Abstract
Astrocytes are emerging as integral functional components of synapses, responding to synaptically released neurotransmitters and regulating synaptic transmission and plasticity. Thus, they functionally interact with neurons establishing tripartite synapses: a functional concept that refers to the existence of communication between astrocytes and neurons and its crucial role in synaptic function. Here, we discuss recent evidence showing that astrocytes are involved in the endocannabinoid (ECB) system, responding to exogenous cannabinoids as well as ECBs through activation of type 1 cannabinoid receptors, which increase intracellular calcium and stimulate the release of glutamate that modulates synaptic transmission and plasticity. We also discuss the consequences of ECB signalling in tripartite synapses on the astrocyte-mediated regulation of synaptic function, which reveal novel properties of synaptic regulation by ECBs, such as the spatially controlled dual effect on synaptic strength and the lateral potentiation of synaptic efficacy. Finally, we discuss the potential implications of ECB signalling for astrocytes in brain pathology and animal behaviour.
Keywords: tripartite synapses, endocannabinoids, astrocytes, gliotransmitter release, neuron–glia, communication
1. Introduction
The endocannabinoid (ECB) system is an important intercellular signalling system involved in a wide variety of physiological processes, including regulation of cardiovascular, gastrointestinal, liver and brain functions. It comprises several components: two types of seven transmembrane-domain G-protein coupled receptors (CB1 and CB2 receptors) that can be activated by exogenous ligands, such as Δ9-tetrahydrocannabinol (THC; the main psychoactive ingredient in marijuana), as well as endogenous transmitters called ECBs, such as anandamide and 2-arachidonoylglycerol, and a number of biosynthetic and degradative enzymes and transporters (for general reviews, see [1–3]).
ECB signalling in the brain affects multiple biological functions such as pain perception, food intake, learning and memory, anxiety and cognitive functions [4,5], through the regulation of synaptic transmission and plasticity as the main functional process. The canonical mechanism underlying ECB regulation of synaptic function occurs through retrograde signalling, i.e. the activity of the post-synaptic neuron leads to production and release of ECBs that diffuse in a retrograde manner across the synapse, and subsequently activate type 1 cannabinoid receptors (CB1Rs) present at the pre-synaptic terminal to inhibit neurotransmitter release [6,7]. CB1Rs are expressed at high levels by different neuronal types throughout the whole brain [3,8], which correlates with the wide implication of ECB signalling in a plethora of physiological and pathological processes.
Besides this canonical mechanism of ECB signalling, recent evidence has demonstrated a novel additional mechanism of modulation of synaptic function by ECBs that involves the participation of astrocytes [9–11]. Astrocytes are the most abundant glial cell type in the nervous system, and for years were considered to be passive elements providing structural and metabolic support for neurons. However, a wealth of evidence supports the notion that astrocytes are integral functional components of synapses, establishing functional tripartite synapses in which astrocytes process synaptic information and regulate synaptic function by reciprocally exchanging information with the neuronal synaptic elements [12,13]. Indeed, astrocytes respond with intracellular calcium elevations to neurotransmitters released from synaptic terminals [14–18]. These calcium elevations have been shown to stimulate the release of gliotransmitters through different mechanisms [19–24], which still remain debated (for recent reviews, see [25–27]).
ECB signalling between neurons and astrocytes has been shown to have consequences relevant for tripartite synapse function, having contributed to the establishment of this novel concept in synaptic physiology. While direct modulation of synaptic activity by ECB signalling has been recently discussed by thorough and excellent reviews [6,28,29], this review will highlight the emerging role of astrocytes in ECB signalling, and will discuss how astrocyte–neuron interaction mediated by ECB signalling in tripartite synapses provides novel mechanisms of synaptic modulation and adds further complexity to the ECB signalling effects.
2. Endocannabinoid signalling in neuron–astrocyte communication
For years, the existence and role of CB1Rs in astrocytes has been a controversial issue. Earlier studies showed discrepancies in the expression of CB1Rs by astrocytes in culture (see [30]), which could arise from the well-known phenotypic changes in astrocytes in different culture conditions. Indeed, several studies indicated the presence of such changes in astrocytes in culture and in situ [30–35]. The extremely low levels of CB1R expression in this cell type questioned their functional relevance in ECB signalling. However, recent evidence has demonstrated that hippocampal and cortical astrocytes in situ express functional CB1Rs that mediate relevant effects on astrocyte–neuron communication and synaptic modulation.
