shown to only occur in association with microglia activation [91,92]. Remarkably, however, the use of the transgenic mice that has one of the fractalkine (FKN) receptor loci replaced by the reporter gene encoding the green fluorescent protein (GFP) [93] in two-photon imaging of thin skull preparations led to breakthrough studies in the healthy, intact brain. These analyses showed that microglial cells continuously monitor the cerebral cortex parenchyma and that the assumed ‘resting’ microglia indeed have extremely motile processes and protrusions, which continuously sample their microenvironment and dynamically interact with other CNS elements [7,94]. These studies overcome the dichotomy of ‘resting’ versus ‘activated’ microglia and enabled the discovery of new and unexpected roles for microglial cells in the CNS. In the following sections, we provide an overview on the exaptation of typical functions of activated microglia such as proinflammatory cytokines release and phagocytosis, which are crucial for the establishment and function of neural circuits (figure 2b).
5. Microglial functions on central nervous system wiring
(a). Microglial regulation of synaptic activity
In vitro studies have shown that microglia are endowed with a wide range of receptors for neurotransmitters, neuropeptides and neuromodulators, including adrenergic receptors, metabotropic and ionotropic glutamate and γ-aminobutyric acid (GABA) receptors, dopamine receptors, bradykinin receptors and several types of purinoceptors (reviewed in [16,95]). These receptors enable microglia to detect ongoing neuronal activity. The importance of microglia–synapse interactions is further substantiated by studies that demonstrate microglial cell release of signalling molecules that modulate synaptic plasticity. For example, the inhibitory neurotransmitter glycine, detected in conditioned media (CM) obtained from microglia cultures, is the putative mediator of the facilitation of long-term potentiation (LTP) in Schaeffer collaterals via N-methyl-d-aspartate receptor (NMDAR) activation. NMDAR activation is evidenced by an increased CamKII immunoreactivity in CA1 neurons, upon stimulation (25 Hz), and in the presence of microglia CM [20]. LTP seems also to be dependent on FKN/CX3CR1 signalling. In CX3CR1 knockout mice (CX3CR1ko), the lack of the constitutive ‘calming’ signalling pathway established between neurons and microglia [82,95] triggers microglial activation, increasing the levels of IL-1β via MAP kinase p38 activation, and also the levels of TNF-α. As a result of microglial activation, CX3CR1ko mice exhibit deficiencies in both associative and spatial memory, resulting from a reduction in LTP-mediated synaptic plasticity [22].
In addition to modulating LTP, microglia have emerged as crucial participants in homeostatic synaptic scaling. TNF-α increases the expression of neuronal AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors [96,97] and modulates the traffic of both AMPA and GABAA receptors [23]. The increased expression of AMPA receptors triggered by TNF-α potentiates synaptic strength, detected as an increase in the amplitude of miniature excitatory postsynaptic currents (mEPSCs) in hippocampal slices [23]. TNF-α is also critical for synaptic plasticity in the visual cortex. In TNF-αko mice, the homeostatic synaptic scaling of mEPSCs is blocked, thus hampering the ocular dominance plasticity that follows the marked decrease in synaptic activity triggered by eye-occlusion [24].
Microglial activation also has less direct effects on the modulation of synaptic transmission. Hippocampal slices treated with lipopolysaccharide (LPS) show a transient increase in the frequency of AMPA receptor-dependent spontaneous excitatory postsynaptic currents (EPSCs). This increased EPSC frequency is associated with microglial activation and is modulated by ATP, which is released by microglia in response to LPS. This microglial-released ATP acts as signal to local astrocytes, which is transduced via the astrocytic purinoceptor P2RY1 [21]. Astrocytic P2RY1 activation in turn triggers glutamate release [98,99], which yields an increased frequency of EPSCs, through the activation of mGluR5 in astrocytes [21]. By means such as this, microglial activation may modulate both the threshold and intensity of synaptic activity in local networks.
(b). Microglia–synapse interactions are crucial for neural circuitry integration
In vivo studies have shown that proper microglia–synapse interactions have physiological consequences for both neural circuitry establishment and functioning. In adult mice, Wake and colleagues showed that microglial processes distributed through cortical layers II/III make specific and direct contact to synaptic terminals. Microglia–synapse contacts are established in an activity-dependent manner: deprivation of visual stimuli trigger an acute retraction of microglia processes within the visual cortex, and the frequency of these microglia–synapse contacts was significantly reduced upon spontaneous neuronal activity impairment induced by tetrodotoxin (TTX) or reduction of body temperature. Interestingly, prolonged microglia–synapse contacts were observed upon middle cerebral artery occlusion, and the majority of synaptic terminals contacted for longer periods by microglia remained intact [25], possibly suggesting a protective role for microglia in ischaemic regions.
