Abstract
Carbon nanotubes (CNTs) have shown much promise in neurobiology and biomedicine. Their structural stability and ease of chemical modification make them compatible for biological applications. In this review, we discuss the effects that chemically functionalized CNTs, applied as colloidal solutes or used as strata, have on the morpho-functional properties of astrocytes, the most abundant cells present in the brain, with an insight into the potential use of CNTs in neural prostheses.
Keywords: carbon nanotubes, graft copolymers, astrocytes, morphology, glial fibrillary acidic protein, proliferation
1. Carbon nanotubes as biocompatible material
Carbon nanotubes (CNTs) with their many desirable properties (size, strength, flexibility, conductivity, etc.) have shown much promise in biomedical applications, especially in neural prostheses. They are relatively inert and can be chemically modified using various functional groups depending on the application.
CNTs are made up of a single sheet of graphene rolled into a cylinder. They can consist of a single cylinder of graphene, single-walled CNTs (SWCNTs), with diameters ranging from 0.4 to 2 nm, or of multiple coaxial cylinders, multi-walled CNTs (MWCNTs), with their outer diameters ranging from 2 to 100 nm and inner diameters ranging from 1 to 3 nm, and lengths ranging from 1 to several micrometres; these coaxial structures can also assume an intermediate form, i.e. double-walled CNTs. CNTs are produced using a variety of methods, including electric arc, laser ablation and chemical vapour deposition in the presence of catalysts, usually Fe, Ni, Co, Y or Mo, or their combinations (e.g. nickel and yttrium, typically, in approx. 4 : 1 weight ratio). Depending on the conformation of the carbon atoms in the graphene sheet, CNTs have three different configurations, which also dictate their conductivity: armchair, chiral or zigzag. All armchair CNTs are metallic, whereas chiral or zigzag CNTs can either be metallic or semiconducting (reviewed in [1,2]).
To enhance the biocompatibility of the CNTs, as well as their dispersion in aqueous media, they can be modified via non-covalent or covalent attachment of molecules. A wide range of compounds such as DNA, proteins and lipids can be adsorbed onto the CNTs creating non-covalently functionalized CNTs. However, for a permanent attachment, the compounds have to be covalently linked to the CNTs. The most common way to do this is by incubating the CNTs with a strong oxidizing agent, e.g. nitric acid, which adds carboxyl groups to the ends of the CNTs or any defect sites. To obtain CNTs linked to other compounds, this carboxyl group can be converted to an acyl chloride intermediate, which can then be reacted with the compound of interest, for example, polyethylene glycol (PEG) or poly-m-aminobenzene sulfonic (PABS) acid (reviewed in [3]).
2. Astroglia: the homeostatic scaffold of the central nervous system
Astroglia are a highly heterogeneous population of cells of ectodermal (i.e. neural) origin that provide for homeostasis in the central nervous system (CNS) [4]. In the grey matter, astrocytes define the micro-architecture through parcellation of the nervous tissue into relatively independent functional domains known as neuro-vascular units [5]. For example, protoplasmic astrocytes in the CA1 stratum radiatum of the hippocampus occupy separate anatomical domains. Within these domains, astrocytes provide a functional link between neurons and vasculature, and control interstitial space. Peripheral astroglial processes and perisynaptic membrane sheath provide the intimate coverage of the majority (60–80%) [6,7] of synapses, with preferential association with large perforated ones. Perisynaptic glial processes are endowed with a high density of numerous molecules responsible for various homeostatic pathways. These include: (i) interstitial potassium homeostasis, (ii) homeostasis of main neurotransmitters such as glutamate, γ-aminobutyric acid (GABA) and adenosine, (iii) water homeostasis, (iv) pH homeostasis, (v) energy support, and (vi) control over reactive oxygen species, etc. (for details, see [8]).
Conceptually, therefore, astroglia represent a functional scaffold that defines the spatial organization of the synaptic neuronal network and maintains synaptic connectivity. Through numerous receptors expressed in astroglial cells (which essentially are represented by receptors to all neurotransmitters, neuro-modulators and hormones functionally present in the CNS) [9], astrocytes are able to monitor neuronal activity precisely and accordingly adjust their homeostatic support [10]. In addition, astrocytes may contribute to information processing in the CNS and act as secretory elements able to release a multitude of neuroactive substances important for long-lasting regulation of neuronal function and synaptic plasticity [11]. Astroglia are also capable of mounting complex defensive responses to brain injury (generally referred to as reactive astrogliosis) that are fundamental for the progression and outcome of essentially all neurological disorders [12,13].
Because CNTs are considered suitable candidates for drug delivery or brain–machine interface (BMI) applications, it is critically important to understand the effects that CNTs have on astrocytes. CNTs can be used in biological applications either as colloidal solutes or as substrates/strata on which the cells can attach and grow. Here, we review the effects that these two modalities of CNTs have on the morphology and function of astrocytes in culture together with an insight into the potential applications of these CNTs in neural prostheses. We also provide some technical and methodological aspects used in these studies.
