Abstract
Volume transmission is a form of intercellular communication that does not require synapses; it is based on the diffusion of neuroactive substances across the brain extracellular space (ECS) and their binding to extrasynaptic high-affinity receptors on neurons or glia. Extracellular diffusion is restricted by the limited volume of the ECS, which is described by the ECS volume fraction α, and the presence of diffusion barriers, reflected by tortuosity λ, that are created, for example, by fine astrocytic processes or extracellular matrix (ECM) molecules. Organized astrocytic processes, ECM scaffolds or myelin sheets channel the extracellular diffusion so that it is facilitated in a certain direction, i.e. anisotropic. The diffusion properties of the ECS are profoundly influenced by various processes such as the swelling and morphological rebuilding of astrocytes during either transient or persisting physiological or pathological states, or the remodelling of the ECM in tumorous or epileptogenic tissue, during Alzheimer's disease, after enzymatic treatment or in transgenic animals. The changing diffusion properties of the ECM influence neuron–glia interaction, learning abilities, the extent of neuronal damage and even cell migration. From a clinical point of view, diffusion parameter changes occurring during pathological states could be important for diagnosis, drug delivery and treatment.
Keywords: extracellular space, diffusion, tortuosity, astrocytes, extracellular matrix, volume fraction
1. Introduction
The extracellular space (ECS) is the precisely balanced microenvironment that accommodates the cellular components of the brain: neurons and glia. The composition of the ECS, its chemical and biophysical properties, ensures the functionality of these cells in both physiological and pathological states. In addition to the more commonly known process of synaptic transmission, extrasynaptic or ‘volume’ transmission, based on the diffusion of neuroactive substances through the volume of the ESC to their binding sites (often high-affinity receptors) on neighbouring neurons or glial cells, has been recognized in the latter half of the last century as an alternative form of intercellular communication [1–3] and since then it has been frequently studied (for review, see [4,5]). This type of signal transmission is crucial for the proper reaction of astrocytes to neuronal activity and their role in ionic, pH and volume homeostasis as well as for neuronal and glial network interactions involved in the regulation of neuronal transmission and plasticity [6–8].
Extracellular diffusion is the underlying mechanism of both short- and long-distance communication between cells in central nervous tissue (figure 1). An example of short-distance communication is the extrasynaptic interaction among presynaptic terminals, postsynaptic terminals and adjacent astrocytic processes enwrapping the synapse. While the model of a tripartite synapse [10], that suggests the cooperation of all three elements in signal transmission, is widely accepted, a more recent model introduces a fourth partner, the ECS—including its major component, the extracellular matrix (ECM) [9,11,12]. A condensed ECM surrounds the axosomatic synapses on interneurons forming aggrecan-based perineuronal nets (PNNs) [13], whereas other synapses can be covered by brevican-based axonal coats (ACs) [14,15]. ‘Private’- or ‘closed’-type synapses, tightly ensheathed by astrocytic processes and PNNs or ACs, prevail in the already developed nervous tissue and ensure the selectivity and high signal-to-noise ratio of synaptic transmission.
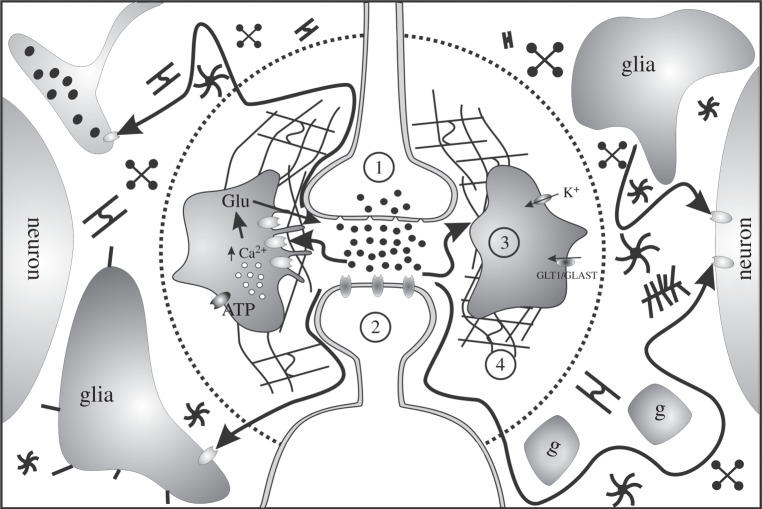
Figure 1.
Schematic of intercellular communication via diffusion. Inside the dotted circle: short-distance diffusion of molecules mediates intercellular communication between presynaptic terminal (1), postsynaptic terminal (2) and glia and their processes ensheathing the synapse (3), and it is affected by the ECS properties and its content, particularly the ECM (4). Outside the dotted circle: neuroactive molecules, which have escaped from the synaptic cleft, reach high-affinity neuron or glia receptors at longer distance. This diffusion is restricted to the ECS volume and hindered by neuronal and astrocytic processes and ECM molecules. g, glial processes; Glu, glutamate; GLT1/GLAST, glutamate transporter. Adapted from [9].
