Abstract
The perisynaptic extracellular matrix (ECM) contributes to the control of the lateral mobility of AMPA-type glutamate receptors (AMPARs) at spine synapses of principal hippocampal neurons. Here, we have studied the effect of the ECM on the lateral mobility of AMPARs at shaft synapses of aspiny interneurons. Single particle tracking experiments revealed that the removal of the hyaluronan-based ECM with hyaluronidase does not affect lateral receptor mobility on the timescale of seconds. Similarly, cross-linking with specific antibodies against the extracellular domain of the GluA1 receptor subunit, which affects lateral receptor mobility on spiny neurons, does not influence receptor mobility on aspiny neurons. AMPARs on aspiny interneurons are characterized by strong inward rectification indicating a significant fraction of Ca2+-permeable receptors. Therefore, we tested whether Ca2+ controls AMPAR mobility in these neurons. Application of the membrane-permeable Ca2+ chelator BAPTA-AM significantly increased the lateral mobility of GluA1-containing synaptic and extrasynaptic receptors. These data indicate that the perisynaptic ECM affects the lateral mobility differently on spiny and aspiny neurons. Although ECM structures on interneurons appear much more prominent, their influence on AMPAR mobility seems to be negligible at short timescales.
Keywords: glutamate receptor, interneurons, extracellular matrix, single particle tracking
1. Introduction
AMPA receptors (AMPARs) are the principal excitatory neurotransmitter receptors in the brain. They are highly mobile within the neuronal membrane owing to lateral diffusion [1,2]. Diffusion and trapping by the postsynaptic scaffold are key factors controlling AMPAR numbers at the synapse, and thereby regulating synaptic strength [2,3]. Immobilization of diffusive receptors at synapses occurs mainly by direct or indirect interaction with intra- and/or extracellular molecules. Intracellular factors include PDZ domain-containing scaffold proteins such as PSD95 and SAP97, which bind AMPARs directly or via auxiliary subunits, e.g. TARPs [4,5]. PDZ binding is reversible and activity-dependent; high synaptic activity, which leads to elevated intracellular Ca2+ and subsequent CaMKII-dependent phosphorylation of stargazin, increases the affinity of AMPARs for PDZ domains and enhances their synaptic accumulation [6,7]. Furthermore, the spine itself may act as a diffusion barrier, because lateral diffusion is restricted at the spine neck, and AMPARs exchanged faster between synaptic and extrasynaptic compartments on aspiny neurons, as measured by fluorescence recovery after photobleaching (FRAP) [8].
As extracellular factors affecting mobility and clustering of AMPARs, components of the extracellular matrix (ECM) are discussed. The brain's ECM is a meshwork of proteins of neuronal and glial origin [9]. Main components are chondroitin sulfate proteoglycans, including brevican and aggrecan that are coordinated by the glycosamine–glycan hyaluronan [10,11]. This ECM is found around most neurons and their synapses in the brain. While it has a loose appearance on excitatory forebrain neurons, it appears as dense, net-like structures around parvalbumin-positive interneurons, where the ECM forms so-called perineuronal nets [11,12]. Owing to its net-like appearance, the ECM has been postulated to define compartments on the neuronal surface that isolate synaptic contacts and control the lateral diffusion of AMPARs. Indeed, experimental removal of the ECM with the glycosidase hyaluronidase increased lateral diffusion and exchange of synaptic versus extrasynaptic AMPARs on principal neurons [13]. Physiologically, ECM removal was associated with decreased paired-pulse depression very likely owing to rapid exchange of desensitized synaptic for naive extrasynaptic AMPARs [13,14]. Thus, on spiny neurons, synaptic availability of AMPARs is defined by interplay between membrane-associated cytoplasmic scaffolds, i.e. the PSD, spine morphology and ECM-based surface compartments. Here, we wondered whether on aspiny interneurons the lack of spines as diffusion barriers might be functionally compensated by ECM structures. To test this, we analysed the influence of the ECM on lateral mobility and short-term plasticity of aspiny neurons.
2. Material and methods
A detailed description of chemicals and antibodies used in this study is provided in the electronic supplementary material.
(a). Neuronal cultures, fluorescence recovery after photobleaching experiments
Preparations of primary cultures from embryonic rat hippocampi (E18), their transfection with Effectene and matrix digestion procedure are described in the electronic supplementary material. Protocol for immunostainings has been described previously [13,14]. Protocol for single particle tracking (SPT) of AMPARs and its analysis are described in the electronic supplementary material. Set-up and methods to analyse FRAP were described previously [13].
(b). Electrophysiology
Whole-cell patch-clamp recordings were performed and analysed as described in the electronic supplementary material.
(c). Statistics
Data are expressed as mean ± s.e.m. or as median and interquartile range (IQR, 25%/75%). Statistical analysis was performed with Graph Pad Prism (GraphPad Software v. 5.0, USA). Statistical tests are indicated within the figure descriptions. Significant differences correspond to p-values: *p < 0.05, **p < 0.005 and ***p < 0.001.
