Abstract
Astrocytes may express ionotropic glutamate and gamma-aminobutyric acid (GABA) receptors, which allow them to sense and to respond to neuronal activity. However, so far the properties of astrocytes have been studied only in a few brain regions. Here, we provide the first detailed receptor analysis of astrocytes in the murine ventrobasal thalamus and compare the properties with those in other regions. To improve voltage-clamp control and avoid indirect effects during drug applications, freshly isolated astrocytes were employed. Two sub-populations of astrocytes were found, expressing or lacking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. AMPA receptor-bearing astrocytes displayed a lower Kir current density than cells lacking the receptors. In contrast, all cells expressed GABAA receptors. Single-cell RT-PCR was employed to identify the receptor subunits in thalamic astrocytes. Our findings add to the emerging evidence of functional heterogeneity of astrocytes, the impact of which still remains to be defined.
Keywords: thalamus, heterogeneity, astrocytes, AMPA receptors, NMDA receptors, GABAA receptors
1. Introduction
Early studies in cell culture suggested that astrocytes comprise a relatively uniform cell population. However, more recent work performed in acute brain slices or with freshly isolated cells indicates that astrocyte morphology and physiology varies within and between different regions of the central nervous system (CNS) [1,2]. Most of our current knowledge about astrocyte function has been derived from studying only a few brain areas, such as the neocortex, hippocampus, cerebellum and optic nerve. The thalamus, an important relay station for sensory information in the CNS, is one of those regions in which the properties of astrocytes remain largely unexplored. Here, we give an overview of the expression of functional ionotropic glutamate and gamma-aminobutyric acid (GABA) receptors by astrocytes across brain regions and compare their properties with those that we have observed in the thalamus.
2. Heterogeneity in the expression of astrocytic AMPA receptors
Patch-clamp analyses from several laboratories have proved the presence of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in astrocytes of the cerebellum, neocortex and brain stem. In Bergmann glial cells, the inwardly rectifying current/voltage (I/V) relationship indicated the presence of Ca2+ permeable AMPA receptors, which was confirmed with Ca2+ imaging [3]. The Ca2+ elevations activated by kainate, an agonist at AMPA/kainate receptors, were abolished by AMPA receptor antagonists or in Ca2+-free bath solution. Transcript analysis revealed that Bergmann glia express GluA1 and GluA4 but lack the GluA2 subunit, which entails a high Ca2+-permeability and inward rectification of AMPA receptor channels [4,5]. Neuronal activity was sufficient to elicit fast elevations of the intracellular Ca2+ concentration [Ca2+]i in Bergmann glia [6]. This neuron-to-glia signalling was mediated by fast ectopic release of glutamate from climbing fibre presynapses, which are closely apposed to the glial receptors [7]. To look for the physiological impact of the Ca2+ permeable glial receptors they were made Ca2+ impermeable through viral overexpression of the GluA2 subunit. As a result, the fine lamellae of Bergmann glial cells underwent profound changes: synapse ensheathment was lost, glutamate clearance of the synaptic cleft was impaired and Purkinje cells were now innervated by more than one climbing fibre [8]. Full ablation of AMPA receptors (GluA1 and GluA4) from Bergmann glial cells led to retraction of processes from the synapses, disturbed glutamate buffering and altered synaptic transmission between parallel fibres and Purkinje cells. Consequences on the behavioural level included impaired motor performance and changes in eye blink conditioning [9]. On the other hand, AMPA receptor activation in Bergmann glia leads to glutamate receptor interacting protein (GRIP)-induced stimulation of serine racemase and d-serine release, which regulates neuronal migration via activation of N-methyl-d-aspartate (NMDA) receptors [10].
Astrocytes in layer II of the somatosensory cortex were investigated in situ and after acute isolation. In the presence of the AMPA receptor modulator cyclothiazide (CTZ) [11], application of glutamate or fibre stimulation evoked rapidly desensitizing responses that were blocked by 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX), an antagonist of AMPA/kainate receptors [12]. Targeted over-expression of GluA2 under control of the human glial fibrillary acidic protein (GFAP) promoter led to morphological changes of glial processes and reduced numbers of neurons not only in the cerebellum (cf. above) but also the neocortex [13]. AMPA and NMDA receptors in cortical astrocytes may activate the enzyme haem oxygenase-2 and produce carbon monoxide to regulate cerebral vasodilatation at the glia–vascular unit [14]. AMPA receptors are also present in astrocytes of the brain stem [15,16].
In contrast to the above findings, astrocytes in the CA1 region of the hippocampus lack functional AMPA receptors. An early study reported Ca2+ responses upon application of AMPA receptor agonists in situ [17], but no evidence for functional receptors was found when employing freshly isolated astrocytes. Neither rapid agonist application nor co-application of agonists with CTZ evoked any responses, and hippocampal astrocytes were even devoid of transcripts encoding the receptors [18]. However, AMPA receptors are present in radial glia-like cells in the subgranular zone (SGZ) of the dentate gyrus. These cells have astrocyte properties and represent stem cells for adult neurogenesis [19]. The receptors were exclusively located at the processes but not the glial soma. Single-cell RT-PCR confirmed expression of the GluA1 and GluA2 subunits [20].