In 2008, our group demonstrated that CB1Rs expressed in hippocampal astrocytes in situ could be activated by exogenous cannabinoid ligands as well as by ECBs released by neurons. This activation increased astrocyte Ca2+ levels through the mobilization of Ca2+ from internal stores [9]. The intracellular signalling pathway underlying this effect exhibited specific characteristics. While it is well known that CB1Rs are preferentially coupled to pertussis toxin-sensitive Gi/o proteins that regulate cAMP levels [3], ECB-induced astrocyte Ca2+ elevations were mediated by CB1Rs coupled to Gq/11 proteins that activate phospholipase C and produce inositol triphosphate [9].
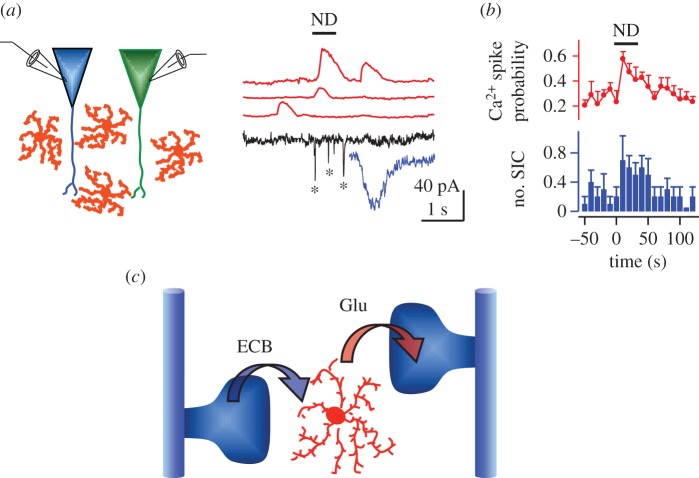
This study also provided evidence that the increased astrocyte Ca2+ levels evoked by ECBs released from pyramidal neurons stimulated the release of glutamate from astrocytes. This gliotransmitter activated NMDA receptors (NMDARs), evoking slow inward currents (SICs) in adjacent CA1 pyramidal neurons [9]. These results indicate that neurons and astrocytes communicate via ECB signalling and suggest the existence of intercellular communication pathways mediated by ECB–glutamate signalling in which astrocytes serve as a bridge for non-synaptic interneuronal communication (figure 1).
Figure 1.
Endocannabinoids released by neurons elevate calcium and stimulate glutamate release in astrocytes. (a) Stimulating ECB release by neuronal depolarization (ND; horizontal bar) of a CA1 hippocampal pyramidal neuron elevates astrocyte Ca2+ levels (red traces) and increase the number of slow inward currents (SICs; marked with asterisks) in an adjacent neuron (black trace); a single SIC is expanded (blue trace). (b) The astrocyte Ca2+ spike probability (red) and the mean number of neuronal SICs (blue) were enhanced after the neuronal depolarization (horizontal bar). (c) Schematic drawing representing the ECB-mediated neuron–astrocyte signalling and the subsequent glutamate-mediated astrocyte–neuron communication. Adapted from [9].
The presence of functional CB1Rs that, upon activation, evoke astrocyte Ca2+ elevations in situ has also been recently demonstrated in human brain tissue [36], suggesting that ECB-mediated neuron–astrocyte signalling and its functional consequences, originally observed in rodents, are also present in human brain. The fact that astrocytes express functional CB1Rs has important implications for our current understanding of the cellular basis of the behavioural effects of exogenous cannabinoids and ECBs.
3. Role of astrocytes in the regulation of synaptic transmission by endocannabinoids
The canonical mechanism by which ECBs regulate synaptic transmission is through retrograde signalling from the post-synaptic neuron to the pre-synaptic terminal. ECBs produced and released during the activity of the post-synaptic cell activate pre-synaptic CB1Rs that suppress neurotransmitter release, leading to short- and long-term synaptic plasticity [6,8,29,37–39]. Nevertheless, recent studies in goldfish [40], lamprey spinal cord [41] and mouse hippocampal slices [10] have reported that CB1R activation can also transiently enhance neurotransmission. Cannabinoid and ECB effects in brain physiology have been largely thought to be exclusively mediated by CB1Rs present in neurons. However, recent evidence has challenged this idea, proposing additional mechanisms based on the role of astrocytes as intermediary cells between post-synaptic neurons and pre-synaptic terminals that provide distinct and indirect mechanisms for ECB-induced synaptic transmission regulation.
