Abstract
Astrocytes dynamic interactions with neurons play an active role in neurotransmission. The gap junction (GJ) subunits connexins 43 and 30 are strongly expressed in astrocytes and have recently been shown to regulate synaptic activity and plasticity. However, the specific role of connexin 43 in the morphological and electrophysiological properties of astrocytes in situ as well as in synaptic transmission remains unknown. Here, we show that connexin 43, a major determinant of astroglial GJ coupling, regulates astrocyte cell volume, but has no impact on astroglial passive membrane properties. Furthermore, we demonstrate that connexin 43 modulates glutamatergic synaptic activity of hippocampal CA1 pyramidal cells. This regulation involves changes in synaptically released glutamate, with no alteration in neuronal excitability or postsynaptic function. These results reveal connexin 43 as a critical player in neuroglial interactions by supporting synaptic efficacy.
Keywords: astrocytes, connexin, synapses, hippocampus
1. Introduction
Recent data suggest that astrocytes play an important role in behaviour and cognitive functions [1–3]. Indeed, astrocytes integrate neuronal inputs through their membrane channels, receptors and transporters, and can transmit information by clearing or releasing a number of neuroactive substances. Such astroglial function can modulate neuronal excitability, synaptic activity and plasticity, as well as network activity. However, the molecular mechanisms of such regulations remain unclear.
In astrocytes, the high expression level of the two main gap junction (GJ) proteins connexins 30 and 43 (Cx), together with their role in intercellular communication, is thought to contribute to brain homeostasis through nutrient transport, as well as glutamate clearance and potassium spatial buffering [3,4], and suggests that they play an important role in neuroglial interactions. This hypothesis is supported by neurodevelopmental defects [5], impaired neuronal plasticity [6], accelerated hippocampal spreading depression, enhanced locomotor activity and increased exploratory behaviour [7] in astrocyte-targeted Cx43 knockout mice, as well as altered neurogenesis [8], potassium buffering [9], energy metabolism [10], synaptic activity and plasticity [11], and dysmyelination and vacuolation [12] in Cx43 and Cx30 double knockout mice.
Cx subunits assemble to form hemichannels that align between adjacent cells to form intercellular GJ channels. They mediate direct cell-to-cell diffusion of ions and small signalling molecules (less than 1.5 kDa), providing electrical and metabolic coupling between connected cells [3]. Cx functions, however, extend beyond GJ communication, and also include extracellular exchanges mediated by hemichannels, as well as channel-independent functions involving cell adhesion or signalling [4,13]. Interestingly, Cx43 differs from Cx30 in several aspects, including temporal and regional distribution patterns [4], biophysical properties, intracellular C-terminal domain [14], regulation by neuronal activity [15] and contribution to behaviour [7,16,17]. Therefore, determining whether each Cx confers specific features to astrocytes, and unravelling the underlying differential regulations of neurotransmission are crucial issues. We recently found that there was a non-channel function of Cx30 which, by controlling the astroglial coverage of synapses, sets synaptic strength through alteration of astroglial glutamate transport [18]. While a few Cx43 functions in astrocyte biology and brain development have been described involving intercellular communication, release of gliotransmitters through hemichannels, and neuroglial proliferation and migration through adhesive properties [4,13], the specific role of Cx43 in synaptic transmission is currently unknown. We here show that Cx43 enhances hippocampal excitatory synaptic activity through modulation of synaptically released glutamate levels.
2. Material and methods
(a). Animals
Experiments were carried out according to the guidelines of the European Community Council Directives of 1 January 2013 (2010/63/EU), and all efforts were made to minimize the number of animals used and their suffering. Experiments were performed in the hippocampus of wild-type and Cx43fl/fl:hGFAP-Cre (Cx43−/−) mice (provided by K. Willecke, University of Bonn, Germany), with conditional deletion of Cx43 in astrocytes, as previously described [17]. Mice of both genders and littermates were used at postnatal days 16–25, unless otherwise stated.
(b). Electrophysiology
Acute transverse hippocampal slices (300–400 μm) were prepared as previously described [19]. Slices were maintained at room temperature in a storage chamber that was perfused with an artificial cerebrospinal fluid (ACSF) containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3 and 11 glucose, saturated with 95% O2 and 5% CO2, for at least 1 h prior to recording. Slices were then transferred to a submerged recording chamber mounted on an Olympus BX51WI microscope and were perfused with ACSF at a rate of 1.5 ml min−1 at room temperature. All experiments were performed in the presence of picrotoxin (100 µM), and a cut was made between CA1 and CA3 to prevent the propagation of epileptiform activity, except when indicated. Astroglial whole-cell patch-clamp recordings, intercellular coupling experiments and synaptic electrophysiology were performed as previously described [11,18]. Evoked postsynaptic responses were induced by stimulating Schaffer collaterals (0.1 Hz) in CA1 stratum radiatum with ACSF-filled glass pipettes. Somatic whole-cell recordings were obtained from visually identified CA1 pyramidal cells and astrocytes, using 5–10 MΩ glass pipettes filled with either (in mM): 105 K-gluconate, 30 KCl, 10 HEPES, 10 phosphocreatine, 4 ATP-Mg, 0.3 GTP–Tris, 0.3 EGTA (pH 7.4, 280 mOsm l−1); or, for synaptic activity recordings (in mM): 115 CsMeSO3, 20 CsCl, 10 HEPES, 2.5 MgCl2, 4 Na2ATP, 0.4 NaGTP, 10 Na-phosphocreatine, 0.6 EGTA, 0.1 spermine, 5 QX314 (pH 7.2, 280 mOsm l−1). Recordings were acquired with Axopatch-1D amplifiers (Molecular Devices, USA), digitized at 10 kHz, filtered at 2 kHz, stored and analysed on computer using Pclamp9 and Clampfit9 softwares (Molecular Devices).