3. Effect of carbon nanotubes as colloidal solutes on astrocytes
One of the challenges with the use of CNTs, owing to their hydrophobic nature, has been to disperse them in aqueous solutions, which is a prerequisite for many biological applications. As alluded to in §1, one way to circumvent this problem is to chemically functionalize the CNTs with water-soluble polymers such as PEG or PABS, rendering the resulting graft co-polymers water soluble. Detailed reviews on the biofunctionalization of the CNTs are available elsewhere [14]. Previous work in our group by Ni et al. [15] achieved selected neurite outgrowth by treating neonatal rat hippocampal neurons grown on polyethyleneimine (PEI)-coated glass coverslips with colloidal solutes of water-soluble SWCNT (ws-SWCNTs) graft copolymers, either SWCNT-PEG or SWCNT-PABS. These effects on neurite outgrowth were due to an imbalance between exocytotic incorporation of vesicles into the plasma membrane of neurons, and the endocytotic retrieval, as we showed in a follow up study by Malarkey et al. [16]: as endocytosis was specifically hampered by CNTs, there was an enhanced outgrowth of selected neurites. As an extension to these findings, an in vivo study was done wherein the application of SWCNT-PEG at the site of injury decreased lesion volume, increased neurofilament-positive fibres and corticospinal tract fibres in the lesion, and did not increase reactive astrogliosis, assessed by glial fibrillary acidic protein (GFAP) immunoreactivity (GFAP-ir), in an acute spinal cord injury model performed in adult Sprague–Dawley rats; CNTs also modestly improved hindlimb locomotor recovery [17]. Although the in vivo study gave us an insight into the effects of ws-SWCNTs on astrocytes, they have not been thoroughly investigated. Hence, we designed a subsequent study to systematically assess the effects of ws-SWCNTs on the morphology and function of astrocytes in culture, which is reviewed next.
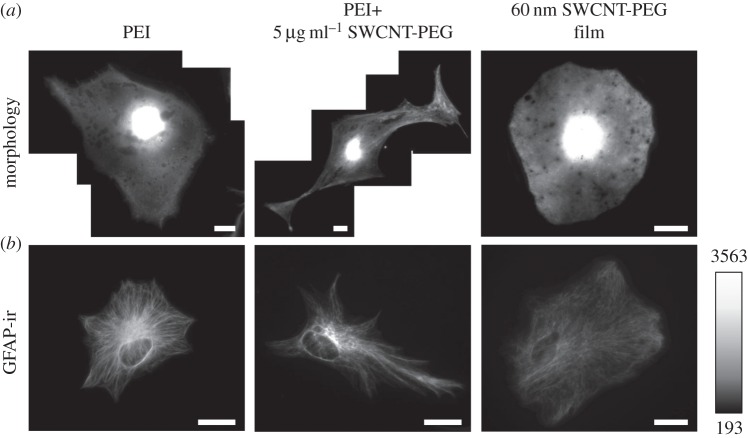
To investigate the effects that the dispersible colloidal solutes of CNTs have on astrocytes in culture, graft copolymers of SWCNTs chemically functionalized with PEG (figure 1) have been used as described by Gottipati et al. [12]. We used astrocytes isolated from the visual cortex of 0–2 day old C57BL/6 pups and plated them onto glass coverslips pre-coated with PEI in the absence or the presence of SWCNT-PEG and systematically assessed the changes in the morphology of astrocytes. After 3 days in culture, we loaded the cells with calcein, a vital fluorescent dye, and imaged them live using a fluorescence microscope (figure 2a). Based on the calcein images, the area and perimeter of the astrocytes were measured, the values of which were used to calculate the form factor (FF), defined by 4π(area)/(perimeter)2 [21]. Treatment of astrocytes with SWCNT-PEG (5 µg ml−1), resulted in an increasing trend of their cell area and a significant increase in the perimeter together with a significant decrease in the FF, the latter reporting on cell stellation, compared to the untreated astrocytes (table 1).
Figure 1.