The ‘open’ type of synapse, lacking in the ECM covering, has a higher potential for plastic changes and appears more frequently in the immature brain. These synapses are a major source of neuroactive substances for extrasynaptic transmission as they allow the escape of mediators from the synaptic cleft to the ECS, a phenomenon known as ‘spill-over’. The neuroactive substances then further diffuse through the ECS and affect glial cells or neighbouring synapses. The described process is the underlying mechanism of synaptic crosstalk [16], a heterosynaptic communication which can play a role in long-term potentiation or depression, modulation of inhibition in the hippocampus and release of mediators or hormones (e.g. oxytocin, dopamin or glutamate) [17–19]. The terms volume or extrasynaptic transmission have been coined to encompass both synaptic crosstalk as well as long-distance communication by extracellular diffusion of other neuroactive substances; namely ions (e.g. potassium), small molecules like ATP, gaseous transmitters (nitric oxide), hormones, trophic and growth factors [3,9].
2. Diffusion parameters of the extracellular space
In comparison to free medium, diffusion of molecules through the ECS is restricted by its volume and hindered by its geometry and content. To describe the diffusion in the brain tissue, the original equation for Fick's second law had to be modified by introducing three ECS diffusion parameters: ECS volume fraction α (α = ECS volume/total tissue volume), tortuosity λ (λ2 = free/apparent diffusion coefficient) and non-specific uptake k' [4,20]. While the term ECS volume fraction represents the space available for diffusion, tortuosity describes the hindrance of the substance diffusing within the brain ECS. Typical values observed in healthy young adult human or rodent cortex are α ∼ 0.20–0.22 and λ ∼ 1.5–1.6 [4]. The hindrance of the diffusing molecule is due to the presence of diffusion barriers within the brain ECS compared to diffusion in free medium. Until now, several reasons for increased λ have been suggested: increased diffusion path length due to circumvention of the diffusing molecule around cells and their processes, trapping in dead-space microdomains, effects of membrane charges, viscosity and hindrances resulting from the presence of ECM molecules in narrow ECS clefts [4,21]. Non-specific uptake k' represents the loss of diffusing molecules from the ECS either into capillaries or across cell membranes.
The diffusion in the ECS is heterogeneous in different brain regions and in some areas also anisotropic, i.e. more facilitated in a certain direction than in others [4]. Anisotropic diffusion is a typical feature of myelinated white matter, with preferential diffusion along myelinated axons [22]. Anisotropy has also been found in the molecular layer of the cerebellum [23], the hypothalamic supraoptic nucleus (SON) [24], the auditory cortex [25] and the dentate gyrus of the hippocampus [26]; more recent studies did not confirm anisotropy in CA1 region [27,28]. Diffusion anisotropy is not present in the immature unmyelinated corpus callosum but appears as myelination proceeds [22]. Anisotropic properties of the tissue may also disappear, as seen in the SON during lactation due to the reorganizing of glial processes [24], or in the dentate gyrus during ageing as a result of demyelination, rearrangement of astrocytic processes or loss of the ECM [29].
3. Methods for detection of extracellular diffusion
Although introduced over 30 years ago [20], the real-time iontophoresis (RTI) method remains the most comprehensive method for studies on the diffusion of small molecules in ECS. RTI uses tetramethylammonium cation (TMA+) as an extracellular marker, which is applied iontophoretically into the tissue. Time-dependent changes in concentration of TMA+ following an iontophoretic pulse are detected within a known distance by a TMA+ ion-sensitive microelectrode [20]. This is the only method able to determine the absolute values of all three diffusion parameters from measurements in tissue slices as well as in vivo. However, in regions with anisotropic diffusion, the real value of the extracellular volume fraction has to be re-calculated from the data obtained from measurements in all three axes [22,23].
The other methods used to study ECS volume fraction and tortuosity (for review, see [4,21]) include the measurement of tissue resistance, detecting intrinsic optical signals by light transmittance and/or scattering (IOS method), the measurement of the apparent diffusion coefficients (ADCs) of fluorescently labelled large molecules by integrative optical imaging (IOI), fluorescent recovery after photobleaching (FRAP) and the measurement of the apparent diffusion coefficient of water (ADCW) by diffusion-weighted MRI (DW-MRI). In comparison with RTI, these methods are less comprehensive as they either measure only one of the diffusion parameters or determine only relative changes in the ECS volume fraction, but they can be useful in certain studies. For example, IOI, first described by Nicholson & Tao [30], represents a valuable tool in detecting tortuosity values for diffusion of large molecules. Presuming that the fluorescent label is firmly attached to the diffusing substance, IOI can be used with a wide range of substances [21]; moreover, it also enables a real-time registration of diffusion anisotropy in brain tissue [27,31]. The original FRAP method, primarily developed to monitor lateral diffusion in cell membranes, has been improved by the introduction of two-photon excitation microscopy and is currently exploited for detecting the tortuosity of large and small fluorescently labelled molecules in vivo [32,33]. Most recently the development of microfibre optic techniques has enabled fluorescent measurement of the volume fraction [34]. DW-MRI, which measures ADCW, is a suitable method for animal and patient studies as it is non-invasive and is already commonly used for clinical MRI. As there is free movement of water across the cell membrane, DW-MRI measures both intra- and extracellular values of ADCW. Moreover, ADCW reflects both ESC α and λ changes [35]. Thus, the comprehensive studies correlating the results of DW-MRI and RTI methods are useful for understanding the mechanism underlying ADCW changes.