(c). Immunohistochemistry and immunoblotting
Immunohistochemistry, immunoblotting, sulforhodamine 101 astroglial labelling on acute living slices and quantifications were performed as previously described [11,18]. The used antibodies included Homer1 rabbit polyclonal antibody (1 : 200, AB160 003, Synaptic Systems), VGlut1 guinea pig polyclonal antibody (1 : 500, AB5905, Chemicon), and all other antibodies as previously described [11,18]. For quantification of VGlut1–Homer1 co-localization, images of VGlut1- and Homer1-stained slices were acquired with a confocal laser-scanning microscope (Leica TCS SP5) at 0.1 µm interval. The images were first deconvoluted with Huyens software, using microspheres (size ≈ 0.175 ± 0.005 μm) to approximate the point spread function (PS-Speck Microscope point source kit 7220, Molecular Probes). Deconvoluted images were then filtered by segmentation using a multi-dimensional image analysis interface [20] implemented in Metamorph (Molecular Devices). Co-localization between VGlut1 and Homer1 puncta was determined using masks.
(d). Statistics
Two-tailed unpaired t-tests were used for between-group comparisons. Statistical significance for within-group comparisons was determined by two-way repeated measures ANOVA. All statistical analysis was performed in GraphPad Prism v. 6.
3. Results
(a). Cx43 contribution to morphological and electrophysiological properties of astrocytes in situ
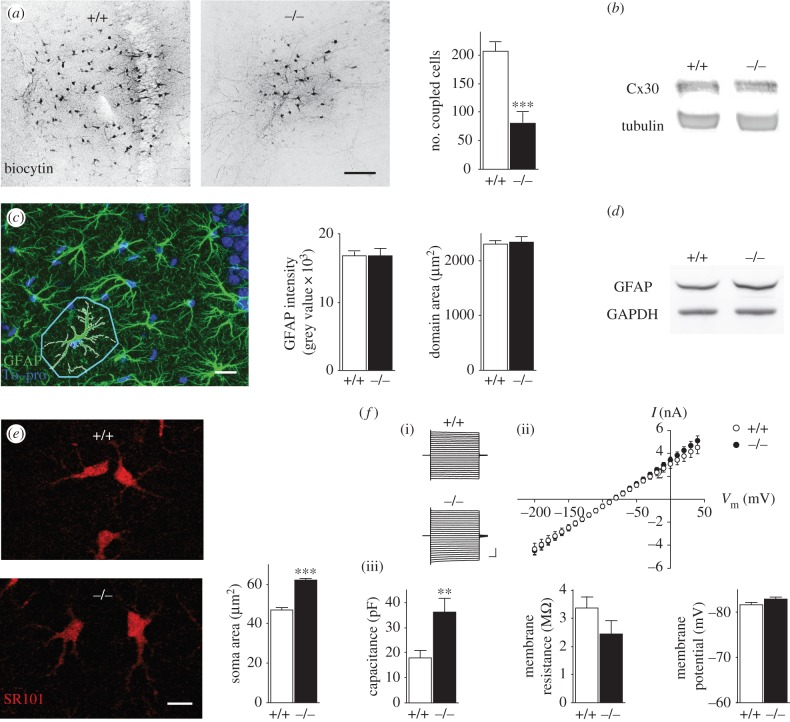
Because the primary function of Cx43 is to form GJ channels, we first examined the contribution of Cx43 to hippocampal–astroglial GJ coupling, by analysing the diffusion of biocytin, a GJ channel-permeable tracer, after whole-cell patch clamp of CA1 astrocytes. We found that Cx43 greatly contributed to astroglial intercellular communication, because biocytin diffusion into astrocytes was reduced by approximately 60% in Cx43fl/fl:hGFAP-Cre mice, with conditional deletion of Cx43 in astrocytes (Cx43−/−) when compared with wild-type (figure 1a). This reduction resulted specifically from Cx43 deletion, because Cx43−/− mice did not show compensatory upregulation of hippocampal Cx30 protein levels at three weeks old (figure 1b), although this was observed at later stages (three month old mice, relative density Cx30/GAPDH (arbitrary units): wild-type 0.8815 ± 0.2171, n = 4; Cx43−/− 3.241 ± 0.4890, n = 4; p < 0.05, unpaired t-test) as previously reported in the cortex [17]. Because Cx43 interacts with cytoskeleton proteins and regulates astroglial morphology in vitro [21], we investigated whether Cx43 alters the morphology of hippocampal astrocytes in situ. GFAP immunolabelling of Cx43−/− astrocytes was unchanged in the CA1 area of the hippocampus and revealed similar astrocytic domain size (figure 1c). However, Cx43−/− astrocytes displayed enlarged soma areas (approx. 30%), detected by sulforhodamine 101 labelling (figure 1d), indicating an increased astrocytic volume, as previously reported for Cx30−/−Cx43−/− astrocytes [11]. We then investigated whether Cx43 deficiency altered typical functional electrophysiological features of astrocytes, and found no effect on intrinsic membrane properties such as resting membrane potential, membrane resistance or passive whole-cell current patterns (linear current–voltage relationship, figure 1e). However, Cx43−/− astrocytes displayed a strong increase in membrane capacitance (approx. 100%; figure 1e,f), consistent with the increased soma area (figure 1d).