The establishment of microglia–synapse contacts seems to be modulated at the onset of neural circuitry development, as originally demonstrated by Tremblay and colleagues. They took advantage of the critical period of development of the visual cortex, in which the establishment of connections between layer II and V1 neurons become sensitive to exogenous stimuli. At the peak of critical period sensitivity, at postnatal day 28, they noted an increase in both the density of microglial cells, and in the area occupied by microglial processes in the visual cortex. These results revealed dynamic interactions of microglia, which contact multiple synapse-associated elements, as visualized by electron microscopy and two-photon imaging. Remarkably, visual stimuli deprivation promotes microglia accumulation around larger dendritic spines that undergo shrinking due to degeneration, preceding their engulfment by microglia. By contrast, when deprivation ceases, microglia cells tend to contact spines that transiently grow, similar to what is observed in control animals [12].
(c). Microglial functions on synaptic pruning
Another key function performed by microglial cells during neural circuitry establishment is synaptic pruning. In the hippocampus of juvenile mice, fragments of PSD95—a marker of excitatory postsynaptic density—were observed inside clathrin-coated and non-clathrin-coated vesicles of CX3CR1+ cells, demonstrating that microglia actively engulf synaptic material [13]. The role of microglial cells on synaptic pruning seems to be pivotal for hippocampal circuitry maturation; deficiencies of FKN/CX3CR1 signalling in CX3CR1ko mice result in a markedly reduced frequency of spontaneous excitatory postsynaptic potential currents (sEPSC). Furthermore, in CX3CR1ko mice, long-term depression (LTD) upon Schaeffer collateral stimuli was enhanced. Moreover, at neonatal stages, it has been shown that mice lacking FKN signalling are more susceptible to seizures [13]. Notably, the reduced synaptic pruning at neonatal stages results in persistent deficits detected as a reduced number of multi-synapse boutons in the hippocampus of adult CX3CR1ko mice. These structural changes appear to hamper brain connectivity, resulting in lack of synchronization of oxygen consumption and neuronal activity in the hippocampus and prefrontal cortex. In turn, such deficits in brain connectivity have been reported to impair social interaction and increase grooming behaviour [100].
Microglia also engulf presynaptic inputs during retinogeniculate formation [14]. At neonatal stages, retinal ganglion cells establish synaptic connections with thalamic neurons, localized in the dorsal lateral geniculate nucleus (dLGN) [101–103], and spontaneous retinal activity plays critical role in the refinement of this circuitry [104–108]. Microglial cells specifically engulf presynaptic terminals, characterized by the lack of mitochondria and expression of the vesicular glutamate transporter VGlut2, at the peak of retinogeniculate pruning (P5). As previously observed in the cerebral cortex, microglia engulfment of synaptic elements also occurs in an activity-dependent manner, and either blockade or increase in neuronal activity by TTX and forskolin, respectively, augmented the engulfment of synaptic elements in the retinogeniculate nucleus. The mechanisms underlying synaptic pruning have also been characterized in a series of elegant experiments. Synaptic material engulfment by microglial cells relies on the complement component 3 (C3) and its receptor CR3, whose expression levels are increased at P5 in synapses within the dLGN [14,109]. Markedly, the disruption of this signalling pathway leads to malformation of retinogeniculate circuitry [14]. Accordingly, complement components were also shown to play a key role on microglial-mediated clearance of neurites in vitro. Linnartz and colleagues [110] have shown that enzymatic removal of sialic acid residues from cultured neurons triggers the binding of microglia-derived opsonin C1q to neurites. In turn, microglial cells recognize alterations in neuronal glycocalyx and phagocytose these structures via C3R. These results suggest that molecules present in neuronal glycocalyx are key elements in the modulation of microglia-mediated phagocytosis during synaptic pruning [110,111].
(d). Microglia-mediated phagocytosis and neural circuitry shaping
Microglia-mediated phagocytosis of newborn neurons also has been shown as an important process for the shaping of neural circuitry. During development, programmed cell death occurs among several cells of neuronal lineage [112]. These cells undergo apoptosis, triggered by proteolytic cascades mediated by caspases, resulting in DNA fragmentation and membrane blebbing [113,114]. These apoptotic cells include both postmitotic neurons and proliferating neuroblasts that are effectively removed from CNS parenchyma by microglia. In the developing cerebellum, the microglial release of superoxides triggers cell death in a fraction of newborn neurons, and the resulting apoptotic cells (caspase3+) are phagocytized by microglia [11].