3. Evidence for NMDA receptors in astrocytes
The application of NMDA to hippocampal and cortical astrocytes in situ evoked inward currents at negative voltages and increases in [Ca2+]i, which suggested functional expression of these receptors [21,22]. However, the responses might have been due to indirect effects, because NMDA also activated neurons leading to fluctuations in [K+]o or release of other messenger molecules. Indeed, subsequent analyses of astrocytes freshly isolated from the hippocampus did not find evidence for NMDA receptors in hippocampal astrocytes [18], while isolated cortical astrocytes responded to application of NMDA [12]. The responses were sensitive to the NMDA receptor antagonists D-AP5 and MK-801, but displayed a very weak Mg2+-sensitivity and a linear I/V relationship. Only some of the cortical astrocytes were sensitive to the GluN2B antagonist ifenprodil [12]. These unique properties indicated expression of the GluN2C and GluN2D subunits, which was in line with the high efficiency of UBP141, an antagonist of GluN2C/D subunits, and a relatively low Ca2+-permeability of the glial receptors [23]. In a GluN2C knockin reporter mouse, NR2C was found in astrocytes of the neocortex and hippocampus [24]. The latter study did not find the essential NR1 subunit in hippocampal astrocytes, which is in line with the absence of functional receptors in that region (see also [25,26]).
4. Abundant expression of GABAA receptors by astrocytes
GABAA receptors are abundantly found in astrocytes throughout brain regions [27]. In acutely isolated hippocampal astrocytes, receptor currents were inhibited by bicuculline and picrotoxin, potentiated by pentobarbital and diazepam, while the inverse agonist methyl-6,7-dimethoxy-4-ethyl-beta-carboline-3-carboxylate (DMCM) mostly decreased the responses. Immunohistochemistry identified α1 and β1 subunits in these cells [28]. GABAA receptors in Bergmann glial cells were not sensitive to diazepam but to pentobarbital, and immunocytochemistry and immunogold electron microscopy revealed expression of α2, α3, γ1 and δ [29,30]. Radial glia-like cells in the SGZ of the dentate gyrus expressed functional GABAA receptors [19], both on soma and processes, that mainly contain α2, α4, β1, γ1 and γ3 [20]. In a subpopulation of these cells, α5, β3 and γ2 were found, which might be important for regulating self-renewal in these stem cells [31]. Similarly, GABAA receptor signalling in the subventricular zone regulates the proliferation of GFAP-positive stem cells [32].
5. Material and methods
(a). Preparation of isolated cells from brain slices
We have previously reported that the very poor space clamp control significantly impedes quantitative analyses of membrane properties in astrocytes in situ [33]. Moreover, drug application in slices may produce indirect effects at the glial membrane, which may complicate the characterization of receptor responses. To circumvent these potential flaws, we chose to use freshly isolated cells in the present study. All experiments were carried out in accordance with local, state and European regulations. Transgenic hGFAP/EGFP mice [34] of either sex (postnatal age (p) 9–13 days) were anaesthetized with isoflurane (Abbott, Wiesbaden, Germany) and decapitated. Brains were removed and transferred to an ice cooled, oxygen bubbled solution containing (in mM): 150 NaCl, 5 KCl, 2 MgCl2, 1 Na-pyruvate, 10 glucose and 10 HEPES (pH 7.4). Horizontal slices (300 µm) containing the ventroposteromedial (VPM) and ventroposterolateral (VPL) nuclei of the thalamus were obtained using a vibratome (VT1200S, Leica, Nussloch, Germany). Slices were stored (1 h) in cold (10°C) standard artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 3 KCl, 2 MgSO4, 2 CaCl2, 10 glucose, 1.25 NaH2PO4 and 26 NaHCO3, bubbled with carbogen (95% O2/5% CO2) and allowed to warm to room temperature (25°C).
Cells were isolated using an enzymatic/mechanical approach as described [35]. In brief, papaine (24 U ml−1) (Sigma, Taufkirchen, Germany) and l-cysteine (0.24 mg ml−1) (Sigma) were added to standard ASCF, continuously bubbled with carbogen. Slices were stored in the papain-containing solution for 8–12 min. Subsequently, slices were transferred to the recording solution consisting of (in mM): 150 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES and 10 glucose, pH 7.38. The VPM and VPL nuclei were dissected under a stereomicroscope (KL200, Zeiss, Jena, Germany) and cells were isolated in the recording chamber with help of tungsten needles and Pasteur pipettes. Cells were allowed to settle for 20 min before analysis.