In a recent study, Navarrete & Araque [10] revealed that ECB signalling modulates synaptic transmission in a dual manner. They showed that depolarizing a single hippocampal pyramidal neuron stimulated the release of ECBs that, by directly activating pre-synaptic CB1Rs, induced a transient depression of synaptic transmission in synapses onto that neuron: a process known as depolarization-induced suppression of excitation (DSE) [37,42–44]. Concomitantly, ECBs released by the stimulated neuron activated CB1Rs in nearby astrocytes, which led to the Ca2+-dependent release of glutamate and subsequent activation of pre-synaptic type I metabotropic glutamate receptors, and to a transient potentiation of synaptic transmission in relatively distant synapses in adjacent neurons [10] (figure 2). Therefore, ECBs may have opposite and complementary neuromodulatory effects: classical depression of synaptic transmission (e.g. DSE) and lateral potentiation of neurotransmission through the activation of astrocytes.
Figure 2.
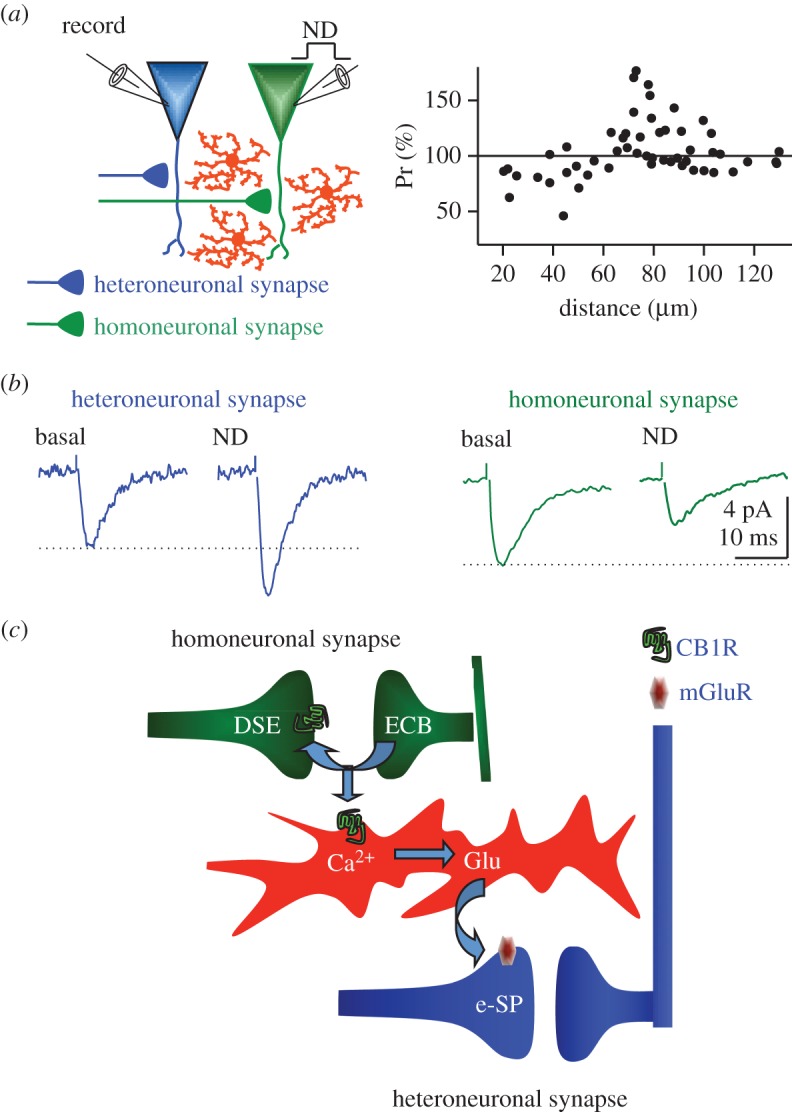
Astrocytes spatially control the synaptic regulation induced by neuron released endocannabinoids. (a) Recording from two CA1 hippocampal pyramidal neurons while simultaneously monitoring astrocyte calcium levels and synaptic transmission properties in single synapses and adjacent neuron (heteroneuronal synapses). ECBs released after depolarization of one neuron depressed or enhanced the probability of neurotransmitter release depending on the distance to the ECB source. (b) While ECBs released by neuronal depolarization (ND) depressed excitatory synaptic currents into the depolarized neuron (homoneuronal synapses; green traces), they enhanced synaptic transmission in adjacent neruons (heteroneuronal synapses; blue traces). (c) Scheme representing the ECB signalling processes that regulates hippocampal synaptic transmission. ECBs released by the post-synaptic neuron directly activate CB1Rs in the pre-synaptic terminal, which leads to synaptic depression in homoneuronal synapses (DSE). In addition, they activate CB1Rs in astrocytes, elevate their intracellular Ca2+ and stimulate the release of glutamate that potentiates neurotransmitter release in heteroneuronal synapses (e-SP). Adapted from [10].