Interestingly, in the adult CNS, microglial-mediated phagocytosis of apoptotic neuroblasts also occurs. In the mammalian brain, two discrete regions exhibit persistent neurogenesis: the subgranular zone (SGZ) in the dentate gyrus of the hippocampus and the SVZ, which lines the walls of the LVs [115–117]. Postnatally generated cells include glutamatergic and GABAergic neurons that populate the dentate gyrus of the hippocampus and are integrated into neural circuitry during learning and memory formation processes. Alternatively, neuroblasts generated in the postnatal SVZ migrate through a long pathway, the rostral migratory stream, towards their final destination in the glomerular and granule cell layers of the olfactory bulb (OB) [118]. Within the OB, neuroblasts differentiate into GABAergic and to a lesser extent glutamatergic cells, which are integrated as juxtaglomerular neurons [115,119,120]. These two neurogenic sites both harbour neural stem cells that in turn give rise to more rapidly dividing transit-amplifying cells (TACs). These rapidly dividing TACs in turn generate lineage-restricted neuroblasts, which are functionally integrated into neuronal circuits [115,121]. Yet despite the abundant production of new neurons in these adult germinative zones, only a small proportion of newborn cells are functionally integrated [122]. The majority of neuronal precursors generated in the hippocampal SGZ undergo apoptosis at early stages, during the transition from TACs to neuroblasts, and these newborn cells are phagocytosed by microglia [15]. Notably, the mechanisms underlying the microglial phagocytosis of newborn cells in this neurogenic niche remain elusive. Tyro3, Axl and Mer (TAM) receptor tyrosine kinases and their ligands Gas6 and protein S have been shown as key elements for the efficient phagocytosis of apoptotic cells in the mature immune, nervous and reproductive systems [123]. As such, the activity of these signalling pathways might govern microglial-mediated phagocytosis in this adult neurogenic niche. Moreover, whereas several studies have addressed the roles of microglia in shaping adult hippocampal circuitry, few have addressed the potentially analogous functions of microglial cells in the SVZ and OB [121,124,125]. Indeed, both the functions of microglia and regulation thereof in these extensive neurogenic niches remain unclear.
6. Concluding remarks
For several decades, microglia were considered the brain immunoeffector cells, chiefly in charge of antigen removal from the neural parenchyma. There is no doubt that microglia play important protective roles in the setting of acute CNS injury. It is also likely that microglial activation ultimately can hamper CNS health in the setting of prolonged activation, such as described in Alzheimer's disease. The fine balance between protective and harmful effects of microglia activation have been discussed in several excellent reviews [6,16,26,89,90]. However, the observation that microglia cells dynamically survey the healthy, intact brain [7] represented a turning point in microglial studies. Many subsequent studies have broadened the implications of this observation and it is now established that microglial cells are critically important for neural circuitry establishment during development. Microglial cells are in intimate contact with both neural projections and synapses. Interestingly, microglia use the same machinery, including cyto- and chemokine release as well as phagocytosis, to shape and refine neural connections. These observations question the dichotomy of ‘resting’ versus ‘activated’ microglia, as well the limited view of microglia as immune effectors and, in so doing, place microglia as integral elements of the CNS.
References
- 1.Jordan FL, Thomas WE. 1988. Brain macrophages: questions of origin and interrelationship. Brain Res. 472, 165–178. ( 10.1016/0165-0173(88)90019-7) [DOI] [PubMed] [Google Scholar]
- 2.Guillemin GJ, Brew BJ. 2004. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J. Leukoc. Biol. 75, 388–397. ( 10.1189/jlb.0303114) [DOI] [PubMed] [Google Scholar]
- 3.Ransohoff RM, Cardona AE. 2010. The myeloid cells of the central nervous system parenchyma. Nature 468, 253–262. ( 10.1038/nature09615) [DOI] [PubMed] [Google Scholar]
- 4.Aguzzi A, Barres BA, Bennett ML. 2013. Microglia: scapegoat, saboteur, or something else? Science 339, 156–161. ( 10.1126/science.1227901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivest S. 2009. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 9, 429–439. ( 10.1038/nri2565) [DOI] [PubMed] [Google Scholar]