4. Glia and extracellular space diffusion parameters
ECS diffusion parameters vary substantially during physiological and pathological states [4,9]. Based on the intensity and duration of the stimuli, the ionic changes are accompanied by cell swelling, resulting in compensatory ECS shrinkage and a concomitant increase of tortuosity, related to a higher concentration of already existing diffusion barriers in the narrower ECS. In various physiological and pathological states, coupled with long-lasting structural changes of the nervous tissue, α and λ often behave independently, e.g. the amount of diffusion barriers can increase or decrease regardless of changes in extracellular volume.
(a). Transient changes
Decreases in ECS volume compensate for neuronal and astrocyte swelling that occurs during both physiological and pathological states. Physiological stimuli, such as extensive neuronal activity, can result in cell swelling that outlasts the activity for many minutes and consequently influences the function of the neural circuit [36]. A recent paper by Xie and co-workers [37] has shown that noradrenergic activity-evoked cell swelling during wakefulness results in the reduction of extracellular/interstitial space by as much as 60%, in comparison with natural sleep or anaesthesia. A larger ECS during sleep increases the convective exchange of cerebrospinal fluid with interstitial fluid, which in turn increases the rate of β-amyloid clearance [37]. The authors hypothesized that a lack of deep and quality sleep might therefore contribute to neurodegenaration processes due to the accumulation of toxic metabolic products during wakefulness and their insufficient clearance.
Swelling of astrocytes is considered to be a major cause of cytotoxic oedema in various pathological conditions [38]. Moreover, astrocyte swelling may be an important event predisposing the brain to further damage, owing to the impairment of protective homeostatic mechanisms and pathological accumulation of cytotoxic substances such as glutamate. The detailed mechanisms of astrocytic swelling and their volume regulation are under intensive investigation. The astrocytic membrane is rich in various ion channels as well as transport proteins involved in the maintenance of ionic and volume homeostasis, and neurotrasmitter uptake. Intracellular increases in osmolarity, due to transmembrane ion movement, are followed by water influx through either various ion co-transporters or specialized aquaporin channels [39,40]. Cell swelling, accompanied by an α decrease and a λ increase, was evoked by hypotonic stress or increased potassium [41–43], spreading depression [44–46] or ischaemia/anoxia [42,47,48]. RTI measurements have shown that the extent of the evoked decrease in α and increase in λ depends on the stimulus duration and intensity. From the control value of 0.20–0.25, α decreases to 0.13 during repetitive electrical stimulation, prolonged neuronal excitation evoked by chronic pain, mild hypotonic stress or 10 mM K+ application, with no or only small increases in λ (from 1.50 to 1.60). More profound changes have been found in spreading depression or terminal ischaemia/anoxia, where α decreases to 0.07–0.05 and λ increases above 2.00 (for review, see [4]).
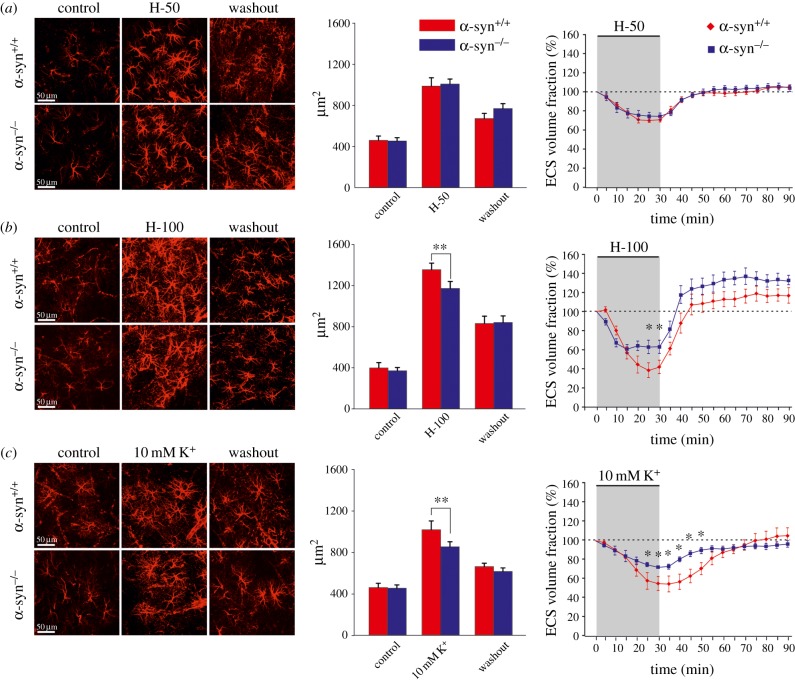
Further research investigating the mechanisms underlying the development of brain oedema was initiated by the identification of aquaporin channels, and their role in water transport [49,50]. Complete removal of aquaporin 4 channels (AQP4) or deficiency in the AQP4 anchoring protein α-syntrophin (α-syn) reduces the extent of brain oedema [49,51]. Recent data indicate an important role of a complex AQP4/TRPV4 (transient receptor potential cation channel, subfamily V, member 4) in osmolarity sensing and in the process of regulatory volume decrease [52]. Interestingly, at resting state, ECS volume in AQP4 or α-syn knockout mice (AQP4−/− or α-syn−/−, respectively) is larger than that found in control animals [42,53]. The higher initial value of α was proposed to be a protective mechanism against the fast increase in concentration of neurotoxic substances [54], and thus may contribute to a better outcome in AQP4−/− and α-syn−/− animals in pathological states. Using in vitro and in vivo experimental models of cell swelling, Dmytrenko et al. [42] showed that AQP4-mediated water influx plays a role predominantly during pathological states (global ischaemia and strong hypotonic stress) or in states associated with increased potassium concentration, but not during milder stimuli modelling physiological neuronal activity (figure 2).
