Abstract
Although it is considered to be the most complex organ in the body, the brain can be broadly classified into two major types of cells, neuronal cells and glial cells. Glia is a general term that encompasses multiple types of non-neuronal cells that function to maintain homeostasis, form myelin, and provide support and protection for neurons. Astrocytes, a major class of glial cell, have historically been viewed as passive support cells, but recently it has been discovered that astrocytes participate in signalling activities both with the vasculature and with neurons at the synapse. These cells have been shown to release d-serine, TNF-α, glutamate, atrial natriuretic peptide (ANP) and ATP among other signalling molecules. ATP and its metabolites are well established as important signalling molecules, and astrocytes represent a major source of ATP release in the nervous system. Novel molecular and genetic tools have recently shown that astrocytic release of ATP and other signalling molecules has a major impact on synaptic transmission. Via actions at the synapse, astrocytes have now been shown to regulate complex network signalling in the whole organism with impacts on respiration and the sleep–wake cycle. In addition, new roles for astrocytes are being uncovered in psychiatric disorders, and astrocyte signalling mechanisms represents an attractive target for novel therapeutic agents.
Keywords: glia, astrocyte, adenosine, synapse, sleep, depression
1. Introduction
Astrocytes have historically been viewed as passive support cells, but recently it has been discovered that astrocytes participate in signalling activities both with the vasculature and with neurons at the synapse. However, the concept of astrocytes as active participants is not entirely new: in 1895 Santiago Ram'on y Cajal proposed that astrocytes control sleep and waking states [1]. Cajal hypothesized that astrocytic processes act as insulators surrounding neurons to facilitate sleep, and then retracting to allow neuronal communication facilitating wakefulness [2]. A century of research since the time of Cajal has provided support for parts of his original suggestion. We now know that astrocytes have both structural and functional links with neurons and via these links are able to modulate complex behaviours including sleep [3] and contribute to disorders of the brain including depression [4].
2. Astrocyte signalling
As mentioned above, Cajal systematically studied astrocytes from a structural standpoint [2], and until very recently, our understanding of astrocytic morphology has been largely based on Cajal's metal impregnation methods, or on glial fibrillary acidic protein (GFAP) staining. These methods both permit static endpoint assessment of cells, providing only a snapshot of their structure and function. Novel labelling and imaging technologies have revealed that the structure of astrocytes is far more complicated than previously appreciated. In conjunction with GFAP staining, researchers filled astrocytes with fluorescent dyes, revealing that GFAP staining only represented 15% of the true astrocytic volume and that astrocytes extend numerous fine processes virtually filling the surrounding neuropil [5]. These studies also revealed that astrocytes occupy non-overlapping spatial territories, and that a single astrocyte contacts hundreds of neuronal processes and multiple neuronal cell bodies within these territories [5].
In addition to their contact with neurons, astrocytes are known to line the vasculature with their endfeet [6]. This position between neurons and blood vessels allows astrocytes to mediate neurovascular coupling, the process by which neuronal activity and metabolic demands are coupled to blood flow. Astrocytes play essential roles in brain energy homeostasis and metabolism which is again mediated by their close link with the vasculature, and astrocytes are known to express the machinery required for the uptake of glucose from blood vessels.
By controlling the metabolic and ionic milieu surrounding neurons, astrocytes can dramatically impact neuronal activity. The numerous processes of a single astrocyte contact tens of thousands of synapses, and examinations of diverse brain regions have shown that up to 50% of excitatory synapses are closely coupled to an astrocytic process [7]. This close connection between synapses and astrocytic processes is both structural and functional, and has been termed the tripartite synapse (figure 1) [8]. Evidence from many groups of researchers now supports the concept that astrocytes act as both ‘listening’ and ‘talking’ participants in the tripartite synapse via multiple regulated signalling pathways [9].
Figure 1.
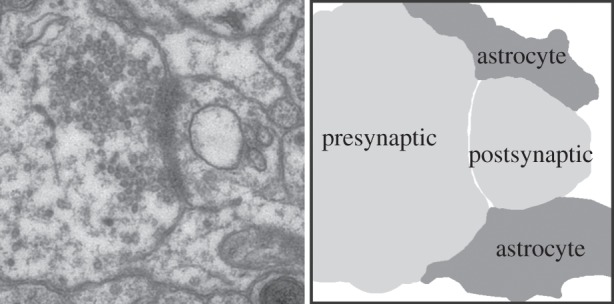
The tripartite synapse composed of physically and functionally interacting synaptic terminals and astrocyte processes. The electron micrograph on the left shows a hippocampal synapse surrounded by astrocyte process end feet. On the right, outlines of the components of the tripartite synapse clearly show the astrocytes end feet (dark grey) tightly surrounding the presynaptic and postsynaptic compartments (light grey), providing a tight seal around the sight of neurotransmission.