(b). Electrophysiological recordings
Experiments on isolated cells employed a customized concentration clamp device connected to an EPC-7 amplifier and TIDA software (Heka Lambrecht, Germany) as described elsewhere [35]. In some experiments, a high Ca2+ solution was used in which NaCl was substituted with N-methyl-d-glucamine (NMDG; 100 mM) and Ca2+ (50 mM). Astrocytes were visualized with an inverted microscope (Axiovert 135, Zeiss) equipped with differential interference contrast and epifluorescence. For recordings in slices, specimens were transferred to a recording chamber and continuously perfused with carbogenated ACSF-based recording solution. Visualization of cells was achieved using upright microscopes (Eclipse E600FN, Nikon and Axioskop FS, Zeiss). Pipettes were manufactured from borosilicate glass (4–6 MΩ; Science Products, Hofheim, Germany) and filled with a solution containing (in mM): 130 KCl, 0.5 CaCl2, 2 MgCl2, 5 1,2-bis(o-aminophenoxy)ethane-N,N,N,N-tetraacetic acid (BAPTA), 10 HEPES, and 3 Na2-ATP, pH 7.25. Currents were sampled at 0.1–30 kHz and filtered at 3 or 10 kHz. Holding potential was −70 mV unless stated otherwise. Holding currents in normal bath solution and in the presence of the K+ channel blockers Ba2+/quinine amounted to +208 ± 31 pA and −63 ± 18 pA (n = 56), respectively. Input and access resistance were continuously checked by applying 10 mV test pulses. The liquid junction potential was not corrected for. Recordings were performed at room temperature.
(c). Drug application
Drug application to isolated cells was performed in HEPES-buffered recording solution, supplemented with the K+ channel blockers quinine + BaCl2 or BaCl2 + 4-aminopyridine (4-AP) + tetraethyl ammonium chloride (TEA). Drugs were purchased from Abcam (Cambridge, UK; CTZ, muscimol, bicuculline), Sigma (kainate) and Tocris (Bristol, UK; GYKI 53655, IEM 1460). 4-[2-(phenylsulfonylamino)ethylthio]-2,6-difluoro-phenoxyacetamide (PEPA) was a kind gift from Dr M. Sekiguchi (National Institute of Neuroscience, Tokyo, Japan). PEPA and CTZ were dissolved in dimethyl sulfoxide (DMSO) at 100 mM before dilution (final DMSO concentration was 0.1%).
(d). Recordings in situ and single-cell RT-PCR
Horizontal slices (200 µm) containing the VPM and VPL nuclei were prepared in sucrose-containing solution as reported elsewhere [35]. Subsequent to recording in situ, the cytoplasm of individual astrocytes was harvested and RT-PCR was performed (for details see the electronic supplementary material and [36]).
(e). Data analysis
The membrane conductance at −130 mV was calculated according to the equation: gMem = I−130mV/(−130 mV − Vr), with I−130mV being the current at −130 mV and Vr the resting potential. The Ca2+-permeability of astrocytic AMPA receptors was calculated using an extended constant field equation [35,37]. The rectification index (RI) of AMPA receptor responses was calculated according to the equation
where I is the membrane current at the indicated voltage and Erev the reversal potential of the receptor response. Data are given as mean ± standard error of the mean. Statistical significance was assessed with Student's t-test with an error probability of p ≤ 0.05.
6. Results
(a). Astrocytes in the thalamus express functional AMPA receptors
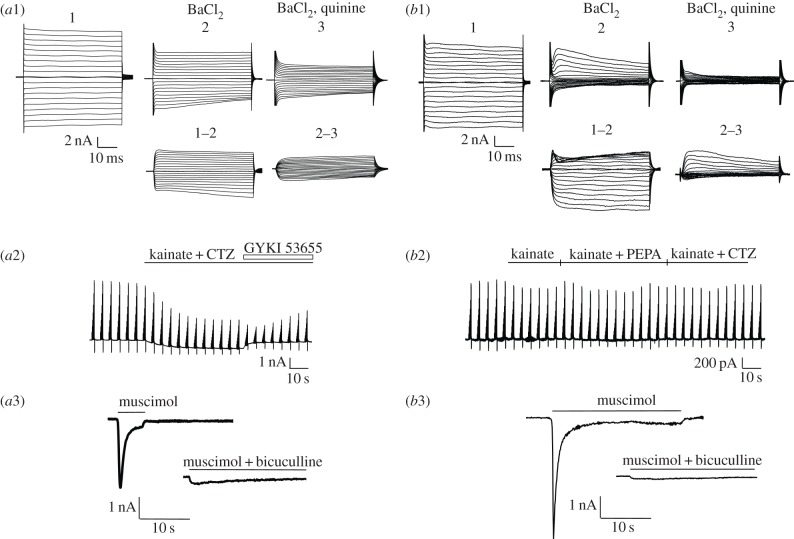
Astrocytes acutely isolated from the thalamus of hGFAP/EGFP mice were identified by their bright endogenous fluorescence, extended process branching and linear current–voltage (I/V) relationships upon de- and hyperpolarization of the membrane. Resting potential and input resistance of the isolated astrocytes amounted to −71.5 ± 0.5 mV and 23.2 ± 1.2 MΩ (n = 81), respectively. The cells were exposed to the AMPA/kainate receptor agonist, kainate (500 µM), supplemented with CTZ (100 µM) or PEPA (100 µM), which modulate AMPA receptors containing the flip or flop splice variants, respectively [11,38]. Prior to application of agonists and modulators, the large astrocytic Kir and leak conductances were blocked by BaCl2 (100 µM) and quinine (200 µM), or BaCl2, TEA (10 mM) and 4-AP (4 mM) [33]. The blocking solutions depolarized the astrocytes by 16.3 ± 1.3 mV and increased their input resistance to 198 ± 21 MΩ (n = 81; figure 1a). At the holding potential of −70 mV, application of kainate generated inward currents of 4.9 ± 1.3 pA pF−1 (n = 17). In the same cells, co-application of kainate with CTZ led to a potentiation of the responses to 575 ± 69% (figure 1b). Among all astrocytes tested (n = 73), a majority (59%) responded to the co-application of kainate and CTZ (henceforth termed GluA+ cells; mean amplitude 26.0 ± 4.1 pA pF−1) (figure 1b), while the remaining cells did not respond (GluA− cells). The threshold of current changes in the presence of AMPA receptor modulators was set at 20 pA. I/V relations of the kainate/CTZ-induced receptor responses of GluA+ cells were linear (RI, 0.99 ± 0.06; n = 18) with a reversal potential close to zero (−1.45 ± 1.43 mV) (figure 1d, left). To test for the expression of flop splice variants, PEPA was co-applied with kainate, which potentiated the kainate-induced currents to 583 ± 46% (n = 4) (figure 1b). The receptor responses induced by kainate and CTZ were almost completely blocked by the AMPA receptor-specific antagonist GYKI 53655 (100 µM; inhibition by 96.3 ± 2.0%, n = 13) (figure 1c).