Figure 1.
Role of Cx43 in morphological and electrophysiological properties of hippocampal astrocytes. (a) Sample images and quantification of GJ-mediated biocytin coupling in CA1 stratum radiatum astrocytes from wild-type (+/+, n = 5) and Cx43−/− (−/−, n = 5) hippocampal slices. Scale bar, 100 µm. (b) Representative Cx30 immunoblot analysis in hippocampal extracts from juvenile +/+ (n = 3) and −/− (n = 3) mice. Equivalent loading was assessed by tubulin levels. (c) Sample image of hippocampal immunostaining with the astrocytic marker GFAP and the nucleus marker To-pro, illustrating the astroglial domain area (delineated in light blue). Quantification of GFAP staining and astroglial domain area in +/+ (n = 15 and n = 88, respectively) and −/− (n = 20 and n = 99, respectively) mice. Scale bar, 20 µm. (d) Representative GFAP immunoblot analysis in hippocampal extracts from +/+ (n = 3) and −/− (n = 3) mice. Equivalent loading was assessed by GAPDH levels. (e) Sample images of sulforhodamine 101 (SR101) labelling of astrocytes and quantification of their soma size in +/+ (n = 186) and −/− (n = 181) mice. Scale bar, 10 µm. (f)(i) Representative astroglial whole-cell current profiles evoked by 150 ms voltage steps (−200 to +40 mV; scale bar, 2 pA, 20 ms) and (ii) quantification of current–voltage (I/V) plots and (iii) intrinsic membrane properties of +/+ (n = 7 and 10, respectively) and −/− (n = 8 and 11, respectively) astrocytes. Asterisks indicate statistical significance (Student's unpaired t-test or two-way repeated measures ANOVA (f), ***p < 0.001, **p < 0.01).
(b). Cx43 modulates hippocampal excitatory synaptic activity
To investigate the role of Cx43 in synaptic strength, we measured evoked basal synaptic responses at CA1 Schaffer collateral synapses in acute hippocampal slices from Cx43−/− mice. By comparing the amplitude of the presynaptic fibre volley (input) with the slope of the field excitatory postsynaptic potentials (fEPSPs; output), we found an approximately 30% reduction in synaptic transmission in Cx43−/− mice (figure 2a). Such modulation of synaptic activity occurred at the single synapse level, because minimal stimulation, activating single or very few synapses, successfully evoked excitatory postsynaptic currents (EPSCs) with decreased amplitude, but no change in failure rate (figure 2b). The decreased synaptic transmission did not result from major developmental defects, because hippocampi from Cx43−/− mice showed no gross anatomical alterations. The overall cytoarchitecture and layered structure of the hippocampi from Cx43−/− mice appeared normal (figure 2c). Similarly, the number of CA1 pyramidal cells and astrocytes, assessed by immunostaining, was unchanged (figure 2d), as previously reported in Cx30−/−Cx43−/− mice [11]. Finally, the levels of the pre- and postsynaptic proteins synaptophysin and PSD-95, respectively, assessed by Western blot analysis in whole hippocampal extracts (figure 2e), as well as the density of stratum radiatum excitatory synapses, in which presynaptic vesicular glutamate transporter 1 (VGlut1) was juxtaposed to postsynaptic Homer1 (figure 2f), were also unchanged in these mice, suggesting similar total structural synaptic contacts.
Figure 2.
Cx43 regulates hippocampal excitatory synaptic strength. (a) Input–output curves for basal synaptic transmission, illustrated in the sample traces and the graph below in wild-type (+/+, n = 25) and Cx43−/− mice (−/−, n = 17). Scale bar, 0.2 mV, 10 ms. (b) EPSCs from CA1 pyramidal cells in response to minimal stimulation, illustrated by (i) plots of EPSC amplitude versus trial number and (ii) superimposed traces recorded at −70 mV, and quantified by (iii) mean amplitude of successful events (EPSC potency) and failure rate in +/+ (n = 12) and −/− (n = 12) mice. Scale bar, 10 pA, 20 ms. (c) Hippocampal layered structure revealed by immunostaining with the nucleus marker To-pro in +/+ and −/− mice. Scale bar, 200 µm. (d) Illustration of hippocampal slices double-stained with To-pro and S100 (astrocytic marker), and quantification of CA1 pyramidal neurons (To-pro+ and S100− cells in CA1 pyramidal cell layer) and astrocytes (S100+ cells in CA1 stratum radiatum) density in +/+ (n = 9 and 16 fields, respectively) and −/− (n = 12 and 16 fields, respectively) mice. Scale bar, 100 µm. (e) Sample immunoblot analysis of the pre- and postsynaptic proteins synaptophysin and PSD95, respectively, in hippocampal extracts from +/+ (n = 3) and −/− (n = 3) mice. Equivalent loading was assessed by tubulin levels. (f) Number of excitatory synapses assessed by co-localized clusters (merge, yellow) of VGlut1 (red) and Homer1 (green) labelling in the hippocampus of +/+ (n = 37 fields) and −/− (n = 48 fields) mice. Analysis was performed on binary images (masks). Scale bar, 2 µm. Asterisks indicate statistical significance (two-way repeated measures ANOVA (a) and Student's unpaired t-test, *** p < 0.001; *p < 0.05).