- 6.Prinz M, Priller J, Sisodia SS, Ransohoff RM. 2011. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 14, 1227–1235. ( 10.1038/nn.2923) [DOI] [PubMed] [Google Scholar]
- 7.Nimmerjahn A, Kirchhoff F, Helmchen F. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. ( 10.1126/science.1110647) [DOI] [PubMed] [Google Scholar]
- 8.Perry VH, O'Connor V. 2010. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. Asn Neuro 2, e00047 ( 10.1042/AN20100024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boche D, Perry VH, Nicoll JA. 2013. Review: activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 39, 3–18. ( 10.1111/nan.12011) [DOI] [PubMed] [Google Scholar]
- 10.Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. 2013. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 16, 543–551. ( 10.1038/nn.3358) [DOI] [PubMed] [Google Scholar]
- 11.Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. 2004. Microglia promote the death of developing Purkinje cells. Neuron 41, 535–547. ( 10.1016/S0896-6273(04)00069-8) [DOI] [PubMed] [Google Scholar]
- 12.Tremblay ME, Lowery RL, Majewska AK. 2010. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527 ( 10.1371/journal.pbio.1000527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paolicelli RC, et al. 2011. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. ( 10.1126/science.1202529) [DOI] [PubMed] [Google Scholar]
- 14.Schafer DP, et al. 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. ( 10.1016/j.neuron.2012.03.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. 2010. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495. ( 10.1016/j.stem.2010.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. 2011. Physiology of microglia. Physiol. Rev. 91, 461–553. ( 10.1152/physrev.00011.2010) [DOI] [PubMed] [Google Scholar]
- 17.Alliot F, Godin I, Pessac B. 1999. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Dev. Brain Res. 117, 145–152. ( 10.1016/S0165-3806(99)00113-3) [DOI] [PubMed] [Google Scholar]
- 18.Ginhoux F, et al. 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. ( 10.1126/science.1194637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kierdorf K, et al. 2013. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280. ( 10.1038/nn.3318) [DOI] [PubMed] [Google Scholar]
- 20.Hayashi Y, Ishibashi H, Hashimoto K, Nakanishi H. 2006. Potentiation of the NMDA receptor-mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia 53, 660–668. ( 10.1002/glia.20322) [DOI] [PubMed] [Google Scholar]
- 21.Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. 2012. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl Acad. Sci. USA 109, E197–E205. ( 10.1073/pnas.1111098109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. 2011. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 31, 16 241–16 250. ( 10.1523/JNEUROSCI.3667-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stellwagen D, Malenka RC. 2006. Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059. ( 10.1038/nature04671) [DOI] [PubMed] [Google Scholar]
- 24.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. 2008. Tumor necrosis factor-α mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58, 673–680. ( 10.1016/j.neuron.2008.04.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. 2009. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980. ( 10.1523/JNEUROSCI.4363-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry VH, Nicoll JA, Holmes C. 2010. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 6, 193–201. ( 10.1038/nrneurol.2010.17) [DOI] [PubMed] [Google Scholar]
- 27.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. 2011. The role of microglia in the healthy brain. J. Neurosci. 31, 16 064–16 069. ( 10.1523/JNEUROSCI.4158-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schafer DP, Lehrman EK, Stevens B. 2013. The ‘quad-partite’ synapse: microglia–synapse interactions in the developing and mature CNS. Glia 61, 24–36. ( 10.1002/glia.22389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao C, Richardson A, Fedoroff S. 1991. Macrophage-like cells originate from neuroepithelium in culture: characterization and properties of the macrophage-like cells. Int. J. Dev. Neurosci. 9, 1–14. ( 10.1016/0736-5748(91)90067-V) [DOI] [PubMed] [Google Scholar]
- 30.Fedoroff S, Hao C. 1991. Origin of microglia and their regulation by astroglia. Adv. Exp. Med. Biol. 296, 135–142. ( 10.1007/978-1-4684-8047-4_14) [DOI] [PubMed] [Google Scholar]