3. Results
(a). Lateral mobility of GluA1 and GluA2 at aspiny synapses is not restricted by the extracellular matrix
The identification of aspiny glutamatergic synapses in our experiments is based on the co-localization of the scaffold protein Homer1 with AMPAR in spiny as well as aspiny neurons (figure 1a–c). In spiny neurons, Homer1 puncta accumulated in spine heads along the entire dendritic tree (figure 1a). In aspiny neurons, Homer1 was distributed in puncta along smooth dendrites and the soma (figure 1b). Co-staining of the surface population of AMPARs by specific antibodies against extracellular epitopes confirmed a higher abundance of GluA1 subunits in aspiny Homer-positive synapses, but no difference in GluA2-containing AMPARs (figure 1c). The majority of aspiny neurons represent GAD65-positive interneurons (electronic supplementary material, figure S1 and table S1). Electrophysiological characterization of the postsynaptic receptor composition revealed that aspiny neurons localize a substantial fraction of Ca2+-permeable AMPARs in their synapses as indicated by the inward-rectifying current–voltage relationship, confirming previous characterizations of the AMPAR population on GABAergic neurons (electronic supplementary material, figure S2) [15–17].
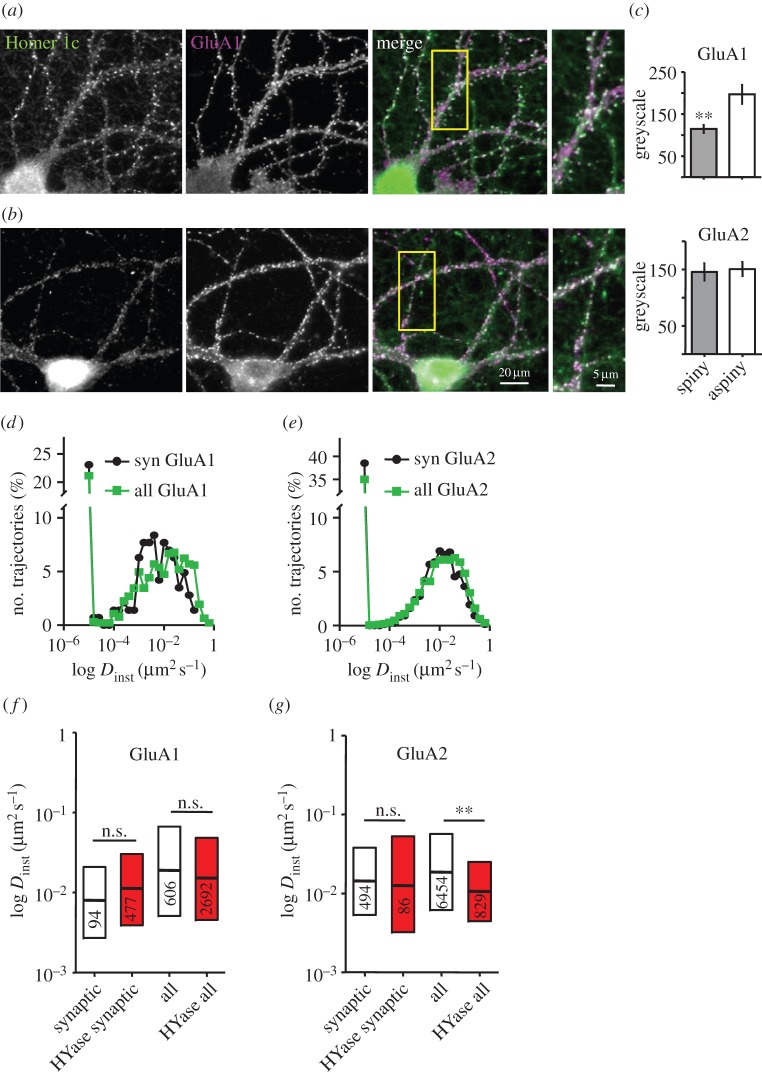
Figure 1.
The lateral mobility of GluA1 and GluA2 around aspiny synapses. (a,b) Dual labelling of Homer 1c (green) and GluA1 (magenta) in a spiny (a) and an aspiny (b) neuron. Boxed regions are magnified on the right. Scale bars apply to (a) and (b). (c) Quantification of synaptic live-staining for GluA1 (upper panel) and GluA2 (lower panel) AMPAR subunits in spiny and aspiny neurons. Data are shown as mean ± s.e.m., p < 0.01, t-test. (d,e) Distribution of instantaneous diffusion coefficients (Dinst) for synaptic (syn) and all trajectories (all) of endogenous GluA1 (d) and GluA2 (e) subunits in aspiny neurons obtained in SPT experiments ((d) synaptic: n = 143, all: n = 928 trajectories, eight cells, (e) synaptic: n = 882, all: n = 10 937 trajectories, two cells). (f,g) Box-plots show Dinst for the mobile fraction (D > 0.001 µm² s−1) of GluA1 (f) and GluA2 (g) in aspiny neurons under control conditions and after exposure to hyaluronidase (HYase). Data are shown as median/interquartile range, p < 0.005; Mann–Whitney test.