(c). Cx43 enhances synaptically released glutamate levels
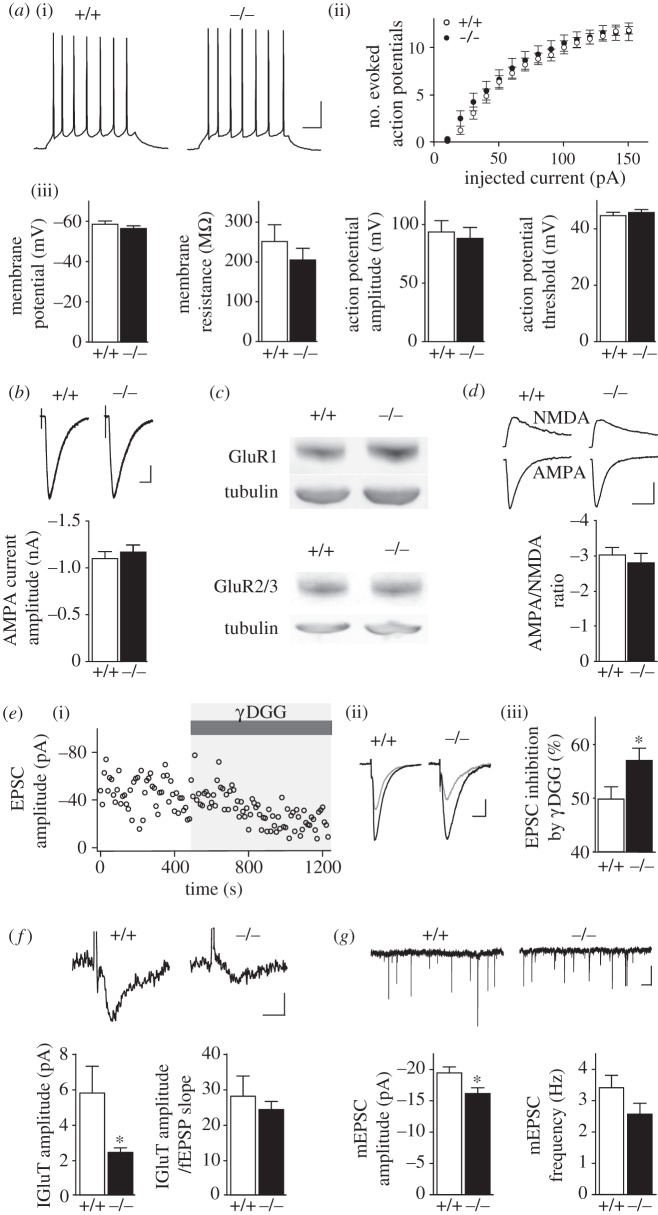
What may cause reduced glutamatergic synaptic transmission in Cx43−/− mice? Analysis of CA1 pyramidal cell intrinsic membrane properties and excitability showed no change in resting membrane potential, membrane resistance, amplitude, threshold and number of action potentials evoked by depolarizing pulses in CA1 pyramidal cells from Cx43−/− mice (figure 3a). Because fEPSPs are mainly mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) activation, we investigated whether the density of functional postsynaptic AMPARs was altered, but found no change in AMPAR-mediated whole-cell currents elicited in CA1 pyramidal cells by local application of high AMPA concentration in the presence of tetrodotoxin and cyclothiazide (figure 3b). Western blot analysis also showed that GluR1 and GluR2/3 AMPAR subunit protein levels were unchanged in the hippocampus of Cx43−/− mice (figure 3c), further indicating unchanged AMPAR density. We then investigated whether the decrease in glutamatergic synaptic transmission was restricted to AMPAR activity, by comparing the AMPAR and N-methyl-d-aspartate receptor (NMDAR) components of the EPSC. The AMPA/NMDA ratio was unchanged in CA1 pyramidal cells from Cx43−/− mice (figure 3d), indicating that the defect in synaptic transmission equally affected the NMDA component of the EPSC. In addition, AMPA and NMDA EPSCs displayed similar decay kinetics, suggesting no change in synaptic glutamate dynamics (AMPA decay time constant: wild-type 19.75 ± 1.76 ms, n = 11; Cx43−/− 20.6 ± 2.57 ms, n = 8; NMDA decay time constant: wild-type 100.8 ± 7.2 ms, n = 11; Cx43−/− 109.9 ± 12.5 ms, n = 8; unpaired t-test). Taken together, these data suggest that the decreased excitatory synaptic transmission in Cx43−/− mice did not result from postsynaptic dysfunction, but may rather involve changes in synaptic glutamate concentration, affecting AMPARs and NMDARs similarly. To test this hypothesis directly, we used γ-d-glutamylglycine (γ-DGG), a low affinity competitive AMPAR antagonist at non-saturating concentration (500 µM), whose potency directly depends on glutamate levels. We found that γ-DGG inhibition of AMPAR-mediated EPSCs was stronger in pyramidal cells from Cx43−/− mice than from wild-type mice (figure 3e), thereby indicating reduced synaptic glutamate levels in Cx43−/− mice. Because astrocytes strongly contribute to glutamate uptake [22], we investigated whether decreased synaptic glutamate in Cx43−/− mice resulted from increased glial glutamate clearance. To do so, we recorded simultaneously synaptically evoked glutamate transporter currents isolated pharmacologically in astrocytes, and neuronal responses (fEPSPs), and found that the amplitude of evoked astroglial glutamate transporter currents was instead decreased by approximately 50% in Cx43−/− mice. However, when normalized to the decreased associated excitatory