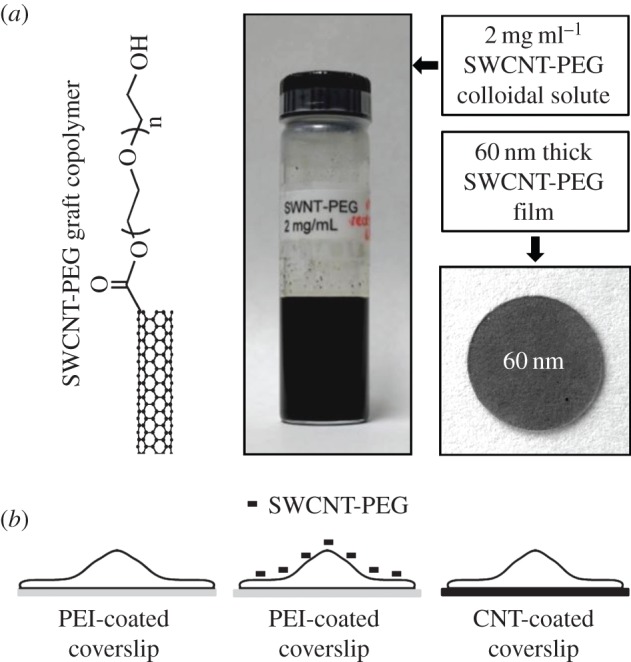
The experimental set-up for the use of solute and film modalities of SWCNT-PEG on astrocytes. (a) Schematic of the SWCNT-PEG graft polymer structure (left), a vial containing SWCNT-PEG colloidal solute at a stock concentration of 2 mg ml−1 (middle) and an image of a glass coverslip (12 mm in diameter) top-coated with 60 nm thick SWCNT-PEG film (right). (b) Scheme to represent the experimental set-up with astrocytes plated onto PEI-coated coverslips in the absence (left) or the presence of SWCNT-PEG colloidal solute (middle) or onto the SWCNT-PEG film (right). Drawings are not to scale. Chemical structure and images in (a) are reproduced (left–right) from Gottipati et al. [12], Lee & Parpura [18] and Malarkey et al. [19], respectively. (Online version in colour.)
Figure 2.
Example images of astrocytes showing the effects induced by the solute and film modalities of SWCNT-PEG on their morphology and GFAP-ir. (a) Images of astrocytes in culture plated onto the PEI-coated coverslips in the absence and the presence of 5 µg ml−1 SWCNT-PEG solute and onto the 60 nm thick SWCNT-PEG film, loaded with calcein, a vital fluorescent dye. (b) Images of astrocytes plated in matching conditions as above and labelled for GFAP using indirect immunocytochemistry. Grey scale is a linear representation of the fluorescence intensities of the pixels in the images, expressed in fluorescence intensity units (i.u.). Scale bar, 20 μm. Adapted from [12,20].
Table 1.
Summary of the effects induced by the two modalities of SWCNT-PEG on cultured mouse cortical astrocytes compared to that on PEI-coated coverslips (configuration as in figure 1) based on the results from Gottipati et al. [12,20]. Dash indicates no change, whereas arrows indicate an increase (up) or decrease (down) in the measurements; ND, not determined measurements/effects.
| cell property | parameter | PEI + 5 µg ml−1 SWCNT-PEG | 60 nm SWCNT-PEG film |
|---|---|---|---|
| morphology | area | ― | ↑ |
| perimeter | ↑ | ― | |
| FF | ↓ | ↑ | |
| GFAP-ir | density | ↑ | ↓ |
| content | ↑ | ― | |
| occupancy | ↑ | ― | |
| vitality | adhesion | ND | ↑ |
| proliferation | ND | ↑ | |
| death | ND | ― |
Similar changes in astrocytic morphology, to those that we observed and reminiscent of stellation, have been linked to an increase in the levels of GFAP with functional consequences on astrocytic physiology [22,23]. To address this, we labelled astrocytes plated onto PEI-coated coverslips in the absence or the presence of 5 µg ml−1 SWCNT-PEG for GFAP using indirect immunocytochemistry (figure 2b). GFAP-ir was visualized using fluorescence microscopy and three GFAP-ir parameters were assessed: density (average fluorescence intensity per pixel of the total area of a cell), content (density × total area of the cell) and occupancy (positive/total cell pixels). We found that SWCNT-PEG caused an increase in the density, content and occupancy of GFAP-ir in astrocytes compared with the untreated cells (table 1). In addition to SWCNT-PEG, we also assessed the effects of SWCNTs chemically functionalized with PABS on astrocytes. We found that SWCNT-PABS caused qualitatively similar effects on the morphology and GFAP-ir of astrocytes; at a concentration of 5 µg ml−1 they caused a significant increase in the area and perimeter of the astrocytes along with a decrease in the FF and also a significant increase in all three GFAP-ir parameters. As the SWCNT backbone is the common feature in the copolymers used, unlike grafts that are substantially different (PEG versus PABS), the generality of data indicates that the SWCNT backbone is necessary for the changes induced by the water-soluble graft copolymers; however, any subtle differences in the effects that SWCNTs might have on astrocytes could be due to the different functional groups attached to the SWCNTs.
Taken together, these studies on astrocytes using colloidal solutes of CNTs show that the CNTs have a potential to modulate the properties of astrocytes by making them larger and stellate in culture while increasing the immunoreactivity of GFAP (summarized in table 1). However, these effects on astrocytes can potentially vary depending on the properties of the CNTs (backbone, functional group, concentration, etc.).