The influence of the ECM on the distribution and surface mobility of AMPARs in interneurons was probed in cultures that had been maintained for more than 21 days in vitro. At this age, dense nets of ECM were detectable around all neurons but were particularly dense around aspiny neurons (electronic supplementary material, figure S3). The ECM was removed with hyaluronidase (HYase) reducing Wisteria floribunda agglutinin staining to 50.3 ± 6.5% of control (electronic supplementary material, figure S3b,c). The digestion of the ECM by HYase occurred within the first 30 min after enzyme application and was not altered after overnight digestion as demonstrated previously for the mobility of AMPARs on glutamatergic neurons [13]. SPT on endogenous surface populations of GluA1- and GluA2-containing AMPARs was employed to test whether the pronounced ECM on interneurons affected the local AMPAR mobility. Antibodies against the N-terminal domain of GluA1 were labelled with quantum dots (QDs), and antibodies against GluA2 were labelled with ATTO647. QDs or ATTO647-molecules co-localizing with overexpressed Homer1c::GFP spots were considered as synaptic. The distribution of the instantaneous diffusion coefficients (Dinst) was shifted to smaller values for GluA1 and GluA2 at aspiny synapses compared with dendrites (figure 1d for GluA1, syn versus all, median: 0.008 µm2 s−1 IQR 0.003/0.021, 94 trajectories, versus 0.019 µm2 s−1 IQR 0.005/0.067, 606 trajectories from eight cells, six cultures p < 0.005; figure 1e for GluA2, syn versus all: 0.014 µm2 s−1 IQR 0.005/0.04, 494 trajectories, versus 0.019 µm2 s−1 IQR 0.006/0.056, 6454 trajectories, 12 cells, six cultures p < 0.005). The fraction of immobile GluA1 and GluA2 subunits at aspiny synapses and extrasynaptic locations was different with a larger fraction of immobilized GluA2-containing receptors in both compartments (figure 1d,e; 38.5%, 55 trajectories for GluA1 and 44.8%, 395 trajectories for GluA2 at synapses and 37.2%, 346 trajectories for GluA1 and 42.2%, 4614 trajectories for GluA2 at dendrites).
In contrast to spiny neurons [13], acute ECM removal with HYase did not change the mobility of synaptic or extrasynaptic GluA1 on aspiny neurons (figure 1f, after HYase synaptic, median: 0.011 µm2 s−1 IQR 0.004/0.030, 477 trajectories; all: 0.015 µm2 s−1 IQR 0.005/0.05, 2692 trajectories, from five cultures). Similarly, endogenous GluA2-containing receptors at shaft synapses were not affected by HYase treatment, but decreased in their mobility outside synapses (figure 1g, after HYase synaptic, median: 0.013 µm2 s−1 IQR 0.003/0.053, 86 trajectories, three cultures; all: 0.011 µm2 s−1 IQR 0.004/0.025, 829 trajectories, two cultures). Higher mobility at extrasynaptic locations than at synapses was preserved after matrix degradation for GluA1 (p < 0.005). These data suggest that ECM has either no strong impact on the local mobility of the endogenous population of GluA1- and GluA2-containing receptors or AMPARs on aspiny synapses have different properties.
(b). Short-term synaptic plasticity in aspiny interneurons is not modulated by the extracellular matrix
To examine AMPAR properties before and after ECM digestion, we probed kinetic parameters found to be affected by ECM digestion [13]. Postsynaptic AMPARs were probed before and after ECM removal by fast iontophoretic Glu application on Homer-positive aspiny synapses. HYase treatment overnight did not alter membrane properties and basic synaptic transmission. Membrane potential, action potential amplitude and width as well as kinetics and frequency of mEPSCs were unchanged after matrix removal (electronic supplementary material, figure S2). Acute incubation of cultures with HYase before patch-clamp experiments also had no effect on the membrane properties, ruling out homeostatic effects of long-term incubation (not shown). Further, we wondered whether long-term ECM removal influences the organization of aspiny glutamatergic synapses. However, no change in rectification index (RI) was observed when comparing synapses before and after ECM digestion (figure 2a, aspiny control versus HYase: 0.42 ± 0.07, n = 19 versus 0.37 ± 0.07, n = 15, p > 0.05). The high variability of the RI points to a quite heterogeneous population of AMPARs expressed in aspiny neurons under our culture conditions, which probably masks ECM-induced changes in the RI. In order to reduce the variability, we used as a control a mouse line expressing GFP under the GAD-65 promoter to identify interneurons. Here, the RI was less variable; however, ECM digestion also had no significant effect (control versus HYase: 0.17 ± 0.04, n = 10 versus 0.23 ± 0.08, n = 3, p > 0.05). Labelling the surface population of GluA1- and GluA2-subunits on aspiny neurons also did not reveal different AMPAR densities before and after digestion (GluA1: 118.1 ± 8.7% of control after HYase, n = 14, p = 0.19; GluA2: 115.4 ± 8.8% of control after HYase, n = 21, p = 0.49). Next, we tested the effect of ECM on the recovery of AMPARs from desensitization. Overnight digestion did not affect recovery from desensitization of AMPARs (figure 2b), indicating that, in contrast to spiny neurons [13], ECM does not influence recovery from desensitization of synaptic receptors or lateral exchange with naive extrasynaptic receptors on aspiny neurons. To exclude effects caused by steady-state desensitization of AMPARs and to focus on the population of activated AMPARs by glutamate iontophoresis, we applied the weak competitive antagonist kynurenic acid (Kyn). Here, only populations of AMPARs exposed to glutamate concentrations that are sufficient to replace Kyn are activated. In the presence of Kyn, recovery from desensitization was significantly slower for synaptic receptors compared with extrasynaptic AMPAR, confirming our SPT experiments that indicated lower mobility inside synapses than outside (figure 2d).