- 31.Fedoroff S, Zhai R, Novak JP. 1997. Microglia and astroglia have a common progenitor cell. J. Neurosci. Res. 50, 477–486. () [DOI] [PubMed] [Google Scholar]
- 32.Perry VH, Hume DA, Gordon S. 1985. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15, 313–326. ( 10.1016/0306-4522(85)90215-5) [DOI] [PubMed] [Google Scholar]
- 33.Lawson LJ, Perry VH, Dri P, Gordon S. 1990. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170. ( 10.1016/0306-4522(90)90229-W) [DOI] [PubMed] [Google Scholar]
- 34.Lawson LJ, Perry VH, Gordon S. 1992. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48, 405–415. ( 10.1016/0306-4522(92)90500-2) [DOI] [PubMed] [Google Scholar]
- 35.Klemsz MJ, McKercher SR, Celada A, Van Beveren C, Maki RA. 1990. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell 61, 113–124. ( 10.1016/0092-8674(90)90219-5) [DOI] [PubMed] [Google Scholar]
- 36.Weisz A, Marx P, Sharf R, Appella E, Driggers PH, Ozato K, Levi BZ. 1992. Human interferon consensus sequence binding protein is a negative regulator of enhancer elements common to interferon-inducible genes. J. Biol. Chem. 267, 25 589–25 596. [PubMed] [Google Scholar]
- 37.Boya J, Calvo JL, Carbonell AL, Borregon A. 1991. A lectin histochemistry study on the development of rat microglial cells. J. Anat. 175, 229–236. [PMC free article] [PubMed] [Google Scholar]
- 38.Boya J, Carbonell AL, Calvo J, Borregon A. 1987. Ultrastructural study on the origin of rat microglia cells. Acta Anatom. 130, 329–335. ( 10.1159/000146465) [DOI] [PubMed] [Google Scholar]
- 39.Cuadros MA, Moujahid A, Quesada A, Navascues J. 1994. Development of microglia in the quail optic tectum. J. Comp. Neurol. 348, 207–224. ( 10.1002/cne.903480204) [DOI] [PubMed] [Google Scholar]
- 40.Moujahid A, Navascues J, Marin-Teva JL, Cuadros MA. 1996. Macrophages during avian optic nerve development: relationship to cell death and differentiation into microglia. Anat. Embryol. 193, 131–144. ( 10.1007/BF00214704) [DOI] [PubMed] [Google Scholar]
- 41.Dalmau I, Finsen B, Tonder N, Zimmer J, Gonzalez B, Castellano B. 1997. Development of microglia in the prenatal rat hippocampus. J. Comp. Neurol. 377, 70–84. () [DOI] [PubMed] [Google Scholar]
- 42.Cuadros MA, Navascues J. 1998. The origin and differentiation of microglial cells during development. Progress Neurobiol. 56, 173–189. ( 10.1016/S0301-0082(98)00035-5) [DOI] [PubMed] [Google Scholar]
- 43.Lelli A, Gervais A, Colin C, Cheret C, Ruiz de Almodovar C, Carmeliet P, Krause KH, Boillee S, Mallat M. 2013. The NADPH oxidase Nox2 regulates VEGFR1/CSF-1R-mediated microglial chemotaxis and promotes early postnatal infiltration of phagocytes in the subventricular zone of the mouse cerebral cortex. Glia 61, 1542–1555. ( 10.1002/glia.22540) [DOI] [PubMed] [Google Scholar]
- 44.Calvo CF, Dobbertin A, Gelman M, Glowinski J, Mallat M. 1998. Identification of CSF-1 as a brain macrophage migratory activity produced by astrocytes. Glia 24, 180–186. () [DOI] [PubMed] [Google Scholar]
- 45.Kwan KY, Sestan N, Anton ES. 2012. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development 139, 1535–1546. ( 10.1242/dev.069963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuadros MA, Rodriguez-Ruiz J, Calvente R, Almendros A, Marin-Teva JL, Navascues J. 1997. Microglia development in the quail cerebellum. J. Comp. Neurol. 389, 390–401. () [DOI] [PubMed] [Google Scholar]
- 47.Marin-Teva JL, Almendros A, Calvente R, Cuadros MA, Navascues J. 1998. Tangential migration of ameboid microglia in the developing quail retina: mechanism of migration and migratory behavior. Glia 22, 31–52. () [DOI] [PubMed] [Google Scholar]
- 48.Rigato C, Buckinx R, Le-Corronc H, Rigo JM, Legendre P. 2011. Pattern of invasion of the embryonic mouse spinal cord by microglial cells at the time of the onset of functional neuronal networks. Glia 59, 675–695. ( 10.1002/glia.21140) [DOI] [PubMed] [Google Scholar]
- 49.Rezaie P, Male D. 1999. Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc. Res. Tech. 45, 359–382. () [DOI] [PubMed] [Google Scholar]
- 50.Rezaie P, Patel K, Male DK. 1999. Microglia in the human fetal spinal cord—patterns of distribution, morphology and phenotype. Dev. Brain Res. 115, 71–81. ( 10.1016/S0165-3806(99)00043-7) [DOI] [PubMed] [Google Scholar]
- 51.Imamoto K, Leblond CP. 1978. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J. Comp. Neurol. 180, 139–163. ( 10.1002/cne.901800109) [DOI] [PubMed] [Google Scholar]