The work by Bardi et al. [24] emphasizes the importance of functionalization of CNTs with respect to the brain tissue tolerance of these nanomaterials. The authors have investigated the interactions between chemically functionalized multi-walled carbon nanotubes (f-MWCNTs) and neural tissue following a cortical stereotactic administration using two different f-MWCNTs, shortened (by oxidation) amino-functionalized MWCNT (oxMWCNT- ) and amino-functionalized MWCNT (MWCNT-
) and amino-functionalized MWCNT (MWCNT-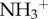 ), in C57BL/6 mice. Coronal brain tissue sections were made and labelled for GFAP at 3 and 30 days post-stereotactic injection. GFAP staining was similar in both f-MWCNTs injected groups compared to the control, 3 days post-injection. However, 30 days post-injection, oxMWCNT-
), in C57BL/6 mice. Coronal brain tissue sections were made and labelled for GFAP at 3 and 30 days post-stereotactic injection. GFAP staining was similar in both f-MWCNTs injected groups compared to the control, 3 days post-injection. However, 30 days post-injection, oxMWCNT-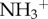 induced higher expression of GFAP, while MWCNT-
induced higher expression of GFAP, while MWCNT-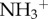 did not lead to an overexpression of GFAP. To further investigate this effect, the authors used a third type of f-MWCNTs that were initially oxidized and then further modified by performing amidation of the carboxylic groups at the surface of the MWCNTs using triethylene diamine (oxMWCNT-amide-
did not lead to an overexpression of GFAP. To further investigate this effect, the authors used a third type of f-MWCNTs that were initially oxidized and then further modified by performing amidation of the carboxylic groups at the surface of the MWCNTs using triethylene diamine (oxMWCNT-amide-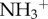 ) which also caused an increased expression of GFAP, 30 days post-injection. The authors attributed this to the remaining unreacted carboxyl groups at the surface of the oxMWCNT-amide-
) which also caused an increased expression of GFAP, 30 days post-injection. The authors attributed this to the remaining unreacted carboxyl groups at the surface of the oxMWCNT-amide-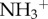 , presumably provoking the observed GFAP response.
, presumably provoking the observed GFAP response.
Thus, while functionalized CNTs could be envisioned as useful injectable materials modulating astrocyte form and function, we still lack detailed information for CNT–brain parenchyma interactions before these novel nanomaterials can be seriously taken into consideration for neural prosthetics.
4. Carbon nanotubes as strata for astrocytic growth
Previous work in our group has used retainable films of CNTs functionalized with different water-soluble polymers (PEI and PEG) as strata for the growth of neural cells [19,25]. It was found that the different graft copolymers have different effects on the morphology of neurons, i.e. neurite outgrowth, number of growth cones and cell body size, depending on the chemical (functional groups attached to the CNTs) or physical (thickness) properties of the CNT films. These findings were further extended to a systematic study on the effects of a subset of CNT films on astrocytes in culture [20], which is reviewed next.
To assess the effects of CNT planar scaffolds on astrocytic growth, we used retainable SWCNT-PEG films of varying thickness (10, 30 and 60 nm) created by spraying a dispersion of SWCNT-PEG onto hot glass coverslips (figure 1). As in our previous work with colloidal solutes and astrocytes, we assessed the changes in the morphology and function of astrocytes plated onto the CNT films in comparison to the cells plated onto PEI-coated coverslips in culture. We loaded the astrocytes with calcein, after 3 days in culture, and assessed their morphological parameters (figure 2a). We found that the cells plated onto the 10 nm films showed no significant difference in their cell area and perimeter but there was a significant increase in the FF, i.e. astrocytes became rounder, when compared to those on PEI. However, the astrocytes plated onto CNT films of higher thicknesses (30 and 60 nm) showed an increase in their cell area and the FF with no apparent difference in perimeter compared to the cells plated onto PEI. These findings imply that the CNT films onto which the astrocytes are plated in culture make them larger and rounder (table 1). We also observed that the FF of the astrocytes plated onto the 10 nm films was higher than that on the 60 nm films, implying that the effects of CNT films on the astrocytes are differently modulated depending on the thickness of the films. We then investigated the effect of these changes in the morphology of astrocytes on the levels of GFAP when plated onto CNT films of varying thickness (figure 2b). The density of GFAP-ir showed a decreasing trend with an increase in the thickness of the CNT films, with a significant difference only in the astrocytes plated onto the 60 nm films, in comparison to that on PEI. The content and occupancy of GFAP-ir, however, remained unchanged across the groups compared. Interestingly, the GFAP-ir occupancy of the cells plated onto the 60 nm films was significantly lower than that on the 10 nm films, further emphasizing that CNT strata of different thicknesses have differential effects on astrocytes.