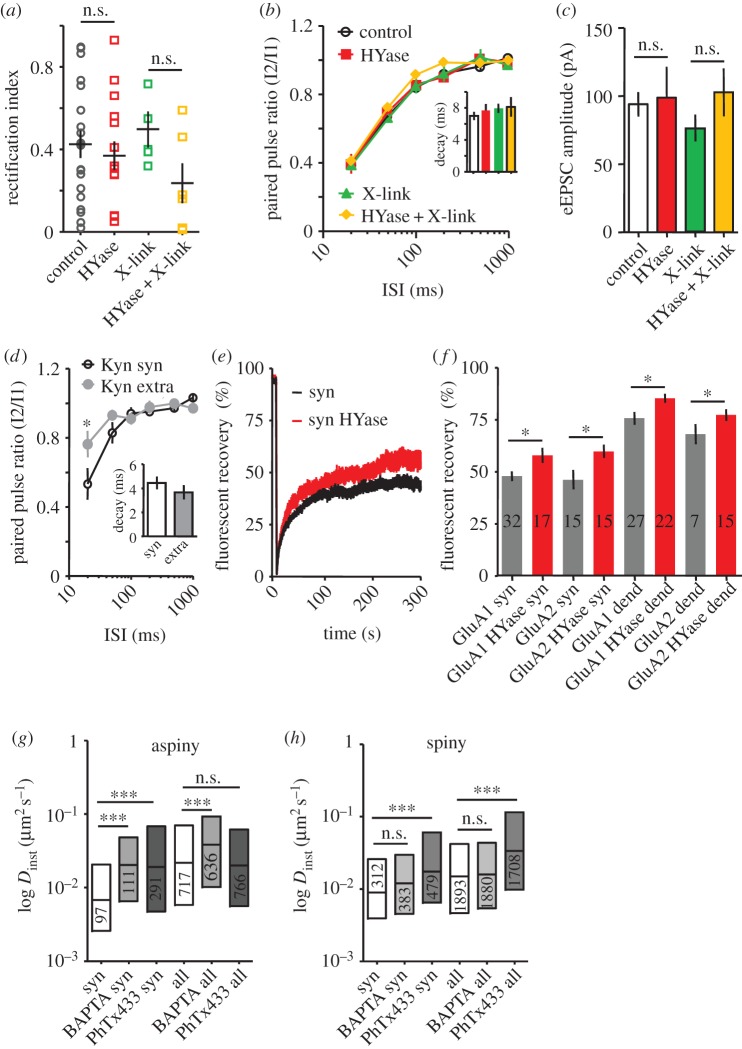
Figure 2.