synaptic transmission, the glutamate transporter current amplitude was unchanged (figure 3f), indicating that the reduced astroglial glutamate uptake in Cx43−/− mice solely reflected weaker glutamatergic synaptic activity. To determine whether transmitter release was altered in Cx43−/− mice, we recorded AMPAR miniature EPSCs (mEPSCs) in CA1 pyramidal cells, and found a reduction in their amplitude, known to reflect vesicular glutamate content and/or postsynaptic receptor activity, but no significant change in their frequency, although there was a tendency for a decrease, likely resulting from decreased amplitudes of miniature events below threshold detection level (less than 5 pA; figure 3g). As we found no change in postsynaptic responses in Cx43−/− mice, these results, consistent with the decreased amplitude but unchanged failure rate of EPSCs evoked by minimal stimulation (figure 2b), suggest a decreased quantal size with no alteration in release probability.
Figure 3.
Cx43 sets synaptic glutamate levels. (a)(i) Representative sample traces of action potentials elicited by current injections (40 pA, 500 ms) in CA1 pyramidal neurons from wild-type (+/+) and Cx43−/− (−/−) slices. Scale bar, 20 mV, 50 ms. Quantification of CA1 pyramidal cells (ii) excitability and (iii) intrinsic membrane properties in +/+ (n = 10) and −/− (n = 11) mice. (b) Whole-cell currents evoked by local AMPA application (5 s, 10 μM S-AMPA + 0.5 μM tetrodotoxin + 100 μM cyclothiazide) in CA1 pyramidal cells from +/+ (n = 13) and −/− (n = 12) mice. Scale bar, 200 pA and 10 s. (c) Sample GluR1 and GluR2/3 immunoblot analysis in hippocampal extracts from +/+ (n = 3) and −/− (n = 3) mice. Equivalent loading was assessed by tubulin levels. (d) Ratios of AMPA to NMDA EPSCs in CA1 pyramidal cells from +/+ (n = 11) and −/− mice (n = 8). Scale bars, 20 pA and 40 ms. (e)(i) EPSC amplitude inhibition over time by γ-DGG (0.5 mM, grey) in a representative cell reveals endogenous synaptic glutamate levels. (ii) Sample EPSCs before (black) and after γ-DGG (grey) application, and (iii) quantification of γ-DGG EPSC mean inhibition in +/+ (n = 13) and −/− (n = 13) mice. Scale bar, 10 pA and 20 ms. (f) Synaptically evoked astrocytic GLT currents in +/+ (n = 5) and −/− (n = 5) mice. Sample traces and quantification of GLT current amplitudes and normalization to the simultaneously recorded excitatory synaptic transmission (fEPSP slope) are shown. Scale bar, 3 pA, 10 ms. (g) mEPSCs amplitude and frequency in CA1 pyramidal cells, with sample traces above, from +/+ (n = 9) and −/− (n = 10) mice. Scale bar, 10 pA, 1 s. Asterisks indicate statistical significance (two-way repeated measures ANOVA (a)(ii) and Student's unpaired t-test, *p < 0.05).
4. Discussion
We here show that Cx43 is a crucial player in the neuroglial dialogue promoting synaptic efficacy of excitatory terminals. By setting the levels of glutamate at synapses, Cx43 modulates excitatory synaptic strength.