Figure 1.
Pharmacological characterization of AMPA receptors in isolated thalamic astrocytes. (a) Membrane currents were recorded after de- and hyperpolarization of the membrane (−160 to +20 mV; 10 mV increments, holding potential −70 mV). Application of BaCl2 (100 µM) reduced K+ currents, and further reduction was achieved by adding quinine (200 µM) to the bath. (b) Application of kainate (500 µM) in the presence of BaCl2 and quinine induced an inward current (–100 pA) at −70 mV (solid line). After wash, kainate was co-applied to the same cell, first with CTZ and then with PEPA, which enhanced the currents to 551% and 500%, respectively. Up- and downward deflections represent membrane currents evoked by repetitively de- and hyperpolarizing the membrane (to −40, −20, 0, 20, 40, 70, 100 and −100 mV; blow-up in inset). Scale bars also apply to (c). (c) Responses induced by kainate (500 µM)/CTZ (100 µM) in the presence of BaCl2/quinine were blocked by the AMPA receptor antagonist, GYKI 53655 (100 µM). (d) Kainate/CTZ-induced responses of the same astrocyte in high Na+ (top left) and high Ca2+ solutions (top right), both supplemented with BaCl2/quinine. Note that in high Ca2+ solution, the receptor current at −70 mV was reduced to 5.5% of the control current obtained in high Na+ solution. The inset gives the response in high Ca2+ solution at higher resolution. The lower panels depict mean I/V relations and SEM of responses evoked by kainate/CTZ under the respective conditions, normalized to maximum amplitudes at −100 mV. In high Na+ and high Ca2+ solution, currents reversed at −1.4 ± 1.4 mV (n = 18) and −43 ± 2 mV (n = 3), respectively.
The Ca2+-permeability of AMPA receptors critically depends on expression of the GluA2 subunit. To investigate this issue, thalamic astrocytes were exposed to the adamantine derivative, IEM 1460 (100 µM), which preferentially blocks AMPA receptors lacking GluA2 [39]. In the presence of IEM 1460, the kainate/CTZ-induced currents were reduced by 42.3 ± 7.8% (n = 13) (not shown), suggesting expression of Ca2+-permeable receptors by thalamic astrocytes. Next, we determined the relative Ca2+-permeability of the receptors by comparing the responses to kainate/CTZ in high Na+ (150 mM) and high Ca2+ (50 mM) bath solutions. In high Ca2+ solution, kainate/CTZ application induced small inward currents in three out of six cells tested. The current density at −70 mV amounted to 3.5 ± 0.7 pA pF−1, which represented 10.9 ± 4.3% of the respective responses elicited in high Na+ solution. The I/V relation of the responses in high Ca2+ solution was outwardly rectifying (RI = 1.58 ± 0.24) and current reversal was significantly shifted towards more negative voltages (to −43 ± 2 mV) as compared with the responses in high Na+ solution (figure 1d, right). PCa/PK was calculated according to an extended constant field equation and amounted to 0.15. Thus, AMPA receptors of juvenile thalamic astrocytes show a low Ca2+-permeability.
To exclude that the lack of kainate/CTZ-sensitivity in GluA− cells was due to cell damage, the same astrocyte was subsequently exposed to the GABAA receptor agonist muscimol. All GluA− cells tested (n = 29) produced receptor responses upon muscimol application (see §6b) (figure 2). Moreover, the responsiveness to kainate/CTZ was tested in thalamic slices. To minimize indirect effects and improve voltage-clamp control of recordings from astrocytes in situ, neuronal activity and gap junction coupling were diminished by adding CdCl2 (30 µM), TTX (0.5 µM), D-AP5 (20 µM), carbenoxolone (100 µM), BaCl2 (100 µM) and quinine (200 µM). Inhibition of coupling was checked by tracer injection [41]. Under these conditions, kainate/CTZ at the above concentrations induced responses in 19 out of 24 cells (data not shown), confirming the co-existence of GluA+ and GluA− astrocytes in the thalamus.
Figure 2.