- 52.Mallat M, Calvo CF, Dobbertin A. 1997. Migration and proliferation of mononuclear phagocytes in the central nervous system. Adv. Exp. Med. Biol. 429, 99–108. ( 10.1007/978-1-4757-9551-6_7) [DOI] [PubMed] [Google Scholar]
- 53.Dalmau I, Vela JM, Gonzalez B, Finsen B, Castellano B. 2003. Dynamics of microglia in the developing rat brain. J. Comp. Neurol. 458, 144–157. ( 10.1002/cne.10572) [DOI] [PubMed] [Google Scholar]
- 54.Fujita S, Tsuchihashi Y, Kitamura T. 1981. Origin, morphology and function of the microglia. Progress Clin. Biol. Res. 59A, 141–169. [PubMed] [Google Scholar]
- 55.Murabe Y, Sano Y. 1981. Thiaminepyrophosphatase activity in the plasma membrane of microglia. Histochemistry 71, 45–52. ( 10.1007/BF00592569) [DOI] [PubMed] [Google Scholar]
- 56.Peters AM, Vassilarou DS, Hows JM, Ballardie FW. 1991. Bone marrow transplantation: effects of conditioning and cyclosporin prophylaxis on microvascular permeability to a small solute (technetium 99 m diethylene triamine penta-acetic acid). Eur. J. Nuclear Med. 18, 199–202. ( 10.1007/BF02262731) [DOI] [PubMed] [Google Scholar]
- 57.Banati RB, Graeber MB. 1994. Surveillance, intervention and cytotoxicity: is there a protective role of microglia? Dev. Neurosci. 16, 114–127. ( 10.1159/000112098) [DOI] [PubMed] [Google Scholar]
- 58.Barron KD. 1995. The microglial cell. A historical review. J. Neurol. Sci. 134(Suppl.), 57–68. ( 10.1016/0022-510X(95)00209-K) [DOI] [PubMed] [Google Scholar]
- 59.Carson MJ, Bilousova TV, Puntambekar SS, Melchior B, Doose JM, Ethell IM. 2007. A rose by any other name? The potential consequences of microglial heterogeneity during CNS health and disease. Neurotherapeutics 4, 571–579. ( 10.1016/j.nurt.2007.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olah M, Biber K, Vinet J, Boddeke HW. 2011. Microglia phenotype diversity. CNS Neurol. Disorders Drug Targets 10, 108–118. ( 10.2174/187152711794488575) [DOI] [PubMed] [Google Scholar]
- 61.Milner R, Campbell IL. 2002. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the α6β1 integrin. J. Neurosci. 22, 1562–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribes S, et al. 2010. Fibronectin stimulates Escherichia coli phagocytosis by microglial cells. Glia 58, 367–376. ( 10.1002/glia.20929) [DOI] [PubMed] [Google Scholar]
- 63.Si Q, Nakamura Y, Kataoka K. 2000. A serum factor enhances production of nitric oxide and tumor necrosis factor-α from cultured microglia. Exp. Neurol. 162, 89–97. ( 10.1006/exnr.2000.7334) [DOI] [PubMed] [Google Scholar]
- 64.Zhao TZ, Xia YZ, Li L, Li J, Zhu G, Chen S, Feng H, Lin JK. 2009. Bovine serum albumin promotes IL-1β and TNF-α secretion by N9 microglial cells. Neurol. Sci. 30, 379–383. ( 10.1007/s10072-009-0123-x) [DOI] [PubMed] [Google Scholar]
- 65.Block ML, Zecca L, Hong JS. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69. ( 10.1038/nrn2038) [DOI] [PubMed] [Google Scholar]
- 66.Lucin KM, Wyss-Coray T. 2009. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64, 110–122. ( 10.1016/j.neuron.2009.08.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giulian D, Haverkamp LJ, Li J, Karshin WL, Yu J, Tom D, Li X, Kirkpatrick JB. 1995. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem. Int. 27, 119–137. ( 10.1016/0197-0186(95)00067-I) [DOI] [PubMed] [Google Scholar]
- 68.Kreutzberg GW. 1996. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318. ( 10.1016/0166-2236(96)10049-7) [DOI] [PubMed] [Google Scholar]
- 69.Streit WJ. 2000. Microglial response to brain injury: a brief synopsis. Toxicol. Pathol. 28, 28–30. ( 10.1177/019262330002800104) [DOI] [PubMed] [Google Scholar]
- 70.Colton CA. 2009. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 4, 399–418. ( 10.1007/s11481-009-9164-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ransohoff RM. 2009. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31, 711–721. ( 10.1016/j.immuni.2009.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colton CA, Gilbert DL. 1987. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 223, 284–288. ( 10.1016/0014-5793(87)80305-8) [DOI] [PubMed] [Google Scholar]
- 73.Gregersen R, Lambertsen K, Finsen B. 2000. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J. Cereb. Blood Flow Metab. 20, 53–65. ( 10.1097/00004647-200001000-00009) [DOI] [PubMed] [Google Scholar]
- 74.Nadeau S, Rivest S. 2000. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J. Neurosci. 20, 3456–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu JS, Amaral TD, Brosnan CF, Lee SC. 1998. IFNs are critical regulators of IL-1 receptor antagonist and IL-1 expression in human microglia. J. Immunol. 161, 1989–1996. [PubMed] [Google Scholar]