Interestingly, this dual effect is spatially controlled, depending on the intercellular signalling pathways activated. While ECBs are known to exert their direct effects at short distances from their sources (less than 20 μm; see [3,10,29,42,45,46]), astrocyte signalling stimulated by ECBs may spread intra- and intercellularly, having far-reaching neuromodulatory effects by releasing gliotransmitters in distal regions, leading to the lateral potentiation of synapses distant from the ECB source [16,47] (figure 2). The exact functional significance of this spatially controlled dual regulation of synaptic transmission is unknown, but it may have important functional implications as a homeostatic mechanism of synaptic transmission. For example, highly active synapses onto a particular neuron could induce the release of ECBs that depress the activity of these synapses. Simultaneously, these ECBs, through the activation of astrocytes, could potentiate less active nearby synapses. The simultaneous depression of strong synapses and the potentiation of weak synapses could produce homogeneity in the synaptic strength of inputs into certain areas. This homeostatic and anti-contrast effect may serve important functions in some areas, like the CA1 of hippocampus, whose functional properties are based on the simultaneous coordinated activity of ensembles of neurons.
4. Role of astrocytes in the regulation of long-term synaptic plasticity mediated by endocannabinoids
Besides the above-described transient regulatory effects of ECBs on synaptic transmission through the stimulation of astrocytes, more recent data indicate that the activation of CB1Rs in astrocytes can control long-lasting synaptic changes in different brain areas.
Plasticity of synapses between excitatory neurons of layer 4 and layer 2/3 in the barrel cortex has been studied for years as a model of spike timing-dependent plasticity, in which the temporal relation between pre- and post-synaptic action potentials controls synaptic plasticity [48–50]. Spike timing-dependent long-term depression (t-LTD) was known to require activation of both CB1Rs and pre-synaptic NMDARs, but how both receptor types cooperatively lead to t-LTD was unknown [48,51–53]. A recent study by Thomas Nevian's laboratory has revealed that astrocytes are crucially involved in this form of synaptic plasticity [11]. ECBs released during t-LTD induction activated CB1Rs in astrocytes, which induced both the release of glutamate in a calcium-dependent manner from these cells and the subsequent activation of pre-synaptic NMDARs that led to t-LTD. These results suggest that astrocytes play a critical role in ECB signalling underlying important synaptic plasticity changes that occur in sensory information processing at the sensory cortex (figure 3b).
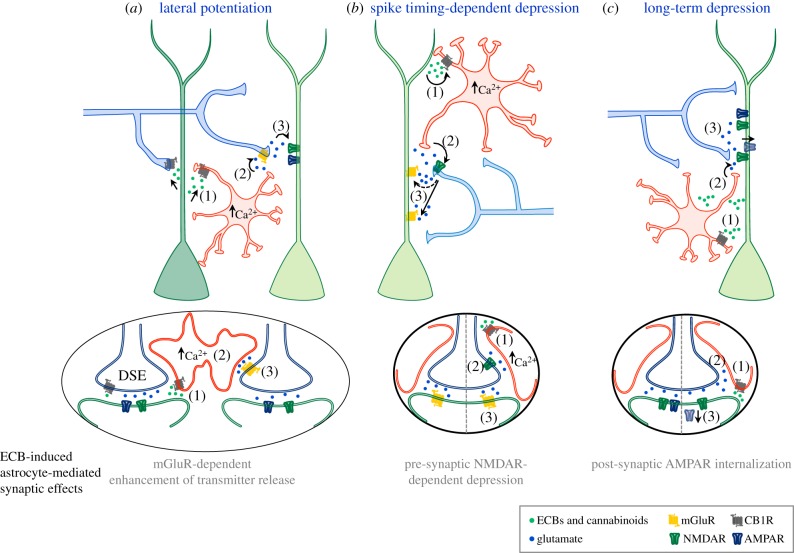
Figure 3.
Signalling mechanisms underlying synaptic regulation by endocannbinoids through stimulation of astrocytes. (a) In the hippocampus, ECBs released by one active pyramidal neuron act directly on pre-synaptic CB1Rs of the synaptic terminals received leading to DSE in homoneuronal synapses. In addition, ECBs activate CB1Rs in astrocytes (1), elevating their intracellular Ca2+ and stimulating the relase of glutamate (2), which potentiates neurotransmitter release in heteroneuronal synapses (3). (b) In the cortex, the coincidence of post-synaptic metabotropic glutamate receptor (mGluR) activation during synaptic activity and Ca2+ influx evoked by back-propagating action potentials in the post-synaptic cell induces the release of ECBs that activate CB1 receptors in astrocytes (1), which stimulates the release of glutamate (2) that activates pre-synaptic NMDA receptors and induces LTD (3). (c) Activation of astrocyte CB1Rs by exogenous cannabinoids (1) stimulate the release of glutamate (2), which activates post-synaptic NMDA receptors (3) that induce the endocytosis of post-synaptic AMPA receptors that leads to LTD. (a–c Adapted from [10,11,54], respectively.)