Astrocytes lacking AMPA receptor responses display larger Kir currents. (a1, b1) Membrane currents were activated as described in figure 1a, and the Ba2+- and quinine-sensitive components were isolated by subtracting corresponding current families as indicated. The GluA+ cell (a1, a2) displayed smaller Ba2+-sensitive currents than the GluA− cell that did not respond to kainate, kainate/PEPA and kainate/CTZ (b1, b2). Note that the reduced outward currents in the presence of kainate and CTZ (a2) most probably reflected a block of glial K+ currents due to Na+ influx through AMPA receptors [40]. Because in GluA+ cells the block of resting currents by Ba2+/quinine is incomplete, this effect is still operative. (a3, b3) Irrespective of the Ba2+-sensitivity and responsiveness to AMPA receptor agonists/modulators, in both astrocytes muscimol (500 µM) induced large inward currents at −70 mV that were blocked by bicuculline (100 µM). Concentrations of kainate, PEPA, CTZ and GYKI 53655 are as described in figure 1.
Interestingly, isolated GluA+ and non-responsive GluA− astrocytes differed in their passive membrane properties and expression of Ba2+-sensitive membrane currents. The resting potentials of GluA+ and GluA− cells were significantly different (−70.3 ± 0.9 mV, n = 43 versus −72.9 ± 0.6 mV, n = 30), whereas the input resistance was the same (GluA+ cells: 26.5 ± 2.2 MΩ, n = 43; GluA− cells: 24.4 ± 1.7 MΩ, n = 30). In GluA+ cells, application of Ba2+ (100 µM) decreased the membrane conductance to 35.4 ± 3.5% with the Ba2+-sensitive Kir current density being 187 ± 38 pA pF−1 (at −130 mV; n = 26), while GluA− cells displayed a much higher Ba2+-sensitivity (reduction to 20.6 ± 2.2%) and Kir current density (395 ± 82 pA pF−1; n = 15) (figure 2a1, b1). Accordingly, GluA− cells showed significantly larger Ba2+-sensitive inward currents than GluA+ cells.
Single-cell transcript analysis of AMPA receptors was performed subsequent to functional characterization of thalamic astrocytes in situ. After reverse transcription we performed a two round multiplex PCR for AMPA receptors and the astrocytic gene, S100β. For the second PCR round, subunit-specific primers were used. The subunits GluA1 to GluA4 were found in 17%, 96%, 43% and 35% of the astrocytes tested (n = 23), respectively. Another five cells did not express any of the subunits, although all cells expressed transcripts for S100β. Subsequently, restriction analysis was performed to distinguish the flip and flop splice variant of the AMPA receptor subunits. Thalamic astrocytes most frequently expressed GluA2 flip (82%) and flop (79%) (n = 22). Among GluA3-expressing cells (n = 10), flop occurred in all, and flip in half of cells. GluA4 flip and flop splice variants were detected with a frequency of 75% each (n = 8). See also the electronic supplementary material.
d-Aspartate (500 µM), a substrate of glial glutamate transporters, also evoked inward currents in 12 of 14 cells tested (45 ± 8 pA; current density 4.6 ± 0.8 pA pF−1; V = −70 mV). All cells responding to kainate/CTZ also showed inward currents upon application of d-aspartate (4.5 ± 0.9 pA pF−1, n = 9, not shown).
(b). GABAA receptors in thalamic astrocytes
As mentioned in §6a, in individual astrocytes the expression of GABAA receptors was investigated after pharmacological characterization of AMPA receptors. The cells were exposed to muscimol (100 µM, n = 7; not shown or 500 µM, n = 44; figure 2a3, b3), which produced desensitizing inward currents corresponding to a Cl− efflux ([Cl−]i = 135 mM). All isolated astrocytes responded to muscimol, regardless of whether they were GluA+ (n = 22) or GluA− (n = 29) cells. The receptor current density of GluA+ and GluA− cells was not different (at 500 µM muscimol: 370 ± 48 pA pF−1; n = 44). Co-application of muscimol (500 µM) with bicuculline (100 µM) reduced the responses to 9.9 ± 2.4% of the control (n = 12) (figure 2a3, b3).
For GABAA receptor transcript analysis, single-cell RT-PCR was performed with primers for α, β and γ subunits, and the marker S100β. α1–α5 were found in 33, 50, 0, 7, 56% (n = 18) and β1 and β2/3 in 86 and 43% (n = 7) of the cells. Among the γ subunits, γ1 (100%) and γ3 (89%) were abundantly expressed, while γ2 was almost absent (1/9 cells).
7. A subpopulation of thalamic astrocytes expresses functional AMPA receptors
Our pharmacological and molecular analyses revealed heterogeneity among thalamic astrocytes with respect to the expression of AMPA receptors. Only about 60% of the isolated cells responded to the application of kainate/CTZ. It is unlikely that the non-responsiveness of GluA− cells was due to cell damage because in all GluA− cells muscimol induced receptor responses, and co-existence of GluA+ and GluA− cells was also found in the more intact slice preparation. The existence of thalamic astrocytes lacking AMPA receptors was confirmed by showing that many S100β-positive astrocytes lack transcripts for AMPA receptors. A similar heterogeneity was reported for astrocytes in the striatum [42].