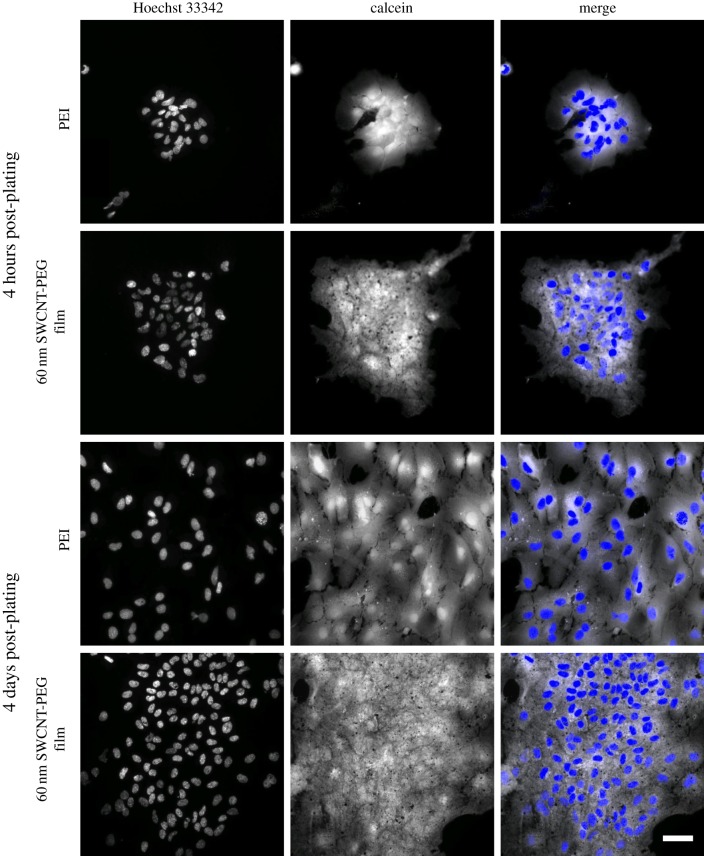
Since changes in the expression of GFAP are often linked to changes in the proliferation characteristics of astrocytes, we assessed this possibility by loading the cells, plated onto PEI- or CNT-coated coverslips, with calcein and Hoechst 33342, a cell permeable nuclear stain, 4 h and 4 days post-plating (figure 3). The 4 h time point was used to estimate the initial plating density as well as the adhesion of astrocytes plated onto the CNT films in comparison to that on PEI, while the 4 day time point was used as an estimate for the proliferation of astrocytes. The cells positive for both calcein and Hoechst were considered live, whereas the cells positive for Hoechst and negative for calcein were considered dead. We calculated the relative density of live cells across the different CNT strata by normalizing the number of live cells in each group and time point to the median number of live cells on the PEI-coated coverslips, 4 h post-plating. We showed that the relative density of live cells plated onto the CNT films, 4 h post-plating, was significantly higher compared with that on PEI (table 1). In addition, the relative density of live cells plated onto the 60 nm films was significantly higher than that on the 10 nm films. These results imply that the astrocytes preferentially adhere to the CNT films compared to PEI and that this adhesion increases with an increase in the thickness of the films. At the 4 day time point, the 10 nm films did not show a significant increase in their relative density of live cells compared with the cells grown on PEI. The cells grown on the thicker (30 and 60 nm) films, however, showed a statistically significant increase, implying that there is an increased proliferation of the astrocytes on the CNT films in comparison to PEI (table 1); this effect was disproportionately higher than expected from the enhanced adhesion of astrocytes onto the CNT films. At both time points the percentage of dead cells, however, was not affected by the films (table 1).
Figure 3.
Example images of astrocytes showing the effects induced by the SWCNT-PEG films on their adhesion and proliferation. Images of astrocytic nuclei labelled with Hoechst 33342, a cell permeable nuclear dye (left column), and the corresponding astrocytes loaded with calcein, a vital fluorescent dye (middle column). Right column shows the merge of the images. (top set) The top two rows show images of astrocytes plated onto the PEI-coated coverslips and onto the 60 nm thick CNT films, respectively, 4 h post-plating and assessing cell adhesion. (bottom set) The bottom two rows show images of astrocytes plated in matching conditions as above, but imaged at the later time point, 4 days post-plating, and assessing cell proliferation. Scale bar, 50 μm. Reproduced from [20]. (Online version in colour.)
Of note, the initial study to assess the potential use of CNTs as strata for astrocytes was done by Lovat et al. [26]. They coated glass coverslips with MWCNTs by first functionalizing the MWCNTs with pyrrolidine groups, applying them to the glass and then removing the functionalization with high temperature to yield non-functionalized MWCNTs that were used as film/stratum for cell growth. Rat hippocampal astrocytes were plated onto these MWCNT-coated coverslips; plain glass coverslips were used as control. They labelled the cells for GFAP to assess the effects of the MWCNT films on astrocytes and reported qualitatively that, after 8 days in culture, they did not find any differences in the morphology and proliferation of astrocytes compared to those on bare glass coverslips [26].