The most common effects of ECBs on synaptic plasticity result in the depression of synaptic transmission [28,29,55]. A recent report proposes that ECBs mediate the long-lasting potentiation of cortical synapses onto the hippocampus [56]. The participation of astrocytes in this potentiation in cortico-hippocampal synapses is unknown. However, data from our laboratory indicate that coincidence of ECB signalling and post-synaptic activity leads to the ECB-induced long-term enhancement of transmitter release at single CA3–CA1 hippocampal synapses through stimulation of astrocytes. This ECB-induced astrocyte-mediated long-term potentiation (LTP) occurs at single synapses in neurons relatively distant from the ECB source (greater than 60 μm), suggesting that ECBs, classically considered to act as retrograde signals that evoke synaptic depression, may also serve as a lateral signal through activation of astrocytes that leads to LTP of synaptic transmission (M Gómez-Gonzalo, M Navarrete, G Perea, A Covelo, M Martín-Fernández, R Shigemoto, R Luján, A Araque 2014, Endocannabinoids induce lateral LTP of transmitter release by stimulation of gliotransmission, unpublished; figure 3a).
5. Impact of astrocytes on the behavioural effects of endocannabinoid signalling
Given the involvement of ECB signalling in a plethora of brain functions and behavioural tasks, the participation of astrocytes in some of these functions is an exciting possibility that largely remains unexplored. A recent ground-breaking study has provided evidence for the necessary role of astroglial CB1Rs in spatial working memory impairment induced by exogenous cannabinoids. Using the powerful tools of recently generated conditional transgenic mice lacking CB1Rs selectively in astrocytes, GABAergic or glutamatergic neurons, Han et al. [54] have shown that the impairment of spatial working memory and the LTD of hippocampal synaptic transmission induced by administration of THC, the major psychoactive ingredient of marijuana, required astrocyte CB1Rs. The mechanistic events proposed for the exogenous cannabinoid effects are that they activate astrocytic CB1Rs and stimulate the release of glutamate that induces LTD by internalization of AMPA receptors, resulting in working memory impairment. This study demonstrates that one of the most common effects of cannabinoid intoxication, the impairment of spatial working memory, is mediated by ECB signalling in astrocytes (figure 3c).
6. Involvement of astrocytes in endocannabinoid signalling in the brain pathology
ECB signalling is altered in numerous neurological and neurodegenerative diseases, which has been proposed to result in the exacerbation or amelioration of certain brain disorders such as multiple sclerosis, post-traumatic stress disorder, traumatic brain injury and Parkinson's disease [57]. While brain disease mechanisms are largely considered to have a neuronal origin, increasing evidence suggests that disturbances of astrocyte–neuron interactions are related to brain disorders [58,59]. Similar to what is indicated above regarding behaviour also applies for pathology. Because alterations of ECB signalling are associated with many brain diseases, the possible involvement of astrocytes does not seem implausible, although it has scarcely been studied. For example, cannabinoids have been shown to influence epileptic activity, although results reporting the proconvulsive or anti-epileptic effects of CB1R ligands are controversial [60,61]. On the other hand, astrocytes have been shown to contribute to epileptiform activity [62–66]. A recent study has shown that pharmacological blockade of CB1 receptors reduced the maintenance of epileptiform discharge, an effect that was abolished when astrocyte calcium elevations were prevented, suggesting that ECB signalling from neurons to astrocytes is involved in the maintenance of hippocampal epileptiform activity [67]. This work opens promising avenues towards defining the participation of ECB signalling and astrocytes as novel joint factors affecting epileptic activity.
Whether alterations of ECB signalling in tripartite synapse properties are associated with other brain diseases requires detailed attention because it may reveal novel mechanisms contributing to brain dysfunctions and may serve to identify new cellular targets in the development of therapeutic strategies for the treatment of brain diseases.
7. Concluding remarks
Astrocytes are emerging as important regulatory elements involved in synaptic function, responding to synaptically released neurotransmitters and regulating synaptic transmission and plasticity. This evidence indicates that astrocytes functionally interact with neurons establishing tripartite synapses and suggest that they play an active role in brain function. Recent studies discussed in this review show that ECB signalling in tripartite synapses has novel consequences relevant to the regulation of synaptic function. These studies have not only decisively contributed to supporting the tripartite synapse concept, but they also have revealed novel properties of intercellular signalling in the nervous system, by showing that astrocytes stimulated by endogenous stimuli (i.e. ECBs physiologically released from neurons) have the ability to mediate interneuronal non-synaptic communication (i.e. releasing glutamate that activates NMDARs [9]), spatially control the regulation of synapses (i.e. through the lateral potentiation of synaptic transmission [10]), necessarily mediate relevant synaptic plasticity phenomena [11] and transform the sign of the regulatory effects of transmitters (i.e. while ECBs directly signalling to synapses induce synaptic depression, astrocytes stimulated by ECBs induce synaptic potentiation) [9,10].