Figure 2.
Changes in astrocyte morphology and extracellular volume evoked by experimental cell swelling. Changes in astrocyte morphology, based on changes in glial fibrillary acidic protein (GFAP) immunoreactivity (left and middle parts of each panel) and the relative changes of the extracellular volume fraction (right part) were determined in the cortex (layers III–IV) of α-syn+/+ and α-syn−/− mice: prior to (control), after a 30 min application of milder (H-50 (a)) and severe (H-100 (b)) hypotonic stress or 10 mM K+ (c) and following a 60 min washout. The bar graphs in the middle indicate cell volume changes expressed as changes in the area corresponding to GFAP immunoreactivity. As there was a significant difference in the control values of the ECS volume between α-syn+/+ and α-syn−/− mice, the control values of all experiments were set to 100% (dotted line) in order to determine the relative changes in ECS volume fraction. The values are presented as mean ± s.e.m. Asterisks (*p < 0.05; **p < 0.01; ***p < 0.001) indicate significant differences between the values in α-syn+/+ and α-syn−/− animals. Adapted from [42]. (Online version in colour.)
(b). Long-term plastic/persisting changes in extracellular space diffusion parameters
Structural rebuilding of tissue and changes in cell morphology are common features of long-lasting physiological as well as pathological conditions. In these states, formation or reduction of diffusion barriers result in alterations of λ, which are not necessarily accompanied by α changes. During the first three postnatal weeks, i.e. in the period of intensive gliogenesis, the value of α in rat grey matter decreases from 0.40 to 0.20 [55,56]. Similarly, during postnatal myelination of rat white matter (corpus callosum), a decrease in α and the establishment of anisotropy corresponds with the period of myelination [22]. In the dentate gyrus of the rat hippocampus, ageing results in a decline in α value, while the averaged λ value slightly decreases [29]. Moreover, anisotropy disappears, which is presumably due to the loss of the typical parallel organization of astrocytic processes. Decrease in α and a loss of typical anisotropy also accompanies plastic rebuilding in the hypothalamic SON during lactation or dehydration [24]. Insufficient clearance of glutamate and facilitated diffusion leads to enhanced crosstalk between glutamate- and GABA-ergic synapses, with a positive feedback mechanism resulting in increased hormone release.