Recent attention has turned to how astrocytes may actively be sending signals to the neurons to which they are coupled, as opposed to passively monitoring the signalling activities of neurons. In addition to the established roles of astrocytes in controlling the ionic environment of the neuropil and controlling the supply of neurotransmitters to synapses, astrocytes are now known to regulate neuronal signalling by direct communication via regulated release of signalling molecules. Astrocytes have now been shown to signal via many chemical transmitters, including classical transmitters, peptides, chemokines and cytokines, and they achieve this diverse communication through a number of different release mechanisms. The varied signalling systems of astrocytes can have complex downstream effects on neurons. For example, glutamate and d-serine released from astrocytes enhances N-methyl-d-aspartate (NMDA) receptor-mediated current and results in excitatory feedback to neurons [10], whereas the release of ATP from astrocytes (converted to extracellular adenosine and acting on presynaptic adenosine A1 receptors) ultimately results in inhibition of synaptic transmission [11].
Multiple mechanisms and modes of release have been proposed for astrocytes signals, and it is thought that these may operate under different physiological or pathological contexts. Studies have described connexin/gap junction [12–14], volume-regulated anion channel [15,16] and exocytotic release [17–19] mechanisms operating in astrocytes.
3. Astrocytes signal via exocytosis
Exocytosis is a well-characterized process of release that is known to occur in multiple cell types. Exocytosis is a regulated process that depends upon docking and fusion of vesicles to the plasma membrane, achieved by the formation of the soluble NSF (N-ethylmaleimide-sensitive fusion) protein attachment protein receptor (SNARE) complex. Proteins that make up the SNARE complex are small, abundant and are primarily membrane bound. Despite diverse structures, all SNARE proteins share a segment in their cytosolic domain called a SNARE motif consisting of 60–70 amino acids that assemble into tight, four-helix bundles called ‘trans’-SNARE complexes. Core SNARE complex machinery proteins are expressed in astrocytes, including synaptobrevin II [20,21] and its homologue cellubrevin [22] and SNAP-23 [20,21]. Ancillary proteins for exocytosis are also expressed in astrocytes, such as Munc 18 [20,23], complexin 2 [20,21] and synaptotagmin IV [23].
Beyond molecular identification of SNARE protein expression, astrocytes have been shown to possess vesicular structures. Electron microscopic studies from tissue have revealed the existence of clear astrocytic vesicles of appropriate diameter in the vicinity of presynaptic terminals [24], which strongly supports the existence of a vesicular pathway of gliotransmission in the intact brain. SNARE proteins have been shown to colocalize with a number of vesicular organelles in cultured astrocytes, including small vesicles positive for vesicular glutamate transporters (VGlut 1–3) [20–23], ATP-storing vesicles [18,25], and d-serine-containing vesicles [26], suggesting the involvement of vesicular mechanisms in the release of these signalling molecules from astrocytes.
A turning point in our understanding of astrocyte signalling resulted from calcium imaging studies showing that cultured astrocytes release glutamate and lead to elevation of calcium in nearby neurons [27]. Several subsequent studies demonstrated the occurrence of this process in acute brain slices [28–33] and in vivo [34]. Since these pioneering studies, astrocytes have been shown to release a number of chemical transmitters, including ATP [35–37], d-serine [10,38], TNF-α [39] and atrial natriuretic peptide (ANP) [40], in a process that is now collectively termed gliotransmission.
4. ATP gliotransmission
ATP is a major extracellular messenger that coordinates the function of astrocytes and communication between them and other cell types [14]. The mechanism by which astrocytes release ATP is not completely understood, but support for an exocytotic mechanism has emerged. In particular, electrophysiological studies have shown calcium-dependent changes in the area of the plasma membrane in single astrocytes, reflecting calcium-regulated vesicle fusion. Quinacrine binding of ATP in peptidergic vesicles has shown that ATP is stored in secretory vesicles with peptides such as ANP within astrocytes [19]. In these studies, ionomycin treatment decreased the total image fluorescence and the number of quinacrine-stained vesicles, suggesting exocytosis of these vesicles following treatment [19]. Other studies have recorded from HEK-293 T cells transfected with a mutated purinergic receptor P2X3 (D266A) that has reduced desensitization while retaining receptor affinity [41]. In D266A expressing HEK-293 T cells neighboured by astrocytes, small, transient, inward currents (STICs) with kinetic properties suggestive of quantal release could be detected [19]. Addition of glutamate to stimulate astrocytes increased the average frequency of STICs in expressing HEK-293 T cells. Because HEK-293 T cells do not respond to glutamate directly, it can be assumed that the increased frequency of recorded STICs resulted from astrocyte release. Calcium free solution reduced STICs in both resting and glutamate-stimulated conditions consistent with the requirement of calcium for SNARE mediated exocytosis. Recent research by Lalo et al. [42] has shown a significant difference in the baseline amplitude of miniature inhibitory currents in wild-type and dn-SNARE mice. This shows conclusive evidence that vesicular release of gliotransmitters may be involved in the long-term homeostatic regulation of inhibitory neurotransmission [42].