Both CTZ and PEPA potentiated the receptor responses in thalamic astrocytes, indicating expression of flip and flop splice variants. GluA+ cells displayed a low PCa/PK-ratio, which was in line with the abundant expression of the GluA2 subunit. Nevertheless, the observation that IEM 1460 decreased the kainate/CTZ-induced responses hinted at the expression of a mosaic of Ca2+-permeable and Ca2+-impermeable receptors. Our findings are at odds with an earlier study that found only GluA1 immunoreactivity in thalamic astrocytes [43].
AMPA receptors in thalamic astrocytes are involved in neuron–glia signalling. Astrocytes in the ventrobasal (VB) thalamus respond to the stimulation of sensory or corticothalamic afferents with Ca2+ elevations, which may induce glutamate release to generate slow inward currents in VB neurons [44]. The astrocytic Ca2+ responses were largely mediated by metabotropic glutamate receptor type 5, but ionotropic receptors were also involved [45]. Thalamic astrocytes have been shown to release glutamate which triggers slow NMDA currents in neighbouring neurons [46], but whether this release occurs through Ca2+-dependent exocytosis [47] still remains to be demonstrated. On the other hand, the influx of Na+ through AMPA receptors and its spread through gap junctions to neighbouring astrocytes might enhance the activity of Na+/K+ ATPase, stimulate glycolysis and increase [Ca2+]i by activation of Na+/Ca2+ exchangers [48–50].
8. Functional impact of GABAA receptor expression in astrocytes
In contrast to the heterogeneous expression of AMPA receptors, all astrocytes in the thalamus displayed responses to the GABAA receptor agonist muscimol. Due to the relatively high [Cl−]i, receptor activation generates Cl− efflux, depolarization and increases in [Ca2+]i in astrocytes [51]. Alternatively, it has been proposed that bicarbonate efflux through astrocyte GABAA receptors, intracellular acidification and block of two pore-domain K+ channels is responsible for the depolarization [52]. Interneurons induce GABAA receptor responses in astrocytes that are maintained by spread of intracellular Cl− through gap junctions, and thus the glial network regulates [Cl−]o and GABAergic synaptic transmission [53,54]. We consistently found a high density of GABAA receptor currents in thalamic astrocytes. The finding that they lack γ2, the subunit that is crucial for synaptic receptors in neurons and NG2 cells [36,55], is in line with a detection of synaptic GABA spillover by the astrocytic receptors. Whether astrocytes in the VB thalamus sense GABA released from the inhibitory input of the reticularis nucleus, and whether astrocytes thereby modulate the generation of oscillatory activity remain to be investigated.
9. Conclusion
Our finding that only a subpopulation of astrocytes in the thalamus expresses AMPA receptors adds to the emerging concept of functional heterogeneity between astrocytes within and across brain regions. Still, research in the neuron–glia field largely ignores this variability in astrocyte properties. Considering the co-existence of distinct astroglial sub-populations not only may give a cue for resolving current controversies arising from opposing findings in different laboratories, it is also indispensable for a faithful definition of the contribution of astrocytes to signalling modes in the healthy and diseased brain.
Supplementary Material
Acknowledgements
We acknowledge excellent technical support by E. de Laittre, MIT Cambridge, USA, and U. Witting, Bonn.
Funding statement
This work was supported by the German Research Foundation (STE 552/3, 552/4) and the EC (EuroEPINOMICS).
References
- 1.Matyash V, Kettenmann H. 2010. Heterogeneity in astrocyte morphology and physiology. Brain Res. Rev. 63, 2–10. ( 10.1016/j.brainresrev.2009.12.001) [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Barres BA. 2010. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 20, 588–594. ( 10.1016/j.conb.2010.06.005) [DOI] [PubMed] [Google Scholar]
- 3.Müller T, Möller T, Berger T, Schnitzer J, Kettenmann H. 1992. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science 256, 1563–1566. ( 10.1126/science.1317969) [DOI] [PubMed] [Google Scholar]
- 4.Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, Sakmann B. 1992. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science 256, 1566–1570. ( 10.1126/science.1317970) [DOI] [PubMed] [Google Scholar]
- 5.Geiger JRP, Melcher T, Koh D-S, Sakmann B, Seeburg PH, Jonas P, Monyer H. 1995. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15, 193–204. ( 10.1016/0896-6273(95)90076-4) [DOI] [PubMed] [Google Scholar]
- 6.Piet R, Jahr CE. 2007. Glutamatergic and purinergic receptor-mediated calcium transients in Bergmann glial cells. J. Neurosci. 27, 4027–4035. ( 10.1523/JNEUROSCI.0462-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui K, Jahr CE, Rubio ME. 2005. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J. Neurosci. 25, 7538–7547. ( 10.1523/JNEUROSCI.1927-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iino M, et al. 2001. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292, 926–929. ( 10.1126/science.1058827) [DOI] [PubMed] [Google Scholar]