An in vivo study by Lu et al. [27] reported the use of electrochemically co-deposited polypyrrole/SWCNT (PPy/SWCNT) films onto Pt microelectrodes for improving the electrode–neural interface in the brains of Sprague–Dawley rats. Six weeks post-implantation, horizontal sections were made from the brain tissue of the rats receiving the film-coated Pt implants and stained for GFAP; uncoated Pt implants were used as the control. The authors quantitatively analysed the GFAP intensity profiles of bare and PPy/SWCNT-coated Pt implants as a function of distance from the interface and showed that the intensity of GFAP immunostaining in the test group was significantly lower than in the control group along a length approximately 100 µm to the implant interface. A recent study by Zhou et al. [28] also used Pt microelectrodes coated with CNTs for neural prostheses applications. The authors coated Pt wires with MWCNT-doped poly(3,4-ethylenedioxythiophene) (PEDOT) composite films and implanted them into Sprague–Dawley rats with uncoated Pt wires as the control. Similar to the above-mentioned study, horizontal sections were made from the tissue surrounding the implants, six weeks post-implantation, and stained for GFAP. Quantitative analysis of GFAP intensity profiles of bare and PEDOT:MWCNT film-coated Pt implants as a function of the distance from the interface indicated that the intensity of GFAP immunostaining in the bare Pt implants group was significantly higher than that of those in the PEDOT:MWCNT-coated Pt implants group along a length 150 µm to the implant interface.
Taken together, these results show that films of CNTs can modulate the morpho-functional properties of astrocytes in culture by making the cells larger and rounder while decreasing the immunoreactivity of GFAP and enhancing the adhesion and proliferation of astrocytes (summarized in table 1). These changes in astrocytic properties are dependent on the physical properties of the films (e.g. film thickness) similar to the work on neurons. Also, the consistent finding across the different research groups that CNT-coated electrodes lead to a reduced activation of astrocytes, as evidenced by the expression of GFAP, together with our data in vitro further emphasize the potential of CNTs in BMI applications.
5. Concluding remarks
It is apparent from the reviewed body of work that chemically functionalized ws-SWCNTs, applied as colloidal solutes or used as strata, can affect the properties of astrocytes in culture, namely their morphology and function. There is a lot of scope for research in this field as astrocytic properties could be specifically modulated depending on need by changing the physical and chemical properties of the CNTs.
An in vivo study in an acute spinal cord injury model performed in adult rats showed that SWCNT-PEG solutes applied at the site of injury can modestly improve hindlimb locomotor recovery [17]. At present, however, it is not apparent how these proof-of-principle experiments in rodents could be realistically implemented in human medicine in the near future. A promising venture is the use of CNTs as coating material for electrodes, i.e. BMI applications. Indeed, CNT-coated electrodes have been shown to outperform traditional brain implants (tungsten and stainless steel electrodes) by improving electrical stimulation and recordings of neurons, both in vitro and in vivo [29,30]. CNT coating can also cause a decrease in reactive astrogliosis [27,28], the adaptive/defensive response of astrocytes to injuries such as a ‘stab’ wound due to electrode implantation leading to a scar formation that prevents the regrowth of damaged neurons, hence providing an opportunity for brain parenchyma recovery.
Some of the future work in the field should include assessing the effects of colloidal solutes on the vitality (adhesion, proliferation and death) of astrocytes, as has been done using CNT films (this would populate the ‘not determined’ category of table 1). Another interesting venture would be to elucidate the dependence of the effects induced by the two modalities of SWCNTs on GFAP, by perhaps using a GFAP knockout model. In other words, is GFAP necessary for the morphological and proliferative changes of astrocytes induced by the different CNT modalities or are such changes independent of GFAP, with the expression of this astrocyte-specific intermediate filament being just a bystander effect?
In addition to CNTs, closely related carbon nanofibres (CNFs) have showed promise in experimental ability to interface with neural cells, which could aid future BMI applications. CNF-based devices have improved detection of the chemical and electrical activity of brain tissue and also stimulation paradigms of it. A trio of examples corroborates this statement: Yu et al. [31] used vertically aligned CNFs (VACNFs) to generate microelectrode arrays, which they used to stimulate neurons and record from these cells in cultured organotypic hippocampal slices. The work by de Asis et al. [32] has shown the advantages of using PPy-coated VACNF microbrush arrays to safely stimulate acute hippocampal slices and enhance evoked electrical activity compared to standard tungsten wire electrodes. Rand et al. [33] have shown the effectiveness of VACNFs for the simultaneous detection of dopamine and serotonin, with better selectivity and lower detection limits for both neurotransmitters compared to a conventional glassy carbon electrode.
In spite of the vast potential that CNTs and other carbon nanomaterials have in biomedical applications, neurotoxicity still remains a concern [34,35]. Hence, exposure limits to these nanomaterials, which are currently unavailable, need to be established before they are used for neural prostheses applications in future.