While these studies are very recent and therefore still limited, a more comprehensive characterization of the consequences of ECB signalling in the properties of tripartite synapses in different brain areas is required to fully understand the potential relevant role in brain function. The development of new transgenic animal models, particularly those specifically affecting astrocyte signalling, will be extremely useful to reveal and define that role. This is clearly exemplified by the recent conditional mutant mice generated by Marsicano's group that specifically lack CB1Rs in astrocytes and that allowed the demonstration of the involvement of astrocytes in ECB signalling mediating particular behavioural tasks [54].
Acknowledgements
We thank members of the Alfonso Araque laboratory at Instituto Cajal, CSIC for helpful discussions.
Funding statement
The authors' work was supported by grants from MINECO (BFU2010-15832; CDS2010-00045), and Cajal Blue Brain, Spain, to A.A. A.D is a JAE-CSIC pre-doctoral fellow.
References
- 1.Ahn K, McKinney MK, Cravatt BF. 2008. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem. Rev. 108, 1687–1707. ( 10.1021/cr0782067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Marzo V. 2009. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 60, 77–84. ( 10.1016/j.phrs.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 3.Piomelli D. 2003. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 4, 873–884. ( 10.1038/nrn1247) [DOI] [PubMed] [Google Scholar]
- 4.Puighermanal E, Busquets-Garcia A, Maldonado R, Ozaita A. 2012. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Phil. Trans. R. Soc. B 367, 3254–3263. ( 10.1098/rstb.2011.0384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skaper SD, Di Marzo V. 2012. Endocannabinoids in nervous system health and disease: the big picture in a nutshell. Phil. Trans. R. Soc. B 367, 3193–3200. ( 10.1098/rstb.2012.0313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. 2009. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89, 309–380. ( 10.1152/physrev.00019.2008) [DOI] [PubMed] [Google Scholar]
- 7.Ohno-Shosaku T, Tanimura A, Hashimotodani Y, Kano M. 2012. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist 18, 119–132. ( 10.1177/1073858410397377) [DOI] [PubMed] [Google Scholar]
- 8.Freund TF, Katona I, Piomelli D. 2003. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 83, 1017–1066. [DOI] [PubMed] [Google Scholar]
- 9.Navarrete M, Araque A. 2008. Endocannabinoids mediate neuron–astrocyte communication. Neuron 57, 883–893. ( 10.1016/j.neuron.2008.01.029) [DOI] [PubMed] [Google Scholar]
- 10.Navarrete M, Araque A. 2010. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126. ( 10.1016/j.neuron.2010.08.043) [DOI] [PubMed] [Google Scholar]
- 11.Min R, Nevian T. 2012. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat. Neurosci. 15, 746–753. ( 10.1038/nn.3075) [DOI] [PubMed] [Google Scholar]
- 12.Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. ( 10.1016/S0166-2236(98)01349-6) [DOI] [PubMed] [Google Scholar]
- 13.Perea G, Navarrete M, Araque A. 2009. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 32, 421–431. ( 10.1016/j.tins.2009.05.001) [DOI] [PubMed] [Google Scholar]
- 14.Porter JT, McCarthy KD. 1996. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 16, 5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasti L, Volterra A, Pozzan T, Carmignoto G. 1997. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 17, 7817–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perea G, Araque A. 2005. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci. 25, 2192–2203. ( 10.1523/JNEUROSCI.3965-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. 2011. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798. ( 10.1016/j.cell.2011.07.022) [DOI] [PubMed] [Google Scholar]
- 18.Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. 2011. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284. ( 10.1038/nn.2929) [DOI] [PubMed] [Google Scholar]
- 19.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. 2004. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7, 613–620. ( 10.1038/nn1246) [DOI] [PubMed] [Google Scholar]