- 9.Saab AS, et al. 2012. Bergmann glial AMPA receptors are required for fine motor coordination. Science 337, 749–753. ( 10.1126/science.1221140) [DOI] [PubMed] [Google Scholar]
- 10.Kim PM, et al. 2005. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc. Natl Acad. Sci. USA 102, 2105–2110. ( 10.1073/pnas.0409723102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Partin KM, Patneau DK, Mayer ML. 1994. Cyclothiazide differentially modulates desensitization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol. Pharmacol. 46, 129–136. [PubMed] [Google Scholar]
- 12.Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. 2006. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J. Neurosci. 26, 2673–2683. ( 10.1523/JNEUROSCI.4689-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuzuki K, Ishiuchi S. 2008. GluR2 expressed by glial fibrillary acidic protein promoter decreases the number of neurons. Front. Biosci. 13, 2784–2796. ( 10.2741/2885) [DOI] [PubMed] [Google Scholar]
- 14.Parfenova H, Tcheranova D, Basuroy S, Fedinec AL, Liu J, Leffler CW. 2012. Functional role of astrocyte glutamate receptors and carbon monoxide in cerebral vasodilation response to glutamate. Am. J. Physiol. Heart Circ. Physiol. 302, H2257–H2266. ( 10.1152/ajpheart.01011.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grass D, Pawlowski PG, Hirrlinger J, Papadopoulos N, Richter DW, Kirchhoff F, Hulsmann S. 2004. Diversity of functional astroglial properties in the respiratory network. J. Neurosci. 24, 1358–1365. ( 10.1523/JNEUROSCI.4022-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDougal DH, Hermann GE, Rogers RC. 2011. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J. Neurosci. 31, 14 037–14 045. ( 10.1523/JNEUROSCI.2855-11.201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter JT, McCarthy KD. 1995. GFAP-positive hippocampal astrocytes in situ respond to glutamatergic neuroligands with increases in [Ca2+]i. Glia 13, 101–112. ( 10.1002/glia.440130204) [DOI] [PubMed] [Google Scholar]
- 18.Matthias K, Kirchhoff F, Seifert G, Hüttmann K, Matyash M, Kettenmann H, Steinhäuser C. 2003. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J. Neurosci. 23, 1750–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang LP, Kempermann G, Kettenmann H. 2005. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol. Cell Neurosci. 29, 181–189. ( 10.1016/j.mcn.2005.02.002) [DOI] [PubMed] [Google Scholar]
- 20.Renzel R, Sadek AR, Chang CH, Gray WP, Seifert G, Steinhauser C. 2013. Polarized distribution of AMPA, but not GABAA, receptors in radial glia-like cells of the adult dentate gyrus. Glia 61, 1146–1154. ( 10.1002/glia.22505) [DOI] [PubMed] [Google Scholar]
- 21.Steinhäuser C, Jabs R, Kettenmann H. 1994. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus 4, 19–36. ( 10.1002/hipo.450040105) [DOI] [PubMed] [Google Scholar]
- 22.Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F. 2001. Astrocytes of the mouse neocortex express functional N-methyl-d-aspartate receptors. FASEB J. 15, 1270–1272. ( 10.1096/fj.00-0439fje) [DOI] [PubMed] [Google Scholar]
- 23.Palygin O, Lalo U, Pankratov Y. 2011. Distinct pharmacological and functional properties of NMDA receptors in mouse cortical astrocytes. Br. J. Pharmacol. 163, 1755–1766. ( 10.1111/j.1476-5381.2011.01374.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karavanova I, Vasudevan K, Cheng J, Buonanno A. 2007. Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-[beta]-galactosidase knock-in mice. Mol. Cell. Neurosci. 34, 468–480. ( 10.1016/j.mcn.2006.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzamba D, Honsa P, Anderova M. 2013. NMDA receptors in glial cells: pending questions. Curr. Neuropharmacol. 11, 250–262. ( 10.2174/1570159X11311030002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verkhratsky A, Kirchhoff F. 2007. NMDA receptors in glia. Neuroscientist 13, 28–37. ( 10.1177/1073858406294270) [DOI] [PubMed] [Google Scholar]
- 27.Velez-Fort M, Audinat E, Angulo MC. 2012. Central role of GABA in neuron–glia interactions. Neuroscientist 18, 237–250. ( 10.1177/1073858411403317) [DOI] [PubMed] [Google Scholar]
- 28.Fraser DD, Duffy S, Angelides KJ, Perez-Velazquez JL, Kettenmann H, MacVicar BA. 1995. GABAA/benzodiazepine receptors in acutely isolated hippocampal astrocytes. J. Neurosci. 15, 2720–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller T, Fritschy JM, Grosche J, Pratt GD, Möhler H, Kettenmann H. 1994. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. J. Neurosci. 14, 2503–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riquelme R, Miralles CP, De Blas AL. 2002. Bergmann glia GABA(A) receptors concentrate on the glial processes that wrap inhibitory synapses. J. Neurosci. 22, 10 720–10 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song J, et al. 2012. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489, 150–154. ( 10.1038/nature11306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Wang Q, Haydar TF, Bordey A. 2005. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 8, 1179–1187. ( 10.1038/nn1522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seifert G, Hüttmann K, Binder DK, Hartmann C, Wyczynski A, Neusch C, Steinhäuser C. 2009. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J. Neurosci. 29, 7474–7488. ( 10.1523/JNEUROSCI.3790-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, Kirchhoff F, Kettenmann H. 2001. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33, 72–86. () [DOI] [PubMed] [Google Scholar]