Funding statement
The authors’ work is supported by National Institutes of Health (The Eunice Kennedy Shriver National Institute of Child Health and Human Development award HD078678).
References
- 1.Bekyarova E, Ni Y, Malarkey EB, Montana V, McWilliams JL, Haddon RC, Parpura V. 2005. Applications of carbon nanotubes in biotechnology and biomedicine. J. Biomed. Nanotechnol. 1, 3–17. ( 10.1166/jbn.2005.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malarkey EB, Parpura V. 2007. Applications of carbon nanotubes in neurobiology. Neurodegener. Dis. 4, 292–299. ( 10.1159/000101885) [DOI] [PubMed] [Google Scholar]
- 3.Lee W, Parpura V. 2009. Carbon nanotubes as substrates/scaffolds for neural cell growth. Prog. Brain Res. 180, 110–125. ( 10.1016/S0079-6123(08)80006-4) [DOI] [PubMed] [Google Scholar]
- 4.Verkhratsky A, Butt AM. 2013. Glial physiology and pathophysiology. New York, NY: John Wiley & Sons. [Google Scholar]
- 5.Bushong EA, Martone ME, Jones YZ, Ellisman MH. 2002. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witcher MR, Kirov SA, Harris KM. 2007. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55, 13–23. ( 10.1002/glia.20415) [DOI] [PubMed] [Google Scholar]
- 7.Reichenbach A, Derouiche A, Kirchhoff F. 2010. Morphology and dynamics of perisynaptic glia. Brain Res. Rev 63, 11–25. ( 10.1016/j.brainresrev.2010.02.003) [DOI] [PubMed] [Google Scholar]
- 8.Verkhratsky A, Nedergaard M. 2014. Astroglial cradle in the life of the synapse. Phil. Trans. R. Soc. B 369, 20130595 ( 10.1098/rstb.2013.0595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parpura V, Verkhratsky A. 2013. Astroglial amino acid-based transmitter receptors. Amino acids 44, 1151–1158. ( 10.1007/s00726-013-1458-4) [DOI] [PubMed] [Google Scholar]
- 10.Verkhratsky A, Parpura V, Rodriguez JJ. 2011. Where the thoughts dwell: the physiology of neuronal-glial ‘diffuse neural net. Brain Res. Rev. 66, 133–151. ( 10.1016/j.brainresrev.2010.05.002) [DOI] [PubMed] [Google Scholar]
- 11.Parpura V, Zorec R. 2010. Gliotransmission: exocytotic release from astrocytes. Brain Res. Rev. 63, 83–92. ( 10.1016/j.brainresrev.2009.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottipati MK, Kalinina I, Bekyarova E, Haddon RC, Parpura V. 2012. Chemically functionalized water-soluble single-walled carbon nanotubes modulate morpho-functional characteristics of astrocytes. Nano Lett. 12, 4742–4747. ( 10.1021/nl302178s) [DOI] [PubMed] [Google Scholar]
- 13.Verkhratsky A, Parpura V. 2010. Recent advances in (patho)physiology of astroglia. Acta Pharmacol. Sinica 31, 1044–1054. ( 10.1038/aps.2010.108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bekyarova E, Haddon RC, Parpura V. 2007. Biofunctionalization of carbon nanotubes. In Biofunctionalization of nanomaterials, vol. 1 (ed. Kumar CSSR.), pp. 41–71. Wienheim, Germany: Wiley-VCH. [Google Scholar]
- 15.Ni Y, Hu H, Malarkey EB, Zhao B, Montana V, Haddon RC, Parpura V. 2005. Chemically functionalized water soluble single-walled carbon nanotubes modulate neurite outgrowth. J. Nanosci. Nanotechnol. 5, 1707–1712. ( 10.1166/jnn.2005.189) [DOI] [PubMed] [Google Scholar]
- 16.Malarkey EB, Reyes RC, Zhao B, Haddon RC, Parpura V. 2008. Water soluble single-walled carbon nanotubes inhibit stimulated endocytosis in neurons. Nano Lett. 8, 3538–3542. ( 10.1021/nl8017912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman JA, Niedzielko TL, Haddon RC, Parpura V, Floyd CL. 2011. Single-walled carbon nanotubes chemically functionalized with polyethylene glycol promote tissue repair in a rat model of spinal cord injury. J. Neurotrauma 28, 2349–2362. ( 10.1089/neu.2010.1409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee W, Parpura V. 2012. Dissociated cell culture for testing effects of carbon nanotubes on neuronal growth. In Neurotrophic factors (ed. Skaper SD.), pp. 261–276. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- 19.Malarkey EB, Fisher KA, Bekyarova E, Liu W, Haddon RC, Parpura V. 2009. Conductive single-walled carbon nanotube substrates modulate neuronal growth. Nano Lett. 9, 264–268. ( 10.1021/nl802855c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottipati MK, Samuelson JJ, Kalinina I, Bekyarova E, Haddon RC, Parpura V. 2013. Chemically functionalized single-walled carbon nanotube films modulate the morpho-functional and proliferative characteristics of astrocytes. Nano Lett. 13, 4387–4392. ( 10.1021/nl402226z) [DOI] [PubMed] [Google Scholar]