Other studies have shown reduction in the release of ATP by inhibitors of anion channels [16], ATP-binding cassette proteins or cystic fibrosis transmembrane conductance regulator [43], gap junctions [13] and P2X7 receptors, which suggests the involvement of multiple pathways in ATP release from astrocytes.
ATP hydrolysis is well understood to lead to the accumulation of adenosine; however, the origin and mechanism of adenosine accumulation in the brain was not clearly revealed until the seminal study of Pascual et al. [44]. These studies used a molecular genetic strategy to perturb gliotransmission via conditional, astrocyte-specific expression of a dominant negative inhibitor of SNARE-dependent membrane fusion (the cytoplasmic tail of synaptobrevin 2; dnSNARE). Recordings from the hippocampal Schaffer collateral-CA1 synapses in mice expressing dnSNARE revealed enhanced synaptic transmission compared to wild-type littermates, or transgenic mice in which transgene expression was prevented by doxycycline in the rodent chow [44]. Also, it has been noted [45] that dnSNARE mice, and mice injected with dnSNARE virus do not show alterations in astrocyte morphology from wild-type controls. These studies went on to show that blocking exocytosis from astrocytes using dnSNARE reduced ATP and its metabolite adenosine, which would normally exert tonic suppression of synaptic transmission.
5. Astrocytic regulation of ATP at synapses
Release of ATP from astrocytes has now been shown to be important for modulation of multiple neuronal signalling pathways with implications for behavioural output. For example, in hypothalamic slices, release of ATP from astrocytes is both necessary and sufficient for noradrenaline-dependent synaptic potentiation [46]. Following adrenergic stimuli, signalling via α1-adrenergic receptors expressed on astrocytes initiates release of ATP onto nearby magnocellular neurosecretory neurons. In turn, ATP activates P2X7 receptors on these neurons, enhancing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor surface expression and the amplitude of miniature excitatory postsynaptic currents [46]. Experiments conducted in the retina have shown a suppression of neuronal activity resulting from astrocytic purinergic signalling [47–49]. Stimulation of photoreceptors in retinal wholemounts leads to glial calcium signalling [48,50] and subsequent ATP release from Müller cells [37,51]. Released ATP is degraded to adenosine, which then acts on A1 receptors to suppress neuronal activity (figure 2). Similarly in the hippocampus, suppressive actions of astrocyte-derived adenosine have been observed via A1-dependent presynaptic inhibition of synaptic transmission [44,52]. Astrocytes and neurons share many similar receptors. Because of this, they use similar signalling pathways, making it difficult to use pharmacological manipulations to distinguish the specific role of astrocytes in the modulation of neurons. Astrocyte-derived ATP-adenosine signalling has been confirmed using glia-specific toxins [52] and astrocyte-selective loading of the calcium chelator BAPTA as well as astrocyte-specific molecular genetics [53].
Figure 2.
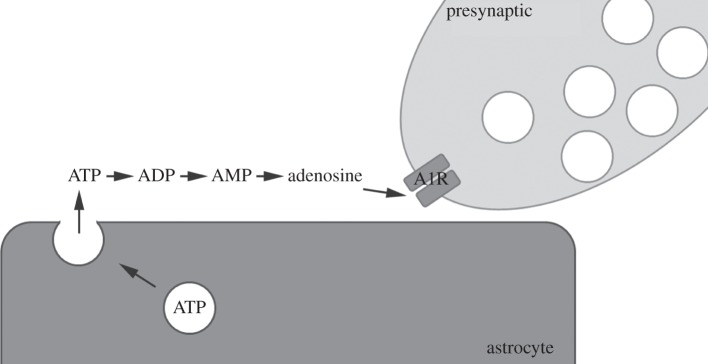
ATP signalling via exocytic release from astrocytes. Upon activation/stimulation astrocytes release ATP via exocytosis. In the extracellular space, ATP is hydrolysed to adenosine, which is then free to act on adenosine receptors. One major site of action is on A1 receptors in the presynaptic terminal.
6. Astrocytic regulation of adenosine in sleep
Activation of the A1 receptor has previously been shown to regulate homeostatic functions of sleep [54]. Adenosine accumulates in the brain with prolonged periods of wakefulness [55]. The selective addition of adenosine directly into the brain induces sleep [56,57] and electrophysiological markers of homeostatic sleep pressure [58], whereas antagonizing adenosine by pharmacological agents promotes wakefulness [59] and attenuates the accumulation of homeostatic sleep pressure [60]. One of the most common indications that adenosine signalling is implicated in the control of human sleep comes from the powerful wake promoting effects of adenosine receptor antagonists including caffeine [61]. In addition, humans with polymorphisms in the adenosine metabolizing enzyme, adenosine deaminase, show reduced adenosine metabolism and exhibit more consolidated sleep [62,63].