- 76.Hanisch UK, Kettenmann H. 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394. ( 10.1038/nn1997) [DOI] [PubMed] [Google Scholar]
- 77.Davoust N, Vuaillat C, Androdias G, Nataf S. 2008. From bone marrow to microglia: barriers and avenues. Trends Immunol. 29, 227–234. ( 10.1016/j.it.2008.01.010) [DOI] [PubMed] [Google Scholar]
- 78.Colton C, Wilcock DM. 2010. Assessing activation states in microglia. CNS Neurol. Disorders Drug Targets 9, 174–191. ( 10.2174/187152710791012053) [DOI] [PubMed] [Google Scholar]
- 79.Graeber MB. 2010. Changing face of microglia. Science 330, 783–788. ( 10.1126/science.1190929) [DOI] [PubMed] [Google Scholar]
- 80.Graeber MB, Streit WJ. 2010. Microglia: biology and pathology. Acta Neuropathol. 119, 89–105. ( 10.1007/s00401-009-0622-0) [DOI] [PubMed] [Google Scholar]
- 81.Hanisch UK. 2002. Microglia as a source and target of cytokines. Glia 40, 140–155. ( 10.1002/glia.10161) [DOI] [PubMed] [Google Scholar]
- 82.Biber K, Vinet J, Boddeke HW. 2008. Neuron–microglia signaling: chemokines as versatile messengers. J. Neuroimmunol. 198, 69–74. ( 10.1016/j.jneuroim.2008.04.012) [DOI] [PubMed] [Google Scholar]
- 83.Rothwell NJ, Hopkins SJ. 1995. Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci. 18, 130–136. ( 10.1016/0166-2236(95)93890-A) [DOI] [PubMed] [Google Scholar]
- 84.Tambuyzer BR, Ponsaerts P, Nouwen EJ. 2009. Microglia: gatekeepers of central nervous system immunology. J. Leukoc. Biol. 85, 352–370. ( 10.1189/jlb.0608385) [DOI] [PubMed] [Google Scholar]
- 85.Rollins BJ. 1997. Chemokines. Blood 90, 909–928. [PubMed] [Google Scholar]
- 86.Bogdan C, Vodovotz Y, Nathan C. 1991. Macrophage deactivation by interleukin 10. J. Exp. Med. 174, 1549–1555. ( 10.1084/jem.174.6.1549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamilton TA, Ohmori Y, Tebo JM, Kishore R. 1999. Regulation of macrophage gene expression by pro- and anti-inflammatory cytokines. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 67, 241–244. ( 10.1159/000028101) [DOI] [PubMed] [Google Scholar]
- 88.Gordon S, Taylor PR. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. ( 10.1038/nri1733) [DOI] [PubMed] [Google Scholar]
- 89.Streit WJ. 2002. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40, 133–139. ( 10.1002/glia.10154) [DOI] [PubMed] [Google Scholar]
- 90.Ransohoff RM, Perry VH. 2009. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145. ( 10.1146/annurev.immunol.021908.132528) [DOI] [PubMed] [Google Scholar]
- 91.Nolte C, Moller T, Walter T, Kettenmann H. 1996. Complement 5a controls motility of murine microglial cells in vitro via activation of an inhibitory G-protein and the rearrangement of the actin cytoskeleton. Neuroscience 73, 1091–1107. ( 10.1016/0306-4522(96)00106-6) [DOI] [PubMed] [Google Scholar]
- 92.Stence N, Waite M, Dailey ME. 2001. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33, 256–266. () [DOI] [PubMed] [Google Scholar]
- 93.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. 2000. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114. ( 10.1128/MCB.20.11.4106-4114.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758. ( 10.1038/nn1472) [DOI] [PubMed] [Google Scholar]
- 95.Kettenmann H, Kirchhoff F, Verkhratsky A. 2013. Microglia: new roles for the synaptic stripper. Neuron 77, 10–18. ( 10.1016/j.neuron.2012.12.023) [DOI] [PubMed] [Google Scholar]
- 96.Beattie MS, Ferguson AR, Bresnahan JC. 2010. AMPA-receptor trafficking and injury-induced cell death. Eur. J. Neurosci. 32, 290–297. ( 10.1111/j.1460-9568.2010.07343.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stellwagen D, Beattie EC, Seo JY, Malenka RC. 2005. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. J. Neurosci. 25, 3219–3228. ( 10.1523/JNEUROSCI.4486-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P. 2006. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-α and prostaglandins. J. Biol. Chem. 281, 30 684–30 696. ( 10.1074/jbc.M606429200) [DOI] [PubMed] [Google Scholar]
- 99.Zeng JW, Liu XH, Zhang JH, Wu XG, Ruan HZ. 2008. P2Y1 receptor-mediated glutamate release from cultured dorsal spinal cord astrocytes. J. Neurochem. 106, 2106–2118. ( 10.1111/j.1471-4159.2008.05560.x) [DOI] [PubMed] [Google Scholar]
- 100.Zhan Y, et al. 2014. Deficient neuron–microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406. ( 10.1038/nn.3641) [DOI] [PubMed] [Google Scholar]