- 35.Seifert G, Steinhäuser C. 1995. Glial cells in the mouse hippocampus express AMPA receptors with an intermediate Ca2+ permeability. Eur. J. Neurosci. 7, 1872–1881. ( 10.1111/j.1460-9568.1995.tb00708.x) [DOI] [PubMed] [Google Scholar]
- 36.Passlick S, Grauer M, Schafer C, Jabs R, Seifert G, Steinhäuser C. 2013. Expression of the γ2-subunit distinguishes synaptic and extrasynaptic GABAA receptors in NG2 cells of the hippocampus. J. Neurosci. 33, 12 030–12 040. ( 10.1523/JNEUROSCI.5562-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer ML, Westbrook GL. 1987. Permeation and block of NMDA acid receptor channels by divalent cations in mouse cultured central neurones. J. Physiol. (London) 394, 501–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekiguchi M, Fleck MW, Mayer ML, Takeo J, Chiba Y, Yamashita S, Wada K. 1997. A novel allosteric potentiator of AMPA receptors: 4-[2- (phenylsulfonylamino)ethylthio]-2,6-difluoro-phenoxyacetamide. J. Neurosci. 17, 5760–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samoilova MV, Buldakova SL, Vorobjev VS, Sharonova IN, Magazanik LG. 1999. The open channel blocking drug, IEM-1460, reveals functionally distinct α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors in rat brain neurons. Neuroscience 94, 261–268. ( 10.1016/S0306-4522(99)00326-7) [DOI] [PubMed] [Google Scholar]
- 40.Schröder W, Seifert G, Hüttmann K, Hinterkeuser S, Steinhäuser C. 2002. AMPA receptor-mediated modulation of inward rectifier K(+) channels in astrocytes of mouse hippocampus. Mol. Cell Neurosci. 19, 447–458. ( 10.1006/mcne.2001.1080) [DOI] [PubMed] [Google Scholar]
- 41.Wallraff A, Odermatt B, Willecke K, Steinhäuser C. 2004. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia 48, 36–43. ( 10.1002/glia.20040) [DOI] [PubMed] [Google Scholar]
- 42.Wang LP, Cheung G, Kronenberg G, Gertz K, Ji S, Kempermann G, Endres M, Kettenmann H. 2008. Mild brain ischemia induces unique physiological properties in striatal astrocytes. Glia 56, 925–934. ( 10.1002/glia.20660) [DOI] [PubMed] [Google Scholar]
- 43.Spreafico R, Frassoni C, Arcelli P, Battaglia G, Wenthold RJ, De Biasi S. 1994. Distribution of AMPA selective glutamate receptors in the thalamus of adult rats and during postnatal development. A light and ultrastructural immunocytochemical study. Dev. Brain Res. 82, 231–244. ( 10.1016/0165-3806(94)90166-X) [DOI] [PubMed] [Google Scholar]
- 44.Pirttimaki TM, Hall SD, Parri HR. 2011. Sustained neuronal activity generated by glial plasticity. J. Neurosci. 31, 7637–7647. ( 10.1523/JNEUROSCI.5783-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parri HR, Gould TM, Crunelli V. 2010. Sensory and cortical activation of distinct glial cell subtypes in the somatosensory thalamus of young rats. Eur. J. Neurosci. 32, 29–40. ( 10.1111/j.1460-9568.2010.07281.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parri HR, Gould TM, Crunelli V. 2001. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat. Neurosci. 4, 803–812. ( 10.1038/90507) [DOI] [PubMed] [Google Scholar]
- 47.Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. 2012. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 4, e00080 ( 10.1042/AN20110061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langer J, Stephan J, Theis M, Rose CR. 2012. Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia 60, 239–252. ( 10.1002/glia.21259) [DOI] [PubMed] [Google Scholar]
- 49.Langer J, Rose CR. 2009. Synaptically induced sodium signals in hippocampal astrocytes in situ. J. Physiol. 587, 5859–5877. ( 10.1113/jphysiol.2009.182279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verkhratsky A, Noda M, Parpura V, Kirischuk S. 2013. Sodium fluxes and astroglial function1. Adv. Exp. Med. Biol. 961, 295–305. ( 10.1007/978-1-4614-4756-6_25) [DOI] [PubMed] [Google Scholar]
- 51.Meier SD, Kafitz KW, Rose CR. 2008. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 56, 1127–1137. ( 10.1002/glia.20684) [DOI] [PubMed] [Google Scholar]
- 52.Ma BF, Xie MJ, Zhou M. 2012. Bicarbonate efflux via GABA(A) receptors depolarizes membrane potential and inhibits two-pore domain potassium channels of astrocytes in rat hippocampal slices. Glia 60, 1761–1772. ( 10.1002/glia.22395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egawa K, Yamada J, Furukawa T, Yanagawa Y, Fukuda A. 2013. Cl− homeodynamics in gap junction-coupled astrocytic networks on activation of GABAergic synapses. J. Physiol. 591, 3901–3917. ( 10.1113/jphysiol.2013.257162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kroeger D, Tamburri A, Amzica F, Sik A. 2010. Activity-dependent layer-specific changes in the extracellular chloride concentration and chloride driving force in the rat hippocampus. J. Neurophysiol. 103, 1905–1914. ( 10.1152/jn.00497.2009) [DOI] [PubMed] [Google Scholar]
- 55.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. 1998. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1, 563–571. ( 10.1038/2798) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.