Sleep and sleep disorders are highly correlated with many psychiatric disorders, and more than 70% of all depressed patients report of difficulties in either the initiation or in maintenance of sleep [64–67]. Closely monitored biometric studies show that hypersomnia, or excessively long sleep episodes, are coupled with daytime sleepiness and frequent napping in 10–40% of patients with diagnosed mood disorders [68,69]. Indeed, alterations in sleep patterns are one of the key diagnostic criteria of depressive disorders. Conversely, depression may be observed as an effect of sleep disorders, showing reciprocity in the relationship between sleep and mood.
7. A role for adenosine and astrocytes in depression
Major depressive disorder (MDD) strikes one in 10 Americans in their lifetime. The effects of mood disorders such as MDD and bipolar disorder on individuals and society rank among the most disabling and costly of all medical illnesses. Numerous antidepressant pharmacotherapies are available in clinical practice, yet many patients undergo trial and error with multiple medications before achieving relief of symptoms. In addition, these pharmacological treatments take weeks to achieve their full efficacy, limiting their application to suicidal patients where rapid relief is necessary. These factors create a tremendous need to improve current treatment options for patients suffering from depression. Interestingly, a clinically employed non-pharmacological intervention that rapidly alleviates symptoms of depression is one or more nights of total sleep deprivation [70]. Sleep deprivation therapy is effective in approximately 60–70% of patients with depression [71,72].
The reduction in depressive symptoms observed following sleep deprivation correlates with the changes that can be seen in the slow wave activity (SWA) on baseline sleep. Changes in the amount of the rebound in right frontal all-night SWA during recovery sleep have also been shown to be significant [73,74]. MDD has been shown to change the sleep homeostat as measured by auditory evoked potential changes causing consequential changes in SWA. Finally, selectively sleep depriving subjects by only interrupting slow wave sleep is still an effective antidepressant treatment in patients with major depression [73,74].
Because the effects of sleep deprivation on depression are not long lasting, sleep deprivation is not always used clinically. However, if the mechanism mediating this action were identified it might be possible to develop therapeutics that target this pathway as a new treatment for certain forms of depression.
Signalling pathways involving adenosine have been linked to depression; however, controversy and inconsistencies exist as to whether adenosine (and its agonists) act in an antidepressant [75] or a depressant (El [76]) manner. A role for adenosine in depression is supported by the observation that 12 h of sleep deprivation elevates adenosine levels in rodent frontal cortex. Additionally, in patients with depression associated with sleep disorders, polymorphisms of the gene encoding the A1 receptor (ADORA-1) have been identified [77,78].
8. Astrocyte adenosine signalling regulates the antidepressant effects of sleep deprivation
We have recently shown that astrocytes are capable of modulating changes in non-rapid eye movement SWA in responses to sleep deprivation. In addition, these studies also showed that astrocytes regulate the amount of time that is spent in recovery sleep following sleep deprivation [3]. Expression of dnSNARE (dominant negative SNARE domain of the vesicle protein VAMP2) selectively within astrocytes reduces extracellular adenosine accumulation [3,79,80]. The dnSNARE mouse also shows reduced activation of A1 receptors. The lack of adenosine accumulation and reduced A1 receptor activation result in a reduction in sleep pressure and the electrophysiological changes associated with sleep deprivation [3].
Based on these findings, we postulated that astrocytes may also play a role in the beneficial effects of sleep deprivation in depressed patients. We demonstrate that sleep deprivation reduces depressive-like symptoms in mouse models of depression with similarity to human depression patients. Specifically, using 12 h of sleep deprivation we were able to show a significant reduction in time spent immobile in forced swim and tail suspension tests. Furthermore, we showed that this reduction in depressive-like symptoms is not observed in dnSNARE mice, A1 receptor knock-out mice or in mice treated with an adenosine receptor antagonist. These findings demonstrate that the anti-depressive effects of sleep deprivation require astrocytic SNARE-dependent signalling that is mediated through the A1 receptor.
9. A general role for astrocytic adenosine regulation in psychiatric disorders
Recent research strongly implicates astrocytes in modulating sleep [3,80,81]. Although very little is known about the direct mechanism, astrocytic adenosine has been shown to be a major player in sleep homeostasis. It is therefore quite logical that the astrocyte can be seen as playing a pivotal role in diseases that have sleep disruption as a hallmark. Many genes and their receptor products have been shown to be linked to adenosine dysregulation and psychiatric disorders (table 1). The next stage of important research will show what populations of cells in specific brain regions are responsible for specific sleep-related disorders.