- 101.Chen C, Regehr WG. 2000. Developmental remodeling of the retinogeniculate synapse. Neuron 28, 955–966. ( 10.1016/S0896-6273(00)00166-5) [DOI] [PubMed] [Google Scholar]
- 102.Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. 2005. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis. Neurosci. 22, 661–676. ( 10.1017/S0952523805225154) [DOI] [PubMed] [Google Scholar]
- 103.Ziburkus J, Guido W. 2006. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J. Neurophysiol. 96, 2775–2784. ( 10.1152/jn.01321.2004) [DOI] [PubMed] [Google Scholar]
- 104.Hooks BM, Chen C. 2006. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52, 281–291. ( 10.1016/j.neuron.2006.07.007) [DOI] [PubMed] [Google Scholar]
- 105.Del Rio T, Feller MB. 2006. Early retinal activity and visual circuit development. Neuron 52, 221–222. ( 10.1016/j.neuron.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 106.Feller MB. 1999. Spontaneous correlated activity in developing neural circuits. Neuron 22, 653–656. ( 10.1016/S0896-6273(00)80724-2) [DOI] [PubMed] [Google Scholar]
- 107.Penn AA, Riquelme PA, Feller MB, Shatz CJ. 1998. Competition in retinogeniculate patterning driven by spontaneous activity. Science 279, 2108–2112. ( 10.1126/science.279.5359.2108) [DOI] [PubMed] [Google Scholar]
- 108.Torborg CL, Hansen KA, Feller MB. 2005. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat. Neurosci. 8, 72–78. ( 10.1038/nn1376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stevens B, et al. 2007. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. ( 10.1016/j.cell.2007.10.036) [DOI] [PubMed] [Google Scholar]
- 110.Linnartz B, Kopatz J, Tenner AJ, Neumann H. 2012. Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J. Neurosci. 32, 946–952. ( 10.1523/JNEUROSCI.3830-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Linnartz B, Neumann H. 2013. Microglial activatory (immunoreceptor tyrosine-based activation motif)- and inhibitory (immunoreceptor tyrosine-based inhibition motif)-signaling receptors for recognition of the neuronal glycocalyx. Glia 61, 37–46. ( 10.1002/glia.22359) [DOI] [PubMed] [Google Scholar]
- 112.de la Rosa EJ, de Pablo F. 2000. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci. 23, 454–458. ( 10.1016/S0166-2236(00)01628-3) [DOI] [PubMed] [Google Scholar]
- 113.Porter AG, Janicke RU. 1999. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6, 99–104. ( 10.1038/sj.cdd.4400476) [DOI] [PubMed] [Google Scholar]
- 114.Yuan J, Yankner BA. 2000. Apoptosis in the nervous system. Nature 407, 802–809. ( 10.1038/35037739) [DOI] [PubMed] [Google Scholar]
- 115.Zhao C, Deng W, Gage FH. 2008. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660. ( 10.1016/j.cell.2008.01.033) [DOI] [PubMed] [Google Scholar]
- 116.Whitman MC, Greer CA. 2009. Adult neurogenesis and the olfactory system. Progress Neurobiol. 89, 162–175. ( 10.1016/j.pneurobio.2009.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ihrie RA, Alvarez-Buylla A. 2011. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 70, 674–686. ( 10.1016/j.neuron.2011.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. 1996. Chain migration of neuronal precursors. Science 271, 978–981. ( 10.1126/science.271.5251.978) [DOI] [PubMed] [Google Scholar]
- 119.Brill MS, et al. 2009. Adult generation of glutamatergic olfactory bulb interneurons. Nat. Neurosci. 12, 1524–1533. ( 10.1038/nn.2416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sequerra EB, Miyakoshi LM, Froes MM, Menezes JR, Hedin-Pereira C. 2010. Generation of glutamatergic neurons from postnatal and adult subventricular zone with pyramidal-like morphology. Cereb. Cortex 20, 2583–2591. ( 10.1093/cercor/bhq006) [DOI] [PubMed] [Google Scholar]
- 121.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. 1997. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma DK, Kim WR, Ming GL, Song H. 2009. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann. NY Acad. Sci. 1170, 664–673. ( 10.1111/j.1749-6632.2009.04373.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lemke G, Burstyn-Cohen T. 2010. TAM receptors and the clearance of apoptotic cells. Ann. NY Acad. Sci. 1209, 23–29. ( 10.1111/j.1749-6632.2010.05744.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caggiano AO, Brunjes PC. 1993. Microglia and the developing olfactory bulb. Neuroscience 52, 717–724. ( 10.1016/0306-4522(93)90420-K) [DOI] [PubMed] [Google Scholar]
- 125.Mercier F, Kitasako JT, Hatton GI. 2002. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J. Comp. Neurol. 451, 170–188. ( 10.1002/cne.10342) [DOI] [PubMed] [Google Scholar]