ECM degradation has no effect on dynamic and subunit composition of AMPARs and short-term plasticity in aspiny neurons, whereas change of intracellular free calcium alters AMPAR dynamics. (a) Rectification index (RI) of AMPAR populations in aspiny synapses measured in control, after ECM digestion by hyaluronidase (HYase), after cross-linking of GluA1 (X-link) and in combination of ECM-digestion and cross-linking (Hyase + X-link, p > 0.05, t-test). (b) Plot of the recovery from desensitization of AMPAR eEPSC induced by iontophoretic Glu application in aspiny neurons as a function of the interstimulus interval (ISI) under indicated conditions. Number of cells in each group are: control = 21, HYase = 11, X-link = 11, HYase + X-link = 9 (F = 1.84, p > 0.05, two-way ANOVA). Inset demonstrates no difference in the decay of eEPSCs under indicated conditions. Data are shown as mean ± s.e.m. (c) Mean ± s.e.m. for amplitude of eEPSCs under indicated conditions (p > 0.05 for all comparisons, Kruskal–Wallis test). (d) Plot of the recovery from desensitization of synaptic (syn) and extrasynaptic (extra) eEPSCs in aspiny neurons in the presence of 500 µM kynurenic acid (Kyn, n = 5, F = 14.22; p < 0.01, two-way ANOVA). Inset demonstrates absence of difference in the decay between synaptic and extrasynaptic eEPSCs. (e,f) FRAP experiment. (f) Normalized fluorescence recovery curve of GluA1::SEP bleached in aspiny synapses under control conditions and after treatment with HYase. (f) Mean ± s.e.m. of averaged normalized recovery in aspiny neurons at 300 s after photobleaching for synaptic (syn) and extrasynaptic locations (dend) without and after HYase treatment for neurons expressing either GluA1::phluorin or GluA2::phluorin as indicated. Number of bleached spots indicated in bars. Comparing synapses or dendrites before and after HYase treatment indicated significant differences as indicated between synaptic and dendritic compartments, t-test. (g,h) Box-plot of the median/interquartile range of instantaneous diffusion coefficients (Dinst) for synaptic (syn) and total (all) GluA1 fractions in aspiny (g) and spiny (h) neurons incubated without or with BAPTA-AM or philantotoxin433 (PhTx433). BAPTA-AM and PhTx433 increased the mobility (Dinst) of synaptic and total fraction of GluA1 in aspiny but not in spiny neurons (p < 0.01 for synaptic and p < 0.005 for all trajectories, Mann–Whitney test).
The existence of a mobile population inside and outside the synapse might still allow exchange of receptors between compartments. To prevent this, we immobilized surface AMPARs by cross-linking [14,18]. Cross-linking of GluA1-containing receptors with antibodies before (X-link) and after acute matrix digestion (HYase + X-link) did not alter paired-pulse ratio (PPR) or recovery from desensitization (figure 2b). None of the treatments affected amplitude or kinetics of the evoked excitatory postsynaptic current (eEPSC; figure 2b inset and 2c) confirming the absence of direct effects of HYase, X-link or the combination on kinetic properties of AMPARs. The variability of the RI in synapses of aspiny neurons may either mask the local dynamic fluctuation of the AMPAR population or simply reflect a rather rigid assembly and/or subunit composition of synaptic receptors in aspiny synapses. Our data indicate that synaptic AMPARs on aspiny neurons are highly confined and their mobility is not modulated by ECM despite the differences in ECM density in comparison with spiny neurons.
These observations argue against the hypothesis that the ECM acts as passive diffusion barrier on aspiny neurons. Nevertheless, we wondered whether an increase of the mobile population of AMPARs might uncover the ECM-mediated compartmentalization. To modulate the mobile AMPAR fraction and its local confinement, we overexpressed pHluorin-tagged GluA1 and GluA2 subunits, a manipulation known to induce an approximately twofold increase in the surface population of GluA1- or GluA2-containing AMPARs [14,19]. The properties of the pHluorin [20] allowed FRAP experiments to be performed to probe the mobility of surface-expressed GluA1- and GluA2-containing AMPARs.
Under these conditions, enzymatic removal of ECM with HYase significantly increased the recovery rate of GluA1::pHluorin and GluA2::pHluorin fluorescence in synaptic and extrasynaptic membrane compartments (figure 2e,f synaptic control versus HYase: GluA1: 48 ± 2%, n = 32 versus 58 ± 4%, n = 17, p = 0.021; GluA2: 46 ± 5%, n = 15 versus 60 ± 3%, p = 0.022 and dendritic control versus HYase: GluA1: 76 ± 3%, n = 27 and 86 ± 2%, n = 22, p = 0.014; GluA2: 66 ± 5%, n = 7 versus 78 ± 3%, p = 0.04, t-test). A similar increase in fluorescence recovery after matrix digestion was observed in spiny synapses [13] confirming the proposed impact of ECM composition on AMPAR surface dynamics. Some limitations of this approach have to be considered. First, the bleached area is determined by the diffraction limit of the microscope (usually ≥ 1 µm²) and hence larger than most postsynapses in cultured neuronal networks. Second, overexpression of fluorescence-tagged proteins induces higher surface dynamics of receptors [21]. Thus, we assume that modulation of the mobile fraction of AMPARs in aspiny neurons might be controlled by intracellular binding partners. In particular, the Ca2+ permeability of the AMPARs prevalent in aspiny neurons (figure 1c and the electronic supplementary material, figure S2) might cause a stronger confinement of the receptors and hence overrule the ECM-based membrane compartmentalization.