Importantly, despite its preponderant contribution to hippocampal astrocytic GJ-mediated intercellular coupling, as previously reported [9,10,17], Cx43 had no significant effect on the main typical electrophysiological properties of astrocytes. These data, akin to observations on disconnected astrocytes lacking both Cx30 and Cx43 [9,11], suggest that such properties, independent of GJ coupling, are intrinsic to astroglial membranes expressing specific ion channels [9,11,23,24]. Two-pore domain K+ channels (K2P), in particular TWIK-1/TREK-1 heterodimers, have indeed recently been reported to mediate astrocyte passive conductance [25,26]. However, we found that Cx43 regulates a fundamental physiological property of astrocytes, i.e. their cell volume, suggesting a role for Cx43 in the redistribution of extracellular ions and water. Such a function is specific to Cx43, because Cx43−/− astrocytes were swollen, similarly to disconnected Cx30−/−Cx43−/− astrocytes [11,12], but unlike Cx30−/− astrocytes [18]. The role of Cx43 in cell volume regulation that we describe here is consistent with other reports proposing that Cx-mediated GJ channels or hemichannels gate the flow of ions and small molecules (less than 1.5 kDa) from the extracellular space to the cytoplasm, which is sufficient to induce an osmotic gradient associated with water co-entry to equilibrate intracellular osmolarity [11,27,28]. Intercellular GJ communication mediated by Cx43 might hence buffer volume changes of individual astrocytes by redistributing ions and water molecules through the astroglial network and releasing them at distant sites, thereby contributing to astroglial cell volume homeostasis [11,27,28]. Notably, Cx43−/− hypertrophic astrocytes were not reactive, in contrast to Cx30−/−Cx43−/− astrocytes [11], suggesting that the remaining Cx30-mediated GJ communication prevents the development of further pathological alterations.
Because astrocytes occupy 50% of the brain volume [29], changes in astrocytic volume classically lead to opposite variations in extracellular space volume, which can affect neuronal activity by altering the concentration and diffusion of extracellular ions and neurotransmitters. Although we found increased astroglial cell volume in Cx43−/− mice, such alteration is, however, unlikely to account for the decreased synaptic transmission induced by astroglial Cx43 deficiency. Indeed, we recently reported that swelling of Cx30−/−Cx43−/− uncoupled astrocytes, associated with decreased extracellular space volume, instead enhances glutamatergic synaptic transmission and neuronal excitability by raising extracellular glutamate and potassium levels and altering synaptic glutamate dynamics [11]. Furthermore, glutamate dynamics in the synaptic cleft is unlikely to be altered in Cx43−/− mice, because we found no change in decay kinetics of evoked AMPA and NMDA EPSCs. Remarkably, the defect in synaptic transmission in Cx43−/− mice results, like in Cx30−/− mice, specifically from reduced synaptic glutamate levels, with no alteration in neuronal excitability or postsynaptic activity [18]. Such regulation of basal synaptic transmission may well underlie the recently reported Cx43 regulation of whisker-related sensory functions and plasticity [6]. However, unlike Cx30 deficiency, Cx43 deletion does not reduce excitatory synaptic transmission by enhancing astroglial glutamate transport owing to changes in morphology [18]. Because we found in Cx43−/− mice alterations in glutamate quantal content, with no change in release probability, our data suggest that Cx43 modulates presynaptic glutamate vesicular pools. Cx43 and Cx30 hence operate through two distinct mechanisms to regulate similarly synaptic transmission.
How such differential Cx43- and Cx30-mediated mechanisms do occur remains unknown. Remarkably, Cx43 and Cx30 GJ channels and hemichannels display distinct biophysical properties and permeability to small physiological molecules such as neurotransmitters or energy metabolites [14,30]. While we recently reported that Cx30 regulation of synaptic transmission relies on an unconventional non-channel function regulating cell adhesion and migration and controlling the synaptic location of astroglial processes [18], it is still unclear whether it is the channel (GJ or hemichannel) or non-channel functions of Cx43 that are involved in the regulation of synaptic activity. Cx43-mediated GJ channels could support excitatory synaptic transmission by providing energy metabolites such as glucose, lactate [10] or glutamine, which all permeate Cx43 channels [31]. Glutamine diffusion through the astroglial GJ network is of particular interest, because it may be involved in the neuroglial glutamine–glutamate cycle [32], necessary to fuel glutamate presynaptic pools and thereby supports excitatory synaptic transmission ([33,34], but see [35]). Alternatively, Cx43 hemichannels could mediate release of energy metabolites or gliotransmitters sustaining excitatory synaptic transmission, because they have recently been shown to be involved in neuronal network activity [36] or memory consolidation [37]. Finally, Cx43 non-channel functions related to protein interactions, cell adhesion or intracellular signalling may also be involved in the regulation of synaptic transmission. In particular, recent studies show that Cx43 can participate to brain development by affecting neural proliferation, migration or differentiation, in part through non-channel functions [5,38,39]. While we and others did not find gross developmental defects in Cx43−/− mice with C57BL6 background [5,6,17,40,41], specific malfunctions related to the maturation of hippocampal presynaptic terminals or to alterations in synapse geometry cannot be excluded.
Importantly, Cx43 expression in astrocytes is regulated by numerous molecules originating from different cell types during various physiological and pathological situations [42], including stress exposure [43], depression [44], sleep recovery [45] or autism [46]. This study therefore suggests that dynamic modulations of Cx43 expression might contribute to synaptic plasticity and behaviour by setting the amount of presynaptic glutamate release.
Acknowledgements
We thank J. Teillon and Y. Dupraz for technical assistance, L. Hennekinne, M. Renner and A. Triller for their help with co-localization analysis, G. Dallérac for helpful comments on the manuscript and K. Willecke for providing the Cx43fl/fl:hGFAP-Cre mice.