Table 1.
Single nucleotide polymorphisms identified in adenosine receptors, transporters and signalling molecules in depression and other neuropsychiatric disorders.
| gene | single nucleotide polymorphism | disorder(s) | reference |
|---|---|---|---|
| ADORA1 | rs17530497 | depression with fatigue | [77] |
| rs12026765 | depression with fatigue | ||
| ADORA2 | 1083C>T | panic disorder | [82] |
| 1976C>T | panic disorder | ||
| rs5751876 | panic disorder | [83] | |
| rs5751862 | anxiety-related personality | ||
| rs2298383 | anxiety-related personality | ||
| rs3761422 | anxiety-related personality | ||
| 1976C>T | caffeine-induced anxiety | [84] | |
| 2592C>T | caffeine-induced anxiety | ||
| rs2236624CC | autism spectrum | [85] | |
| rs3761422 | autism spectrum | ||
| rs5751876 | autism spectrum | ||
| rs35320474 | autism spectrum | ||
| ADA | rs6031682 | depression depression with fatigue |
[77] |
| ADK | rs7924176 | depression with fatigue | [77] |
| ENT1 | rs693955 | depression with morning awakening | [77] |
| rs324148 | depression with morning awakening | ||
| rs6905285 | depression depression with fatigue depression with morning awakening |
||
| ENT3 | rs10999776 | depression with morning awakening | [77] |
| rs2066210 | depression with fatigue | ||
| rs12256138 | depression depression with fatigue depression with morning awakening |
||
| rs780659 | depression depression with morning awakening |
||
| rs780662 | depression | ||
| rs12767108 | depression depression with morning awakening |
||
| rs2487067 | depression |
Recent advancements in the glia field have shown these cells to play a role that is much greater than the ‘glue that holds’ grey matter together. Future research is already being directed towards manipulating specific astrocyte mechanisms relating to neurological disorders. It will be on the foundation of this basic research that some of tomorrow's clinical gains will be made.
References
- 1.Araque A, Navarrete M. 2010. Glial cells in neuronal network function. Phil. Trans. R. Soc. B 365, 2375–2381. ( 10.1098/rstb.2009.0313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Marin V, Garcia-Lopez P, Freire M. 2007. Cajal's contributions to glia research. Trends Neurosci. 30, 479–487. ( 10.1016/j.tins.2007.06.008) [DOI] [PubMed] [Google Scholar]
- 3.Halassa MM, Florian C, Fellin T, Munoz JR, Lee S-Y, Abel T, Haydon PG, Frank MG. 2009. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219. ( 10.1016/j.neuron.2008.11.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hines DJ, Schmitt LI, Hines RM, Moss SJ, Haydon PG. 2013. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl. Psychiatry 3, e212 ( 10.1038/tp.2012.136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushong EA, Martone ME, Jones YZ, Ellisman MH. 2002. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. 2003. Signaling at the gliovascular interface. J. Neurosci. 23, 9254–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ventura R, Harris KM. 1999. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 19, 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. ( 10.1016/S0166-2236(98)01349-6) [DOI] [PubMed] [Google Scholar]
- 9.Haydon PG. 2001. Glia: listening and talking to the synapse. Nat. Rev. Neurosci. 2, 185–193. ( 10.1038/35058528) [DOI] [PubMed] [Google Scholar]
- 10.Panatier A, Theodosis DT, Mothet J-P, Touquet B, Pollegioni L, Poulain DA, Oliet SHR. 2006. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784. ( 10.1016/j.cell.2006.02.051) [DOI] [PubMed] [Google Scholar]
- 11.Haber M, Murai KK. 2006. Reshaping neuron-glial communication at hippocampal synapses. Neuron Glia Biol. 2, 59–66. ( 10.1017/S1740925X06000032) [DOI] [PubMed] [Google Scholar]
- 12.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. 2002. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc. Natl Acad. Sci. USA 99, 9840–9845. ( 10.1073/pnas.152588599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stout CE, Costantin JL, Naus CCG, Charles AC. 2002. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 277, 10 482–10 488. ( 10.1074/jbc.M109902200) [DOI] [PubMed] [Google Scholar]
- 14.Lazarowski ER, Boucher RC, Harden TK. 2003. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 64, 785–795. ( 10.1124/mol.64.4.785) [DOI] [PubMed] [Google Scholar]
- 15.Queiroz G, Gebicke-Haerter PJ, Schobert A, Starke K, von Kügelgen I. 1997. Release of ATP from cultured rat astrocytes elicited by glutamate receptor activation. Neuroscience 78, 1203–1208. ( 10.1016/S0306-4522(96)00637-9) [DOI] [PubMed] [Google Scholar]