(c). Mobility of GluA1 on aspiny neurons is regulated by intracellular Ca2+
In spiny neurons, a transient increase of intracellular Ca2+ via uncaging or strong synaptic activation induces strong immobilization of AMPARs [1,7,14]. To test whether indeed intracellular Ca2+ fluctuations are responsible for the strong confinement of AMPARs on aspiny neurons, we either clamped the intracellular Ca2+ concentration by incubating cultures in BAPTA-AM or blocked the fraction of potentially calcium-permeable AMPARs by philantotoxin 433 (PhTx433) and monitored the mobility of the endogenous receptor populations on spiny and aspiny neurons using SPT. BAPTA increased the mobility of synaptic and extrasynaptic fraction of GluA1 on aspiny neurons (figure 2g, median of Dinst for synaptic GluA1 control versus BAPTA-AM: 0.008 µm2 s−1 IQR 0.001/0.144, 94 trajectories versus 0.020 µm2 s−1 IQR 0.001/0.402, 111 trajectories after incubation with BAPTA-AM, p < 0.01, Mann–Whitney test and for all GluA1 trajectories: control versus BAPTA-AM: 0.019 µm2 s−1 IQR 0.001/0.605, 606 trajectories versus 0.038 µm2 s−1 IQR 0.001/0.630, 636 trajectories, p < 0.005) without affecting the confinement area (electronic supplementary material, figure S4). In spiny neurons, chelating Ca2+ had no effect on the mobility of GluA1 subunits (figure 2h, p > 0.05). Blocking calcium-permeable AMPARs with PhTx433 also mobilized AMPARs inside synapses of aspiny and spiny neurons, but only altered the mobility of extrasynaptic AMPARs in spiny neurons (figure 2g,h, median of Dinst for aspiny synaptic GluA1 + PhTx433: 0.019 µm2 s−1 IQR 0.005/0.069, 291 trajectories; median of Dinst for spiny synaptic GluA1 + PhTx433: 0.017 µm2 s−1 IQR 0.007/0.061, 479 trajectories; for all aspiny GluA1 PhTx433: 0.020 µm2 s−1 IQR 0.006/0.062, 766 trajectories; for all spiny GluA1 PhTx433: 0.034 µm2 s−1 IQR 0.010/0.116, 1708 trajectories).
4. Discussion
Here, we report that aspiny glutamatergic synapses on interneurons in hippocampal cultures contain highly confined AMPARs, which are partially Ca2+-permeable. This Ca2+ permeability might be responsible for the strong confinement within the synapse that is not influenced by interactions with the perisynaptic ECM and hence does not interfere with the AMPAR-mediated short-term plasticity in most of these synapses.
The majority of aspiny neurons in dissociated hippocampal cultures are interneurons [22,23], expressing an AMPAR population (electronic supplementary material, figures S1 and S2) and auxiliary proteins different from those in spine-containing neurons [24,25]. Depending on subunit composition and auxiliary proteins, AMPARs differ substantially in their properties, including rectification, desensitization and recovery from the desensitization [16,24,26]. Here, we confirm that receptors on aspiny neurons show on average faster postsynaptic AMPAR-mediated currents and a slower recovery from desensitization compared with spiny neurons (electronic supplementary material, figure S2), which is reflected in higher numbers of surface-expressed GluA1 subunits (figure 1). This suggests that a considerable fraction (approx. 50%) of AMPARs in aspiny glutamatergic synapses are Ca2+-permeable as confirmed by the sensitivity to PhTx433 (electronic supplementary material, figure S2; [24,27]). This Ca2+ permeability might exert the function of confining AMPARs to synapses and thus explain their limited surface dynamics which is insensitive to ECM removal (figure 1). Similar observations were reported for spiny neurons when GluA1 subunits were overexpressed and Ca2+-permeable GluA1 homomers were introduced in the synapse [14]. This suggests that the ECM-based compartmentalization does not affect the local fraction of mobile receptors in small compartments such as the synaptic contact site defined by Homer staining of aspiny synapses. However, when GluA1 is overexpressed in aspiny neurons, the highly mobile population of AMPARs is increased [18,21], and the ECM acts as a passive diffusion barrier as observed on spiny neurons [13]. Experimental immobilization by cross-linking of endogenous GluA1 receptors does not affect their kinetic properties, and confirms our interpretation that AMPARs in aspiny synapses are more confined than in spiny synapses. This is reminiscent of the behaviour of NMDA-type receptors in spiny synapses [28] and left us with the hypothesis that the local amount of Ca2+-permeable AMPARs might determine the synaptic confinement. Control of intracellular Ca2+ by BAPTA-AM or block of the Ca2+-permeable receptor fraction before tracking AMPARs supports this idea (figure 2g,h). In spiny neurons, we also observed a mobilization of GluA1-containing AMPARs after block of GluA2-lacking receptors (but not with BAPTA). As suggested by the variability of the RI (electronic supplementary material, figure S2), there is also a population of Ca2+-permeable AMPARs expressed in spiny synapses, which might serve a confining function.