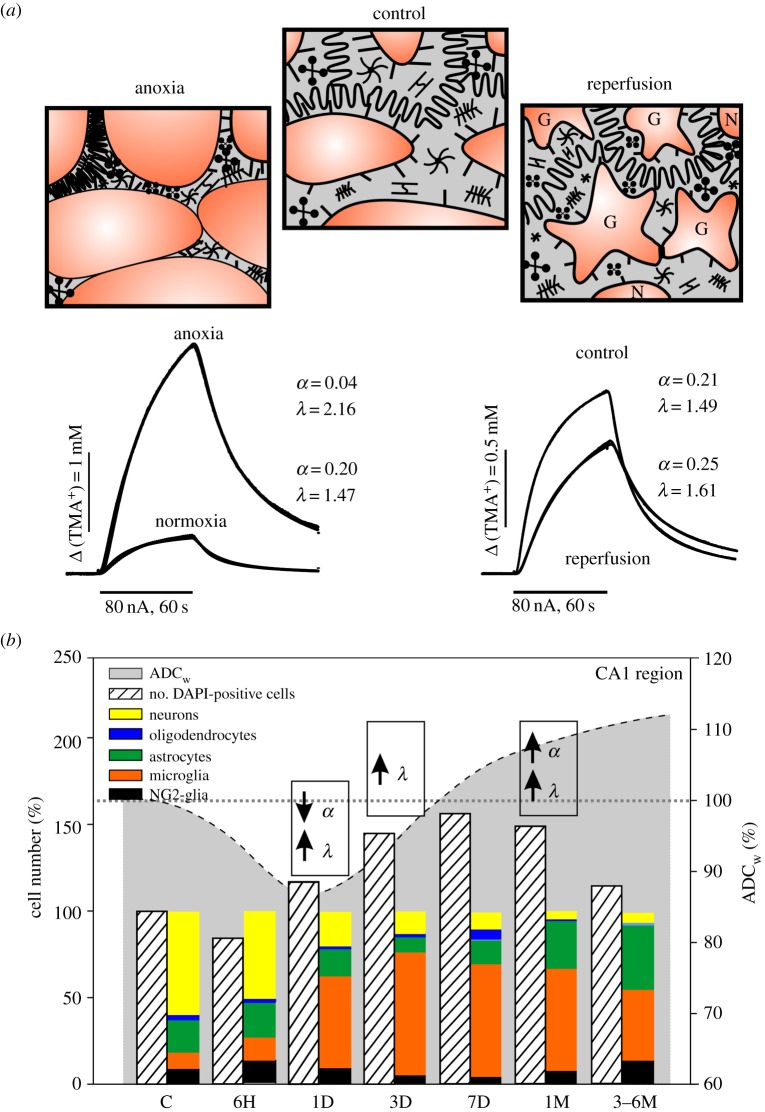
Similarly as in the above-mentioned physiological conditions, α and λ behave independently during chronic pathologies. Post-ischaemic or post-traumatic states are usually associated with enlarged α due to cell death and with increased λ due to the formation of additional diffusion barriers by thicker and hypertrophied astrocytic processes [25,28,47,57]. Besides functional and morphological changes of active astrocytes, we can also detect qualitative and quantitative changes in astrocytic production of ECM molecules, which also contribute to ECS diffusion parameter changes. Anderova et al. [28] correlated the results obtained by DW-MRI and RTI methods in early and late phases of reperfusion after transient hypoxia/ischaemia (H/I), with alterations in cell number/cell type and glial morphology in the rat hippocampus. In the first and third day after H/I induction, neuronal cell death, glial proliferation and developing astrogliosis were accompanied by an ADCW decrease and λ increase, while α was decreased or unchanged. In the late phases of reperfusion (one to six months after H/I), when the CA1 region consisted mainly of microglia, astrocytes and NG2-glia with markedly altered morphology, all three parameters, ADCW, α and λ, were increased (figure 3).
Figure 3.
Effect of acute and chronic phase of ischaemia/anoxia on brain diffusion and cellular composition. (a) Scheme of tissue structure with extracellular diffusion barriers under resting conditions and during the acute and chronic phase of ischaemia/anoxia with corresponding TMA+ diffusion curves and calculated values of extracellular volume fraction α and tortuosity λ. (b) Correlation of the time-dependent changes in the ADC of water (ADCW)—dashed line, α and λ changes and alterations in the cellular composition of the hippocampal CA1 region after induction of transient global hypoxia/ischaemia. Values of ADCW and number of cells in different cell types in the early and chronic phase of reperfusion are expressed as a percentage of the values obtained in controls. Note that acute cell swelling in the early phase evokes an α decrease and λ increase due to the accumulation of obstacles in smaller ECS space. In the chronic phase, creation of new diffusion barriers due to astrogliosis increases λ even in larger ECS volume resulting from cell death. C, control; 6H, 6 h; 1D, 1 day; 3D, 3 days; 7D, 7 days; 1M, 1 month; 6M, 6 months; α, extracellular volume fraction; λ, tortuosity; ADCW, apparent diffusion coefficient of water. Adapted from [28]. (Online version in colour.)
5. Role of the extracellular matrix
The ECS contains three major types of ECM macromolecules that can form large complexes: (i) glycosaminoglycans represented by hyaluronic acid, (ii) chondroitin sulfate proteoglycans (CSPG; especially their lectican and link protein families) and heparan sulfate proteoglycans (HSPG); and (iii) oligomeric glycoproteins like tenascins. The ECM constitutes the true backbone of nervous tissue and acts as a supportive element for neurons and glia. However, the function of ECM molecules is far more complex: the ECM plays an important role in migration, proliferation and differentiation of stem cells, oriented axonal growth, synapse formation and synaptic plasticity, and responds to changes caused by trauma, ageing, epileptogenesis and tumorigenesis, etc. (for review, see [58]). Recent data indicate that the ECM contributes to clustering of signal molecules in functional microdomains [59] and represents an important element affecting signal transmission [12].