- 16.Anderson CM, Bergher JP, Swanson RA. 2004. ATP-induced ATP release from astrocytes. J. Neurochem. 88, 246–256. ( 10.1111/j.1471-4159.2004.02204.x) [DOI] [PubMed] [Google Scholar]
- 17.Bal-Price A, Moneer Z, Brown GC. 2002. Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia 40, 312–323. ( 10.1002/glia.10124) [DOI] [PubMed] [Google Scholar]
- 18.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. 2003. Storage and release of ATP from astrocytes in culture. J. Biol. Chem. 278, 1354–1362. ( 10.1074/jbc.M209454200) [DOI] [PubMed] [Google Scholar]
- 19.Pangrsic T, et al. 2007. Exocytotic release of ATP from cultured astrocytes. J. Biol. Chem. 282, 28 749–28 758. ( 10.1074/jbc.M700290200) [DOI] [PubMed] [Google Scholar]
- 20.Montana V, Ni Y, Sunjara V, Hua X, Parapura V. 2004. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 24, 2633–2642. ( 10.1523/JNEUROSCI.3770-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, et al. 2004. Fusion-related release of glutamate from astrocytes. J. Biol. Chem. 279, 12 724–12 733. ( 10.1074/jbc.M312845200) [DOI] [PubMed] [Google Scholar]
- 22.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. 2004. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7, 613–620. ( 10.1038/nn1246) [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. 2004. Synaptotagmin IV regulates glial glutamate release. Proc. Natl Acad. Sci. USA 101, 9441–9446. ( 10.1073/pnas.0401960101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jourdain P, et al. 2007. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331–339. ( 10.1038/nn1849) [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu X-s, Duan S. 2007. Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953. ( 10.1038/ncb1620) [DOI] [PubMed] [Google Scholar]
- 26.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. 2005. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl Acad. Sci. USA 102, 5606–5611. ( 10.1073/pnas.0408483102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. 1994. Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747. ( 10.1038/369744a0) [DOI] [PubMed] [Google Scholar]
- 28.Porter JT, McCarthy KD. 1996. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 16, 5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angulo MC, Kozlov AS, Charpak S, Audinat E. 2004. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 24, 6920–6927. ( 10.1523/JNEUROSCI.0473-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. 2004. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743. ( 10.1016/j.neuron.2004.08.011) [DOI] [PubMed] [Google Scholar]
- 31.Fiacco TA, McCarthy KD. 2004. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J. Neurosci. 24, 722–732. ( 10.1523/JNEUROSCI.2859-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang N, Xu J, Xu Q, Nedergaard M, Kang J. 2005. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 94, 4121–4130. ( 10.1152/jn.00448.2005) [DOI] [PubMed] [Google Scholar]
- 33.Pirttimaki TM, Hall SD, Parri HR. 2011. Sustained neuronal activity generated by glial plasticity. J. Neurophysiol. 31, 7637–7647. ( 10.1523/JNEUROSCI.5783-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirase H, Qian L, Barthó P, Buzsáki G. 2004. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2, E96 ( 10.1371/journal.pbio.0020096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darby M, Kuzmiski JB, Panenka W, Feighan D, MacVicar BA. 2003. ATP released from astrocytes during swelling activates chloride channels. J. Neurophysiol. 89, 1870–1877. ( 10.1152/jn.00510.2002) [DOI] [PubMed] [Google Scholar]
- 36.Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K. 2003. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc. Natl Acad. Sci. USA 100, 11 023–11 028. ( 10.1073/pnas.1834448100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman EA. 2003. Glial cell inhibition of neurons by release of ATP. J. Neurosci. 23, 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henneberger C, Papouin T, Oliet SHR, Rusakov DA. 2010. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236. ( 10.1038/nature08673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santello M, Bezzi P, Volterra A. 2011. TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001. ( 10.1016/j.neuron.2011.02.003) [DOI] [PubMed] [Google Scholar]
- 40.Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R. 2003. Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J. Neurophysiol. 23, 1580–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabbretti E, Sokolova E, Masten L, D'Arco M, Fabbro A, Nistri A, Giniatullin R. 2004. Identification of negative residues in the P2X3 ATP receptor ectodomain as structural determinants for desensitization and the Ca2+-sensing modulatory sites. J. Biol. Chem. 279, 53 109–53 115. ( 10.1074/jbc.M409772200) [DOI] [PubMed] [Google Scholar]