Interestingly, the RI and hence the population of Ca2+-permeable AMPARs was highly variable among aspiny synapses, probably owing to the heterogeneity of interneurons, different innervating axons [17] or activity-driven changes in accessory subunit compositions [29–31]. A functional explanation for this heterogeneity could be the critical involvement of interneurons in tuning the input–output function of neuronal network activity. Contacts between principal neurons tune the threshold for plasticity, whereas changes in the excitability of interneurons change the gain of plastic changes [32]. How strongly such effects depend on the individual composition of postsynaptic receptor populations remains an open question. The scattering of RI was evident not only between different aspiny neurons, but was also observed between different synapses along an individual aspiny neuron. Whether this is caused by a single axon or different axons was not addressed. Fluctuations of the release probability of individual boutons from the same axon can occur [33] and this could lead to activity-driven shaping of AMPAR compositions as suggested in cerebellar neurons [29]. Accordingly, inputs from different presynaptic synapses might be integrated by the postsynaptic receptor composition and in turn tune the output function of this particular neuron.
Removal of the ECM can influence the receptor dynamics and local receptor density and exchange rate between synaptic and extrasynaptic receptors [13]. In aspiny synapses, another variable seems to be important, which could be the population size of Ca2+-permeable AMPARs and hence their Ca2+-dependent confinement. Binding to intracellular scaffolds and intracellular kinase activity depends on the intracellular fluctuation of free Ca2+ [1,7,14]. This strong confinement might fulfil two functions, first preserving the inhibitory tone (output function) within a neuronal network and second protecting the neuron from excessive Ca2+ influx through Ca2+-permeable AMPARs [34,35]. Accordingly, the function of the ECM seems to be different on aspiny and on spiny neurons. Whereas in spiny neurons, AMPARs seem to be less confined by intracellular binding partners or auxiliary subunits the ECM can function as an obstacle, particularly for the extrasynaptic population, whereas the synaptic population remains unbiased by changes in ECM composition or density [13]. At aspiny neurons, the contributions of mobile AMPARs to modulate synaptic transmission seem to be much more strongly controlled by intracellular interactions and are less influenced by the ECM, at least on the timescale of seconds to minutes that was observed here, despite a much higher density of ECM-like structures around aspiny neurons.
Supplementary Material
Acknowledgements
We thank D. Choquet, E. Hosy for helpful comments and discussions; A. Lenuweit, S. Opitz, H. Wickborn for excellent technical assistance. J.K., R.F. and M.H. designed experiments. J.K., R.F. and M.H. conducted experiments and analysed data. J.K., R.F., E.G. and M.H. designed the concept and wrote the manuscript.
Funding statement
This study was supported by the ERANET/BMBF grant MODDIFSYN and Land Sachsen-Anhalt grant no. LSA MK-IfN-2009-01.
References
- 1.Borgdorff AJ, Choquet D. 2002. Regulation of AMPA receptor lateral movements. Nature 417, 649–653. ( 10.1038/nature00780) [DOI] [PubMed] [Google Scholar]
- 2.Choquet D, Triller A. 2013. The dynamic synapse. Neuron 80, 691–703. ( 10.1016/j.neuron.2013.10.013) [DOI] [PubMed] [Google Scholar]
- 3.Czondor K, Mondin M, Garcia M, Heine M, Frischknecht R, Choquet D, Sibarita JB, Thoumine OR. 2012. Unified quantitative model of AMPA receptor trafficking at synapses. Proc. Natl Acad. Sci. USA 109, 3522–3527. ( 10.1073/pnas.1109818109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bats C, Groc L, Choquet D. 2007. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, 719–734. ( 10.1016/j.neuron.2007.01.030) [DOI] [PubMed] [Google Scholar]
- 5.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. 2002. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl Acad. Sci. USA 99, 13 902–13 907. ( 10.1073/pnas.172511199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. 2007. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron 54, 447–460. ( 10.1016/j.neuron.2007.04.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. 2010. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67, 239–252. ( 10.1016/j.neuron.2010.06.007) [DOI] [PubMed] [Google Scholar]
- 8.Ashby MC, Maier SR, Nishimune A, Henley JM. 2006. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J. Neurosci. 26, 7046–7055. ( 10.1523/JNEUROSCI.1235-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frischknecht R, Seidenbecher CI. 2008. The crosstalk of hyaluronan-based extracellular matrix and synapses. Neuron Glia Biol. 4, 249–257. ( 10.1017/S1740925X09990226) [DOI] [PubMed] [Google Scholar]
- 10.Dityatev A, Schachner M. 2003. Extracellular matrix molecules and synaptic plasticity. Nat. Rev. Neurosci. 4, 456–468. ( 10.1038/nrn1115) [DOI] [PubMed] [Google Scholar]
- 11.Gundelfinger ED, Frischknecht R, Choquet D, Heine M. 2010. Converting juvenile into adult plasticity: a role for the brain's extracellular matrix. Eur. J. Neurosci. 31, 2156–2165. ( 10.1111/j.1460-9568.2010.07253.x) [DOI] [PubMed] [Google Scholar]