The consolidation of the ECM into PNNs and perisynaptic ACs that enwrap neurons and synapses coincides with the termination of juvenile plasticity and the critical period of CNS maturation [60,61]. Enzymatic degradation of ECM complexes or deficiency of certain ECM molecules restores plasticity of the nervous tissue [61–64]. The finding that ECM digestion increases performance in reversal learning without affecting already learned capacities brings forth the interesting challenge of guiding plastic changes using controlled ECM modulation [63]. On the other hand, mature PNNs and perisynaptic ACs create a protective shield attenuating the neurodegeneration evoked by neurotoxicity, oxidative stress or Aβ deposits [14,15,65]. Aggrecan, cartilage link protein Crtl1 and tenascin-R have been identified as the main protective components of PNNs against iron-induced oxidative stress or Alzheimer's disease (AD) pathology [14,65]. The recent study of Lendvai et al. [15] showed preserved ACs and increased brevican and Crtl1 expression in AD patients and transgenic APdE9 mice; this suggests an increased turnover of ECM molecules to maintain synaptic integrity.
Quantitative and qualitative changes of the ECM during development, ageing, wound healing and various pathological states have a profound impact also on extrasynaptic transmission, as they may influence both extracellular volume and tortuosity. The overproduction of ECM during pathological states creates additional diffusion barriers and contributes, together with astrogliosis, to an increase in tortuosity during AD, in injured, tumorous or dysplastic tissue [57,66–68]. On the other hand, accumulation of ECM macromolecules found in these pathological states results in increased values of α. The measurements in dissected samples of human gliomas with different WHO grading showed that the proliferative activity and malignancy grade of tumours were directly proportional to the increasing values of α and λ; the increase in α and λ in high-grade tumours strongly correlated with an increased presence of the ECM molecules, particularly of tenascin and vitronectin [68]. Although all lecticans (aggrecan, neurocan, versican and brevican) have an inhibitory influence on cell motion and axonal growth [69], versican and brevican, which are strongly upregulated in malignant gliomas, show a significant pro-motogenic effect after the cleavage of tumour matrix-metalloproteases and interaction with fibronectin [70,71].
In contrast, a decrease in the amount of ECM content during ageing or a deficit of certain ECM molecules is associated with a significantly smaller α value [29,72]. Our study revealed that the degree of learning impairment during ageing closely correlates with a decrease in ECS volume and changes in the ECM, particularly lower amounts of CSPG and fibronectin [29]. Smaller ECS volume during ageing not only impairs extrasynaptic transmission in the cortex and hippocampus but could also be responsible for the greater susceptibility of the aged brain to pathological events, poorer outcome in clinical therapy and limited regeneration. Interestingly, the ECS volume as well as ADCw is significantly reduced in animals deficient in tenascin-R [72], while behavioural testing has revealed faster reversal learning but not general hippocampal-dependent learning and memory [64].
In other studies, disintegration of the ECM, due to enzymatic treatment or link protein Bral1 deficiency, resulted in significantly facilitated diffusion [73,74]. Using a real-time pressure injection of Ca2+ and Ca2+-selective microelectrodes, Hrabetova et al. [74] showed that normal Ca2+ diffusion in the brain ECS is slowed down in comparison to that of TMA+ and can be enhanced by enzymatic cleavage of CSPG. However, enzymatic treatment did not affect the volume fraction and tortuosity measured by the monovalent cation TMA+. The study on Bral1 deficient mice revealed that the hyaluronan-associated ECM in white matter no longer shows a typical nodal pattern, diffusion along as well as across myelinated fibres is facilitated and CNS nerve conduction is significantly decreased [73]. Both studies show that the ECM serves as a ‘trap’ for Ca2+ ions around synapses or Na+ ions in the nodes of Ranvier. Reduction of diffusion barriers may thus influence axonal signal propagation [73], local diffusion and concentration of Ca2+ ions with all physiological consequences [74].
With the development of new therapeutic strategies using macromolecules or viral vectors as carriers for drug delivery comes the important task of determining the diffusion properties of nervous tissue and their possible interactions with large molecules of the ECM. The measurement of an effective diffusion coefficient (D*) by the IOI method has revealed that D* for lactoferrin was reduced by approximately 60% compared with mathematical predictions; this reduction was reversed by heparin treatment [75]. Moreover, the correlation of diffusion properties of lactoferrin and structurally similar protein transferrin that does not bind to HSPG enabled the quantification of HSPG binding site density and predicted a low micromolar concentration of these binding sites in the neocortex [75].