- 42.Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y. 2014. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 12, e1001747 ( 10.1371/journal.pbio.1001747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwiebert EM, Egan ME, Hwang T-H, Fulmer SB, Allen SS, Cutting GR, Guggino WB. 1995. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell 81, 1063–1073. ( 10.1016/S0092-8674(05)80011-X) [DOI] [PubMed] [Google Scholar]
- 44.Pascual O, et al. 2005. Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. ( 10.1126/science.1116916) [DOI] [PubMed] [Google Scholar]
- 45.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. 2007. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 27, 6473–6477. ( 10.1523/JNEUROSCI.1419-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WRAKJS, Fisher TE, Bains JS. 2005. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat. Neurosci. 8, 1078–1086. ( 10.1038/nn1498) [DOI] [PubMed] [Google Scholar]
- 47.Newman EA. 2001. Calcium signaling in retinal glial cells and its effect on neuronal activity. Prog. Brain Res. 132, 241–254. ( 10.1016/S0079-6123(01)32080-0) [DOI] [PubMed] [Google Scholar]
- 48.Newman EA. 2005. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J. Neurosci. 25, 5502–5510. ( 10.1523/JNEUROSCI.1354-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurth-Nelson ZL, Mishra A, Newman EA. 2009. Spontaneous glial calcium waves in the retina develop over early adulthood. J. Neurosci. 29, 11 339–11 346. ( 10.1523/JNEUROSCI.2493-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman EA. 2004. Glial modulation of synaptic transmission in the retina. Glia 47, 268–274. ( 10.1002/glia.20030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newman EA, Zahs KR. 1998. Modulation of neuronal activity by glial cells in the retina. J. Neurosci. 18, 4022–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang JM, Wang HK, Ye C-Q, Ge W, Chen Y, Jiang Z-l, Wu C-P, Poo M-m, Duan S. 2003. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982. ( 10.1016/S0896-6273(03)00717-7) [DOI] [PubMed] [Google Scholar]
- 53.Serrano A, Haddjeri N, Lacaille J-C, Robitaille R. 2006. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J. Neurosci. 26, 5370–5382. ( 10.1523/JNEUROSCI.5255-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basheer R, Strecker RE, Thakkar MM, McCarley RW. 2004. Adenosine and sleep-wake regulation. Prog. Neurobiol. 73, 379–396. ( 10.1016/j.pneurobio.2004.06.004) [DOI] [PubMed] [Google Scholar]
- 55.Porkka-Heiskanen T, Strecker RE, McCarley RW. 2000. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 99, 507–517. ( 10.1016/S0306-4522(00)00220-7) [DOI] [PubMed] [Google Scholar]
- 56.Strecker RE, et al. 2000. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav. Brain Res. 115, 183–204. ( 10.1016/S0166-4328(00)00258-8) [DOI] [PubMed] [Google Scholar]
- 57.Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. 2003. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience 122, 1107–1113. ( 10.1016/j.neuroscience.2003.08.006) [DOI] [PubMed] [Google Scholar]
- 58.Benington JH, Kodali SK, Heller HC. 1995. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 692, 79–85. ( 10.1016/0006-8993(95)00590-M) [DOI] [PubMed] [Google Scholar]
- 59.Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. 1981. Adenosine receptors and behavioral actions of methylxanthines. Proc. Natl Acad. Sci. USA 78, 3260–3264. ( 10.1073/pnas.78.5.3260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landolt HP. 2008. Sleep homeostasis: a role for adenosine in humans? Biochem. Pharmacol. 75, 2070–2079. ( 10.1016/j.bcp.2008.02.024) [DOI] [PubMed] [Google Scholar]
- 61.Landolt HP, Retey JV, Tönz K, Gottselig JM, Khatami R, Buckelmüller I, Achermann P. 2004. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology 29, 1933–1939. ( 10.1038/sj.npp.1300526) [DOI] [PubMed] [Google Scholar]
- 62.Mazzotti DR, Guindalini C, Pellegrino R, Barrueco KF, Santos-Silva R, Bittencourt LR, Tufik S. 2011. Effects of the adenosine deaminase polymorphism and caffeine intake on sleep parameters in a large population sample. Sleep 34, 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bachmann V, Klaus F, Bodenmann S, Schafer N, Brugger P, Huber S, Berger W, Landolt H-P. 2012. Functional ADA polymorphism increases sleep depth and reduces vigilant attention in humans. Cereb. Cortex 22, 962–970. ( 10.1093/cercor/bhr173) [DOI] [PubMed] [Google Scholar]