- 12.Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. 1998. Perineuronal nets: past and present. Trends Neurosci. 21, 510–515. ( 10.1016/S0166-2236(98)01298-3) [DOI] [PubMed] [Google Scholar]
- 13.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. 2009. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat. Neurosci. 12, 897–904. ( 10.1038/nn.2338) [DOI] [PubMed] [Google Scholar]
- 14.Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. 2008. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320, 201–205. ( 10.1126/science.1152089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaac JT, Ashby MC, McBain CJ. 2007. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54, 859–871. ( 10.1016/j.neuron.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 16.Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. 1994. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 12, 1281–1289. ( 10.1016/0896-6273(94)90444-8) [DOI] [PubMed] [Google Scholar]
- 17.Toth K, McBain CJ. 1998. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat. Neurosci. 1, 572–578. ( 10.1038/2807) [DOI] [PubMed] [Google Scholar]
- 18.Mondin M, et al. 2011. Neurexin-neuroligin adhesions capture surface-diffusing AMPA receptors through PSD-95 scaffolds. J. Neurosci. 31, 13 500–13 515. ( 10.1523/JNEUROSCI.6439-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heine M, Thoumine O, Mondin M, Tessier B, Giannone G, Choquet D. 2008. Activity-independent and subunit-specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc. Natl Acad. Sci. USA 105, 20 947–20 952. ( 10.1073/pnas.0804007106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashby MC, Ibaraki K, Henley JM. 2004. It's green outside: tracking cell surface proteins with pH-sensitive GFP. Trends Neurosci. 27, 257–261. ( 10.1016/j.tins.2004.03.010) [DOI] [PubMed] [Google Scholar]
- 21.Groc L, Lafourcade M, Heine M, Renner M, Racine V, Sibarita JB, Lounis B, Choquet D, Cognet L. 2007. Surface trafficking of neurotransmitter receptor: comparison between single-molecule/quantum dot strategies. J. Neurosci. 27, 12 433–12 437. ( 10.1523/JNEUROSCI.3349-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosp JA, Struber M, Yanagawa Y, Obata K, Vida I, Jonas P, Bartos M. 2013. Morpho-physiological criteria divide dentate gyrus interneurons into classes. Hippocampus 24, 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klueva J, Meis S, de Lima AD, Voigt T, Munsch T. 2008. Developmental downregulation of GABAergic drive parallels formation of functional synapses in cultured mouse neocortical networks. Dev. Neurobiol. 68, 934–949. ( 10.1002/dneu.20632) [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Lu W, Milstein AD, Nicoll RA. 2009. The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron 62, 633–640. ( 10.1016/j.neuron.2009.05.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. 2003. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 161, 805–816. ( 10.1083/jcb.200212116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiger JR, Lubke J, Roth A, Frotscher M, Jonas P. 1997. Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 18, 1009–1023. ( 10.1016/S0896-6273(00)80339-6) [DOI] [PubMed] [Google Scholar]
- 27.Rozov A, Sprengel R, Seeburg PH. 2012. GluA2-lacking AMPA receptors in hippocampal CA1 cell synapses: evidence from gene-targeted mice. Front. Mol. Neurosci. 5, 22 ( 10.3389/fnmol.2012.00022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. 2004. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat. Neurosci. 7, 695–696. ( 10.1038/nn1270) [DOI] [PubMed] [Google Scholar]
- 29.Bats C, Soto D, Studniarczyk D, Farrant M, Cull-Candy SG. 2012. Channel properties reveal differential expression of TARPed and TARPless AMPARs in stargazer neurons. Nat. Neurosci. 15, 853–861. ( 10.1038/nn.3107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bats C, Farrant M, Cull-Candy SG. 2013. A role of TARPs in the expression and plasticity of calcium-permeable AMPARs: evidence from cerebellar neurons and glia. Neuropharmacology 74, 76–85. ( 10.1016/j.neuropharm.2013.03.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herring BE, Shi Y, Suh YH, Zheng CY, Blankenship SM, Roche KW, Nicoll RA. 2013. Cornichon proteins determine the subunit composition of synaptic AMPA receptors. Neuron 77, 1083–1096. ( 10.1016/j.neuron.2013.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho TP, Buonomano DV. 2009. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron 61, 774–785. ( 10.1016/j.neuron.2009.01.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ermolyuk YS, Alder FG, Henneberger C, Rusakov DA, Kullmann DM, Volynski KE. 2012. Independent regulation of basal neurotransmitter release efficacy by variable Ca2+ influx and bouton size at small central synapses. PLoS Biol. 10, e1001396 ( 10.1371/journal.pbio.1001396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu SJ, Zukin RS. 2007. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 30, 126–134. ( 10.1016/j.tins.2007.01.006) [DOI] [PubMed] [Google Scholar]
- 35.Pellegrini-Giampietro DE, Gorter JA, Bennett MV, Zukin RS. 1997. The GluR2 (GluR-B) hypothesis: Ca2+-permeable AMPA receptors in neurological disorders. Trends Neurosci. 20, 464–470. ( 10.1016/S0166-2236(97)01100-4) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.