6. Conclusion
Extrasynaptic transmission, based on the diffusion of neuroactive substances across the ECS, plays an important role in both physiological and pathological states. It represents not only an alternative way of intercellular communication but also a mechanism that can modify the efficacy of synaptic transmission itself. In order to maintain ionic and volume homeostasis and/or tissue integrity, glial cells promptly react to various physiological or pathological stimuli by cell swelling (transient changes), followed by proliferation and hypertrophy (chronic states). Astrocytic changes together with quantitative and qualitative alterations in the ECM directly influence ECS volume fraction α and tortuosity λ, which in turn affect the diffusion properties of the ECS. In acute states, such as neuronal activity or ischaemia, a cell-swelling-evoked decrease in α is accompanied by an increase in tortuosity λ, due to the crowding of existing diffusion barriers in a smaller space. In the long-term physiological states or chronic pathologies, α and λ often behave independently. Thus, facilitated diffusion may occur in smaller extracellular volume (lactation) or, on the other hand, diffusion may be profoundly hindered even in large ECS (post-ischaemic or post-traumatic tissue). Besides influencing neuronal activity and neuron–glia communication, ECS diffusion parameter changes affect accumulation of neuroactive substances (e.g. glutamate), migration of stem or tumour cells, tissue permeability for drugs and regeneration processes, and represent a valuable source of information for diagnostic purposes.
Funding statement
This study was supported by grant nos. 13-11867S and P304-12-G069 from the Grant Agency of the Czech Republic.
References
- 1.Vizi ES. 1984. Non-synaptic interaction between neurones: modulation of neurochemical transmission. Chichester, UK: Wiley and Sons. [Google Scholar]
- 2.Zoli M, Jansson A, Syková E, Agnati LF, Fuxe K. 1999. Intercellular communication in the central nervous system. The emergence of the volume transmission concept and its relevance for neuropsychopharmacology. Trends Pharmacol. Sci. 20, 142–150. ( 10.1016/S0165-6147(99)01343-7) [DOI] [PubMed] [Google Scholar]
- 3.Fuxe K, Dahlstrom AB, Jonsson G, Marcellino D, Guescini M, Dam M, Manger P, Agnati L. 2010. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 90, 82–100. ( 10.1016/j.pneurobio.2009.10.012) [DOI] [PubMed] [Google Scholar]
- 4.Sykova E, Nicholson C. 2008. Diffusion in brain extracellular space. Physiol. Rev. 88, 1277–1340. ( 10.1152/physrev.00027.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson C, Sykova E. 1998. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207–215. ( 10.1016/S0166-2236(98)01261-2) [DOI] [PubMed] [Google Scholar]
- 6.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. 2008. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322, 1551–1555. ( 10.1126/science.1164022) [DOI] [PubMed] [Google Scholar]
- 7.Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. 2012. Glial regulation of neuronal function: from synapse to systems physiology. J. Neuroendocrinol. 24, 566–576. ( 10.1111/j.1365-2826.2011.02259.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannasch U, Vargova L, Reingruber J, Ezan P, Holcman D, Giaume C, Sykova E, Rouach N. 2011. Astroglial networks scale synaptic activity and plasticity. Proc. Natl Acad. Sci. USA 108, 8467–8472. ( 10.1073/pnas.1016650108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sykova E. 2004. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience 129, 861–876. ( 10.1016/j.neuroscience.2004.06.077) [DOI] [PubMed] [Google Scholar]
- 10.Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. ( 10.1016/S0166-2236(98)01349-6) [DOI] [PubMed] [Google Scholar]
- 11.Dityatev A, Frischknecht R, Seidenbecher CI. 2006. Extracellular matrix and synaptic functions. Results Probl. Cell Differ. 43, 69–97. ( 10.1007/400_025) [DOI] [PubMed] [Google Scholar]
- 12.Dityatev A, Rusakov DA. 2011. Molecular signals of plasticity at the tetrapartite synapse. Curr. Opin. Neurobiol. 21, 353–359. ( 10.1016/j.conb.2010.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruckner G, et al. 1993. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8, 183–200. ( 10.1002/glia.440080306) [DOI] [PubMed] [Google Scholar]
- 14.Morawski M, Bruckner G, Jager C, Seeger G, Matthews RT, Arendt T. 2012. Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer's disease neuropathology. Brain Pathol. 22, 547–561. ( 10.1111/j.1750-3639.2011.00557.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lendvai D, et al. 2013. Neurochemical mapping of the human hippocampus reveals perisynaptic matrix around functional synapses in Alzheimer's disease. Acta Neuropathol. 125, 215–229. ( 10.1007/s00401-012-1042-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asztely F, Erdemli G, Kullmann DM. 1997. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron 18, 281–293. ( 10.1016/S0896-6273(00)80268-8) [DOI] [PubMed] [Google Scholar]
- 17.Oliet SH, Piet R, Poulain DA. 2001. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292, 923–926. ( 10.1126/science.1059162) [DOI] [PubMed] [Google Scholar]