- 64.Wulff K, Gatti S, Wettstein JG, Foster RG. 2010. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 11, 589–599. ( 10.1038/nrn2868) [DOI] [PubMed] [Google Scholar]
- 65.Menet JS, Rosbash M. 2011. When brain clocks lose track of time: cause or consequence of neuropsychiatric disorders. Curr. Opin. Neurobiol. 21, 849–857. ( 10.1016/j.conb.2011.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jagannath A, Peirson SN, Foster RG. 2013. Sleep and circadian rhythm disruption in neuropsychiatric illness. Curr. Opin. Neurobiol. 23, 888–894. ( 10.1016/j.conb.2013.03.008) [DOI] [PubMed] [Google Scholar]
- 67.Spiegelhalder K, Regen W, Nanovska S, Baglioni C, Riemann D. 2013. Comorbid sleep disorders in neuropsychiatric disorders across the life cycle. Curr. Psychiatry Rep. 15, 364 ( 10.1007/s11920-013-0364-5) [DOI] [PubMed] [Google Scholar]
- 68.Nierenberg AA, Alpert JE, Pava J, Rosenbaum JF, Fava M. 1998. Course and treatment of atypical depression. J. Clin. Psychiatry 59(Suppl. 18), 5–9. [PubMed] [Google Scholar]
- 69.Baldwin DS, Papakostas GI. 2006. Symptoms of fatigue and sleepiness in major depressive disorder. J. Clin. Psychiatry 67(Suppl. 6), 9–15. [PubMed] [Google Scholar]
- 70.Germain A, Kupfer DJ. 2008. Circadian rhythm disturbances in depression. Hum. Psychopharmacol. 23, 571–585. ( 10.1002/hup.964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Svendsen K. 1976. Sleep deprivation therapy in depression. Acta Psychiatr. Scand. 54, 184–192. ( 10.1111/j.1600-0447.1976.tb00111.x) [DOI] [PubMed] [Google Scholar]
- 72.Hemmeter UM, Hemmeter-Spernal J, Krieg J-C. 2010. Sleep deprivation in depression. Expert Rev. Neurother. 10, 1101–1115. ( 10.1586/ern.10.83) [DOI] [PubMed] [Google Scholar]
- 73.Goldstein MR, Plante DT, Hulse BK, Sarasso S, Landsness EC, Tononi G, Benca RM. 2011. Overnight changes in waking auditory evoked potential amplitude reflect altered sleep homeostasis in major depression. Acta Psychiatr. Scand. 125, 468–477. ( 10.1111/j.1600-0447.2011.01796.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Landsness EC, Goldstein MR, Peterson MJ, Tononi G, Benca RM. 2011. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J. Psychiatr. Res. 45, 1019–1026. ( 10.1016/j.jpsychires.2011.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos ARS, Rodrigues ALS. 2004. Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci. Lett. 355, 21–24. ( 10.1016/j.neulet.2003.10.040) [DOI] [PubMed] [Google Scholar]
- 76.El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois J-M. 2003. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc. Natl Acad. Sci. USA 100, 6227–6232. ( 10.1073/pnas.1034823100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gass N. 2010. Contribution of adenosine related genes to the risk of depression with disturbed sleep. J. Affect Disord. 126, 134–139. ( 10.1016/j.jad.2010.03.009) [DOI] [PubMed] [Google Scholar]
- 78.Sperlagh B, Csolle C, Ando RD, Goloncser F, Kittel A, Baranyi M. 2012. The role of purinergic signaling in depressive disorders. Neuropsychopharmacol. Hung 14, 231–238. [PubMed] [Google Scholar]
- 79.Deng Q, Terunuma M, Fellin T, Moss SJ, Haydon PG. 2011. Astrocytic activation of A1 receptors regulates the surface expression of NMDA receptors through a Src kinase dependent pathway. Glia 59, 1084–1093. ( 10.1002/glia.21181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. 2011. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J. Neurophysiol. 31, 6956–6962. ( 10.1523/JNEUROSCI.5761-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frank MG. 2013. Astroglial regulation of sleep homeostasis. Curr. Opin. Neurobiol. 23, 812–818. ( 10.1016/j.conb.2013.02.009) [DOI] [PubMed] [Google Scholar]
- 82.Hamilton SP, Slager SL, de Leon AB, Heiman GA, Klein DF, Hodge SE, Weissman MM, Fyer AJ, Knowles JA. 2004. Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology 29, 558–565. ( 10.1038/sj.npp.1300311) [DOI] [PubMed] [Google Scholar]
- 83.Hohoff C, et al. 2010. Adenosine A(2A) receptor gene: evidence for association of risk variants with panic disorder and anxious personality. J. Psychiatr. Res. 44, 930–937. ( 10.1016/j.jpsychires.2010.02.006) [DOI] [PubMed] [Google Scholar]
- 84.Alsene K, Deckert J, Sand P, de Wit H. 2003. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 28, 1694–1702. ( 10.1038/sj.npp.1300232) [DOI] [PubMed] [Google Scholar]
- 85.Freitag CM, Agelopoulos K, Huy E, Rothermundt M, Krakowitzky P, Meyer J, Deckert J, Gontard A, Hohoff C. 2010. Adenosine A(2A) receptor gene (ADORA2A) variants may increase autistic symptoms and anxiety in autism spectrum disorder. Eur. Child Adolesc. Psychiatry 19, 67–74. ( 10.1007/s00787-009-0043-6) [DOI] [PubMed] [Google Scholar]