Funding statement
This work was supported by grants from the HFSPO (Career Development Award), ANR (Programme Jeunes Chercheurs and Programme Blanc Neurosciences), Fédération pour la Recherche sur le Cerveau (FRC), INSERM and La Pitié Salpêtrière hospital (Translational research contract) to N.R., from Fondation pour la Recherche Médicale (FRM) to O.C. and from French Research Ministry and Deutsche Forschungsgemeinschaft for U.P.
References
- 1.Ben Achour S, Pascual O. 2012. Astrocyte–neuron communication: functional consequences. Neurochem. Res. 37, 2464–2473. ( 10.1007/s11064-012-0807-0) [DOI] [PubMed] [Google Scholar]
- 2.Halassa MM, Haydon PG. 2010. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 72, 335–355. ( 10.1146/annurev-physiol-021909-135843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pannasch U, Rouach N. 2013. Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci. 36, 405–417. ( 10.1016/j.tins.2013.04.004) [DOI] [PubMed] [Google Scholar]
- 4.Theis M, Sohl G, Eiberger J, Willecke K. 2005. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 28, 188–195. ( 10.1016/j.tins.2005.02.006) [DOI] [PubMed] [Google Scholar]
- 5.Wiencken-Barger AE, Djukic B, Casper KB, McCarthy KD. 2007. A role for connexin43 during neurodevelopment. Glia 55, 675–686. ( 10.1002/glia.20484) [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Yu HX, Sun ML, Wang Y, Xi W, Yu YQ. 2014. Astrocyte-restricted disruption of connexin-43 impairs neuronal plasticity in mouse barrel cortex. Eur. J. Neurosci. 39, 35–45. ( 10.1111/ejn.12394) [DOI] [PubMed] [Google Scholar]
- 7.Frisch C, Theis M, De Souza Silva MA, Dere E, Sohl G, Teubner B, Namestkova K, Willecke K, Huston JP. 2003. Mice with astrocyte-directed inactivation of connexin43 exhibit increased exploratory behaviour, impaired motor capacities, and changes in brain acetylcholine levels. Eur. J. Neurosci. 18, 2313–2318. ( 10.1046/j.1460-9568.2003.02971.x) [DOI] [PubMed] [Google Scholar]
- 8.Kunze A, et al. 2009. Connexin expression by radial glia-like cells is required for neurogenesis in the adult dentate gyrus. Proc. Natl Acad. Sci. USA 106, 11 336–11 341. ( 10.1073/pnas.0813160106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. 2006. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 26, 5438–5447. ( 10.1523/JNEUROSCI.0037-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. 2008. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322, 1551–1555. ( 10.1126/science.1164022) [DOI] [PubMed] [Google Scholar]
- 11.Pannasch U, Vargova L, Reingruber J, Ezan P, Holcman D, Giaume C, Sykova E, Rouach N. 2011. Astroglial networks scale synaptic activity and plasticity. Proc. Natl Acad. Sci. USA 108, 8467–8472. ( 10.1073/pnas.1016650108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF. 2009. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J. Neurosci. 29, 7743–7752. ( 10.1523/JNEUROSCI.0341-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias LA, Wang DD, Kriegstein AR. 2007. Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448, 901–907. ( 10.1038/nature06063) [DOI] [PubMed] [Google Scholar]
- 14.Harris AL. 2001. Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 34, 325–472. ( 10.1017/S0033583501003705) [DOI] [PubMed] [Google Scholar]
- 15.Roux L, Benchenane K, Rothstein JD, Bonvento G, Giaume C. 2011. Plasticity of astroglial networks in olfactory glomeruli. Proc. Natl Acad. Sci. USA 108, 18 442–18 446. ( 10.1073/pnas.1107386108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dere E, De Souza-Silva MA, Frisch C, Teubner B, Sohl G, Willecke K, Huston JP. 2003. Connexin30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes. Eur. J. Neurosci. 18, 629–638. ( 10.1046/j.1460-9568.2003.02784.x) [DOI] [PubMed] [Google Scholar]
- 17.Theis M, et al. 2003. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J. Neurosci. 23, 766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannasch U, et al. 2014. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat. Neurosci. 17, 549–558. ( 10.1038/nn.3662) [DOI] [PubMed] [Google Scholar]
- 19.Pannasch U, Sibille J, Rouach N. 2012. Dual electrophysiological recordings of synaptically-evoked astroglial and neuronal responses in acute hippocampal slices. J. Vis. Exp. 69, e4418 ( 10.3791/4418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racine V, Sachse M, Salamero J, Fraisier V, Trubuil A, Sibarita JB. 2007. Visualization and quantification of vesicle trafficking on a three-dimensional cytoskeleton network in living cells. J. Microsc. 225, 214–228. ( 10.1111/j.1365-2818.2007.01723.x) [DOI] [PubMed] [Google Scholar]
- 21.Olk S, Zoidl G, Dermietzel R. 2009. Connexins, cell motility, and the cytoskeleton. Cell Motil. Cytoskeleton 66, 1000–1016. ( 10.1002/cm.20404) [DOI] [PubMed] [Google Scholar]
- 22.Tzingounis AV, Wadiche JI. 2007. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neurosci. 8, 935–947. ( 10.1038/nrn2274) [DOI] [PubMed] [Google Scholar]