- 21.Wilms H, Hartmann D, Sievers J. 1997. Ramification of microglia, monocytes and macrophages in vitro: influences of various epithelial and mesenchymal cells and their conditioned media. Cell Tissue Res. 287, 447–458. ( 10.1007/s004410050769) [DOI] [PubMed] [Google Scholar]
- 22.Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML. 2004. Loss of glial fibrillary acidic protein results in decreased glutamate transport and inhibition of PKA-induced EAAT2 cell surface trafficking. Mol. Brain Res. 124, 114–123. ( 10.1016/j.molbrainres.2004.02.021) [DOI] [PubMed] [Google Scholar]
- 23.Wang YF, Hatton GI. 2009. Astrocytic plasticity and patterned oxytocin neuronal activity: dynamic interactions. J. Neurosci. 29, 1743–1754. ( 10.1523/JNEUROSCI.4669-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardi G, et al. 2013. Functionalized carbon nanotubes in the brain: cellular internalization and neuroinflammatory responses. PLoS ONE 8, e80964 ( 10.1371/journal.pone.0080964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu H, Ni Y, Mandal SK, Montana V, Zhao B, Haddon RC, Parpura V. 2005. Polyethyleneimine functionalized single-walled carbon nanotubes as a substrate for neuronal growth. J. Phys. Chem. B 109, 4285–4289. ( 10.1021/jp0441137) [DOI] [PubMed] [Google Scholar]
- 26.Lovat V, Pantarotto D, Lagostena L, Cacciari B, Grandolfo M, Righi M, Spalluto G, Prato M, Ballerini L. 2005. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 5, 1107–1110. ( 10.1021/nl050637m) [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Li T, Zhao X, Li M, Cao Y, Yang H, Duan YY. 2010. Electrodeposited polypyrrole/carbon nanotubes composite films electrodes for neural interfaces. Biomaterials 31, 5169–5181. ( 10.1016/j.biomaterials.2010.03.022) [DOI] [PubMed] [Google Scholar]
- 28.Zhou H, Cheng X, Rao L, Li T, Duan YY. 2013. Poly(3,4-ethylenedioxythiophene)/multiwall carbon nanotube composite coatings for improving the stability of microelectrodes in neural prostheses applications. Acta Biomater. 9, 6439–6449. ( 10.1016/j.actbio.2013.01.042) [DOI] [PubMed] [Google Scholar]
- 29.Ansaldo A, Castagnola E, Maggiolini E, Fadiga L, Ricci D. 2011. Superior electrochemical performance of carbon nanotubes directly grown on sharp microelectrodes. ACS Nano 5, 2206–2214. ( 10.1021/nn103445d) [DOI] [PubMed] [Google Scholar]
- 30.Keefer EW, Botterman BR, Romero MI, Rossi AF, Gross GW. 2008. Carbon nanotube coating improves neuronal recordings. Nat. Nanotechnol. 3, 434–439. ( 10.1038/nnano.2008.174) [DOI] [PubMed] [Google Scholar]
- 31.Yu Z, McKnight TE, Ericson MN, Melechko AV, Simpson ML, Morrison B., 3rd 2007. Vertically aligned carbon nanofiber arrays record electrophysiological signals from hippocampal slices. Nano Lett. 7, 2188–2195. ( 10.1021/nl070291a) [DOI] [PubMed] [Google Scholar]
- 32.de Asis ED, Jr, Nguyen-Vu TD, Arumugam PU, Chen H, Cassell AM, Andrews RJ, Yang CY, Li J. 2009. High efficient electrical stimulation of hippocampal slices with vertically aligned carbon nanofiber microbrush array. Biomed. Microdevices 11, 801–808. ( 10.1007/s10544-009-9295-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rand E, et al. 2013. A carbon nanofiber based biosensor for simultaneous detection of dopamine and serotonin in the presence of ascorbic acid. Biosens. Bioelectron. 42, 434–438. ( 10.1016/j.bios.2012.10.080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belyanskaya L, Weigel S, Hirsch C, Tobler U, Krug HF, Wick P. 2009. Effects of carbon nanotubes on primary neurons and glial cells. Neurotoxicology 30, 702–711. ( 10.1016/j.neuro.2009.05.005) [DOI] [PubMed] [Google Scholar]
- 35.Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A. 2007. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett. 168, 121–131. ( 10.1016/j.toxlet.2006.08.019) [DOI] [PubMed] [Google Scholar]