- 20.Crippa D, Schenk U, Francolini M, Rosa P, Verderio C, Zonta M, Pozzan T, Matteoli M, Carmignoto G. 2006. Synaptobrevin2-expressing vesicles in rat astrocytes: insights into molecular characterization, dynamics and exocytosis. J. Physiol. 570, 567–582. ( 10.1113/jphysiol.2005.094052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montana V, Ni Y, Sunjara V, Hua X, Parpura V. 2004. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 24, 2633–2642. ( 10.1523/JNEUROSCI.3770-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo DH, et al. 2012. Trek-1 and best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151, 25–40. ( 10.1016/j.cell.2012.09.005) [DOI] [PubMed] [Google Scholar]
- 23.Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. 2012. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro 4, AN20110061 ( 10.1042/AN20110061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beppu K, Sasaki T, Tanaka KF, Yamanaka A, Fukazawa Y, Shigemoto R, Matsui K. 2014. Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 81, 14–20. ( 10.1016/j.neuron.2013.11.011) [DOI] [PubMed] [Google Scholar]
- 25.Hamilton NB, Attwell D. 2010. Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 11, 227–238. ( 10.1038/nrn2803) [DOI] [PubMed] [Google Scholar]
- 26.Parpura V, Zorec R. 2010. Gliotransmission: exocytotic release from astrocytes. Brain Res. Rev. 63, 83–92. ( 10.1016/j.brainresrev.2009.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volterra A, Meldolesi J. 2005. Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 6, 626–640. ( 10.1038/nrn1722) [DOI] [PubMed] [Google Scholar]
- 28.Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. 2012. Endocannabinoid signaling and synaptic function. Neuron 76, 70–81. ( 10.1016/j.neuron.2012.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chevaleyre V, Takahashi KA, Castillo PE. 2006. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 29, 37–76. ( 10.1146/annurev.neuro.29.051605.112834) [DOI] [PubMed] [Google Scholar]
- 30.Stella N. 2004. Cannabinoid signaling in glial cells. Glia 48, 267–277. ( 10.1002/glia.20084) [DOI] [PubMed] [Google Scholar]
- 31.Moldrich G, Wenger T. 2000. Localization of the CB1 cannabinoid receptor in the rat brain. an immunohistochemical study. Peptides 21, 1735–1742. ( 10.1016/S0196-9781(00)00324-7) [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez JJ, Mackie K, Pickel VM. 2001. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat caudate putamen nucleus. J. Neurosci. 21, 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salio C, Doly S, Fischer J, Franzoni MF, Conrath M. 2002. Neuronal and astrocytic localization of the cannabinoid receptor-1 in the dorsal horn of the rat spinal cord. Neurosci. Lett. 329, 13–16. ( 10.1016/S0304-3940(02)00549-9) [DOI] [PubMed] [Google Scholar]
- 34.Molina-Holgado F, Pinteaux E, Moore JD, Molina-Holgado E, Guaza C, Gibson RM, Rothwell NJ. 2003. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J. Neurosci. 23, 6470–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter L, Stella N. 2003. Endothelin-1 increases 2-arachidonoyl glycerol (2-AG) production in astrocytes. Glia 44, 85–90. ( 10.1002/glia.10270) [DOI] [PubMed] [Google Scholar]
- 36.Navarrete M, Perea G, Maglio L, Pastor J, Garcia de Sola R, Araque A. 2013. Astrocyte calcium signal and gliotransmission in human brain tissue. Cereb. Cortex 23, 1240–1246. ( 10.1093/cercor/bhs122) [DOI] [PubMed] [Google Scholar]
- 37.Alger BE. 2002. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog. Neurobiol. 68, 247–286. ( 10.1016/S0301-0082(02)00080-1) [DOI] [PubMed] [Google Scholar]
- 38.Wilson RI, Nicoll RA. 2002. Endocannabinoid signaling in the brain. Science 296, 678–682. ( 10.1126/science.1063545) [DOI] [PubMed] [Google Scholar]
- 39.Kreitzer AC, Regehr WG. 2002. Retrograde signaling by endocannabinoids. Curr. Opin. Neurobiol. 12, 324–330. ( 10.1016/S0959-4388(02)00328-8) [DOI] [PubMed] [Google Scholar]
- 40.Cachope R, Mackie K, Triller A, O'Brien J, Pereda AE. 2007. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron 56, 1034–1047. ( 10.1016/j.neuron.2007.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song J, Kyriakatos A, El Manira A. 2012. Gating the polarity of endocannabinoid-mediated synaptic plasticity by nitric oxide in the spinal locomotor network. J. Neurosci. 32, 5097–5105. ( 10.1523/JNEUROSCI.5850-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson RI, Nicoll RA. 2001. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592. ( 10.1038/35069076) [DOI] [PubMed] [Google Scholar]
- 43.Kreitzer AC, Regehr WG. 2001. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29, 717–727. ( 10.1016/S0896-6273(01)00246-X) [DOI] [PubMed] [Google Scholar]