- 23.Dallerac G, Chever O, Rouach N. 2013. How do astrocytes shape synaptic transmission? Insights from electrophysiology. Front. Cell Neurosci. 7, 159 ( 10.3389/fncel.2013.00159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schools GP, Zhou M, Kimelberg HK. 2006. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J. Neurophysiol. 96, 1383–1392. ( 10.1152/jn.00449.2006) [DOI] [PubMed] [Google Scholar]
- 25.Hwang EM, et al. 2014. A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat. Commun. 5, 3227 ( 10.1038/ncomms4227) [DOI] [PubMed] [Google Scholar]
- 26.Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, Kimelberg HK, Chen H. 2009. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J. Neurosci. 29, 8551–8564. ( 10.1523/JNEUROSCI.5784-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holthoff K, Witte OW. 2000. Directed spatial potassium redistribution in rat neocortex. Glia 29, 288–292. () [DOI] [PubMed] [Google Scholar]
- 28.Quist AP, Rhee SK, Lin H, Lal R. 2000. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol. 148, 1063–1074. ( 10.1083/jcb.148.5.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura R, Harris KM. 1999. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 19, 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen DB, Braunstein TH, Nielsen MS, Macaulay N. 2014. Distinct permeation profiles of the connexin 30 and 43 hemichannels. FEBS Lett. 588, 1446–1457. ( 10.1016/j.febslet.2014.01.036) [DOI] [PubMed] [Google Scholar]
- 31.Giaume C, Tabernero A, Medina JM. 1997. Metabolic trafficking through astrocytic gap junctions. Glia 21, 114–123. () [DOI] [PubMed] [Google Scholar]
- 32.Schousboe A, Westergaard N, Hertz L. 1993. Neuronal-astrocytic interactions in glutamate metabolism. Biochem. Soc. Trans. 21, 49–53. [DOI] [PubMed] [Google Scholar]
- 33.Billups D, Marx MC, Mela I, Billups B. 2013. Inducible presynaptic glutamine transport supports glutamatergic transmission at the calyx of Held synapse. J. Neurosci. 33, 17 429–17 434. ( 10.1523/JNEUROSCI.1466-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, Reimer RJ. 2014. A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron 81, 888–900. ( 10.1016/j.neuron.2013.12.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kam K, Nicoll R. 2007. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J. Neurosci. 27, 9192–9200. ( 10.1523/JNEUROSCI.1198-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, Takano T, Nedergaard M. 2012. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci. Signal. 5, ra8 ( 10.1126/scisignal.2002160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stehberg J, et al. 2012. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 26, 3649–3657. ( 10.1096/fj.11-198416) [DOI] [PubMed] [Google Scholar]
- 38.Elias LA, Kriegstein AR. 2008. Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci. 31, 243–250. ( 10.1016/j.tins.2008.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santiago MF, Alcami P, Striedinger KM, Spray DC, Scemes E. 2010. The carboxyl-terminal domain of connexin43 is a negative modulator of neuronal differentiation. J. Biol. Chem. 285, 11 836–11 845. ( 10.1074/jbc.M109.058750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez Velazquez JL, Frantseva M, Naus CC, Bechberger JF, Juneja SC, Velumian A, Carlen PL, Kidder GM, Mills LR. 1996. Development of astrocytes and neurons in cultured brain slices from mice lacking connexin43. Brain Res. Dev. Brain Res. 97, 293–296. ( 10.1016/S0165-3806(96)00156-3) [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Yamaguchi K, Tatsukawa T, Nishioka C, Nishiyama H, Theis M, Willecke K, Itohara S. 2008. Lack of connexin43-mediated Bergmann glial gap junctional coupling does not affect cerebellar long-term depression, motor coordination, or eyeblink conditioning. Front. Behav. Neurosci. 2, 1 ( 10.3389/neuro.08.001.2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. 2010. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 11, 87–99. ( 10.1038/nrn2757). [DOI] [PubMed] [Google Scholar]
- 43.Irwin LN. 2001. Gene expression in the hippocampus of behaviorally stimulated rats: analysis by DNA microarray. Brain Res. Mol. Brain Res. 96, 163–169. ( 10.1016/S0169-328X(01)00269-8) [DOI] [PubMed] [Google Scholar]
- 44.Bernard R, et al. 2011. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol. Psychiatry 16, 634–646. ( 10.1038/mp.2010.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franco-Perez J, Ballesteros-Zebadua P, Fernandez-Figueroa EA, Ruiz-Olmedo I, Reyes-Grajeda P, Paz C. 2012. Sleep deprivation and sleep recovery modifies connexin36 and connexin43 protein levels in rat brain. Neuroreport 23, 103–107. ( 10.1097/WNR.0b013e32834e8fcb) [DOI] [PubMed] [Google Scholar]
- 46.Fatemi SH, Folsom TD, Reutiman TJ, Lee S. 2008. Expression of astrocytic markers aquaporin 4 and connexin 43 is altered in brains of subjects with autism. Synapse 62, 501–507. ( 10.1002/syn.20519) [DOI] [PMC free article] [PubMed] [Google Scholar]