- 44.Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. 2002. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J. Neurosci. 22, 3864–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. 2001. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31, 463–475. ( 10.1016/S0896-6273(01)00375-0) [DOI] [PubMed] [Google Scholar]
- 46.Chevaleyre V, Castillo PE. 2004. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron 43, 871–881. ( 10.1016/j.neuron.2004.08.036) [DOI] [PubMed] [Google Scholar]
- 47.Serrano A, Haddjeri N, Lacaille JC, Robitaille R. 2006. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J. Neurosci. 26, 5370–5382. ( 10.1523/JNEUROSCI.5255-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bender VA, Bender KJ, Brasier DJ, Feldman DE. 2006. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J. Neurosci. 26, 4166–4167. ( 10.1523/JNEUROSCI.0176-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Bender KJ, Drew PJ, Jadhav SP, Sylwestrak E, Feldman DE. 2009. Endocannabinoid signaling is required for development and critical period plasticity of the whisker map in somatosensory cortex. Neuron 64, 537–549. ( 10.1016/j.neuron.2009.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dan Y, Poo MM. 2006. Spike timing-dependent plasticity: from synapse to perception. Physiol. Rev. 86, 1033–1048. ( 10.1152/physrev.00030.2005) [DOI] [PubMed] [Google Scholar]
- 51.Nevian T, Sakmann B. 2006. Spine Ca2+ signaling in spike-timing-dependent plasticity. J. Neurosci. 26, 11 001–11 013. ( 10.1523/JNEUROSCI.1749-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sjostrom PJ, Turrigiano GG, Nelson SB. 2003. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39, 641–654. ( 10.1016/S0896-6273(03)00476-8) [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Moreno A, Paulsen O. 2008. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat. Neurosci. 11, 744–745. ( 10.1038/nn.2125) [DOI] [PubMed] [Google Scholar]
- 54.Han J, et al. 2012. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050. ( 10.1016/j.cell.2012.01.037) [DOI] [PubMed] [Google Scholar]
- 55.Heifets BD, Castillo PE. 2009. Endocannabinoid signaling and long-term synaptic plasticity. Annu. Rev. Physiol. 71, 283–306. ( 10.1146/annurev.physiol.010908.163149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu JY, Zhang J, Chen C. 2012. Long-lasting potentiation of hippocampal synaptic transmission by direct cortical input is mediated via endocannabinoids. J. Physiol. 590, 2305–2315. ( 10.1113/jphysiol.2011.223511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pertwee RG. 2012. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Phil. Trans. R. Soc. B 367, 3353–3363. ( 10.1098/rstb.2011.0381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takano T, Oberheim N, Cotrina ML, Nedergaard M. 2009. Astrocytes and ischemic injury. Stroke 40, S8–S12. ( 10.1161/STROKEAHA.108.533166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seifert G, Schilling K, Steinhauser C. 2006. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat. Rev. Neurosci. 7, 194–206. ( 10.1038/nrn1870) [DOI] [PubMed] [Google Scholar]
- 60.Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. 2003. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 307, 129–137. ( 10.1124/jpet.103.051920) [DOI] [PubMed] [Google Scholar]
- 61.Smith PF. 2005. Cannabinoids as potential anti-epileptic drugs. Curr. Opin. Invest. Drugs 6, 680–685. [PubMed] [Google Scholar]
- 62.Crunelli V, Carmignoto G. 2013. New vistas on astroglia in convulsive and non-convulsive epilepsy highlight novel astrocytic targets for treatment. J. Physiol. 591, 775–785. ( 10.1113/jphysiol.2012.243378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. 2013. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 36, 174–184. ( 10.1016/j.tins.2012.11.008) [DOI] [PubMed] [Google Scholar]
- 64.Seifert G, Steinhauser C. 2013. Neuron–astrocyte signaling and epilepsy. Exp. Neurol. 244, 4–10. ( 10.1016/j.expneurol.2011.08.024) [DOI] [PubMed] [Google Scholar]
- 65.Tian GF, et al. 2005. An astrocytic basis of epilepsy. Nat. Med. 11, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang N, Xu J, Xu Q, Nedergaard M, Kang J. 2005. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 94, 4121–4130. ( 10.1152/jn.00448.2005) [DOI] [PubMed] [Google Scholar]
- 67.Coiret G, Ster J, Grewe B, Wendling F, Helmchen F, Gerber U, Benquet P. 2012. Neuron to astrocyte communication via cannabinoid receptors is necessary for sustained epileptiform activity in rat hippocampus. PLoS ONE 7, e37320 ( 10.1371/journal.pone.0037320) [DOI] [PMC free article] [PubMed] [Google Scholar]