- 18.Rusakov DA, Savtchenko LP, Zheng K, Henley JM. 2011. Shaping the synaptic signal: molecular mobility inside and outside the cleft. Trends Neurosci. 34, 359–369. ( 10.1016/j.tins.2011.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiss JP, Zsilla G, Vizi ES. 2004. Inhibitory effect of nitric oxide on dopamine transporters: interneuronal communication without receptors. Neurochem. Int. 45, 485–489. ( 10.1016/j.neuint.2003.11.004) [DOI] [PubMed] [Google Scholar]
- 20.Nicholson C, Phillips JM. 1981. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J. Physiol. 321, 225–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolak DJ, Thorne RG. 2013. Diffusion of macromolecules in the brain: implications for drug delivery. Mol. Pharm. 10, 1492–1504. ( 10.1021/mp300495e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vorisek I, Sykova E. 1997. Evolution of anisotropic diffusion in the developing rat corpus callosum. J. Neurophysiol. 78, 912–919. [DOI] [PubMed] [Google Scholar]
- 23.Rice ME, Okada YC, Nicholson C. 1993. Anisotropic and heterogeneous diffusion in the turtle cerebellum: implications for volume transmission. J. Neurophysiol. 70, 2035–2044. [DOI] [PubMed] [Google Scholar]
- 24.Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. 2004. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc. Natl Acad. Sci. USA 101, 2151–2155. ( 10.1073/pnas.0308408100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vorisek I, Hajek M, Tintera J, Nicolay K, Sykova E. 2002. Water ADC, extracellular space volume, and tortuosity in the rat cortex after traumatic injury. Magn. Reson. Med. 48, 994–1003. ( 10.1002/mrm.10305) [DOI] [PubMed] [Google Scholar]
- 26.Mazel T, Simonova Z, Sykova E. 1998. Diffusion heterogeneity and anisotropy in rat hippocampus. Neuroreport 9, 1299–1304. ( 10.1097/00001756-199805110-00008) [DOI] [PubMed] [Google Scholar]
- 27.Hrabetova S. 2005. Extracellular diffusion is fast and isotropic in the stratum radiatum of hippocampal CA1 region in rat brain slices. Hippocampus 15, 441–450. ( 10.1002/hipo.20068) [DOI] [PubMed] [Google Scholar]
- 28.Anderova M, Vorisek I, Pivonkova H, Benesova J, Vargova L, Cicanic M, Chvatal A, Sykova E. 2011. Cell death/proliferation and alterations in glial morphology contribute to changes in diffusivity in the rat hippocampus after hypoxia-ischemia. J. Cereb. Blood Flow Metab. 31, 894–907. ( 10.1038/jcbfm.2010.168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sykova E, Mazel T, Hasenohrl RU, Harvey AR, Simonova Z, Mulders WH, Huston JP. 2002. Learning deficits in aged rats related to decrease in extracellular volume and loss of diffusion anisotropy in hippocampus. Hippocampus 12, 269–279. ( 10.1002/hipo.1101) [DOI] [PubMed] [Google Scholar]
- 30.Nicholson C, Tao L. 1993. Hindered diffusion of high molecular weight compounds in brain extracellular microenvironment measured with integrative optical imaging. Biophys. J. 65, 2277–2290. ( 10.1016/S0006-3495(93)81324-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao F, Nicholson C, Hrabe J, Hrabetova S. 2008. Diffusion of flexible random-coil dextran polymers measured in anisotropic brain extracellular space by integrative optical imaging. Biophys. J. 95, 1382–1392. ( 10.1529/biophysj.107.124743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savtchenko LP, Rusakov DA. 2005. Extracellular diffusivity determines contribution of high-versus low-affinity receptors to neural signaling. Neuroimage 25, 101–111. ( 10.1016/j.neuroimage.2004.11.020) [DOI] [PubMed] [Google Scholar]
- 33.Binder DK, Papadopoulos MC, Haggie PM, Verkman AS. 2004. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J. Neurosci. 24, 8049–8056. ( 10.1523/JNEUROSCI.2294-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Verkman AS. 2010. Microfiberoptic measurement of extracellular space volume in brain and tumor slices based on fluorescent dye partitioning. Biophys. J. 99, 1284–1291. ( 10.1016/j.bpj.2010.06.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vorisek I, Sykova E. 2009. Measuring diffusion parameters in the brain: comparing the real-time iontophoretic method and diffusion-weighted magnetic resonance. Acta Physiol. 195, 101–110. ( 10.1111/j.1748-1716.2008.01924.x) [DOI] [PubMed] [Google Scholar]
- 36.Prokopova-Kubinova S, Sykova E. 2000. Extracellular diffusion parameters in spinal cord and filum terminale of the frog. J. Neurosci. Res. 62, 530–538. () [DOI] [PubMed] [Google Scholar]
- 37.Xie L., et al. 2013. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. ( 10.1126/science.1241224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimelberg HK. 2005. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia 50, 389–397. ( 10.1002/glia.20174) [DOI] [PubMed] [Google Scholar]
- 39.Kimelberg HK. 2004. Water homeostasis in the brain: basic concepts. Neuroscience 129, 851–860. ( 10.1016/j.neuroscience.2004.07.033) [DOI] [PubMed] [Google Scholar]
- 40.MacAulay N, Hamann S, Zeuthen T. 2004. Water transport in the brain: role of cotransporters. Neuroscience 129, 1031–1044. ( 10.1016/j.neuroscience.2004.06.045) [DOI] [PubMed] [Google Scholar]
- 41.Sykova E, Vargova L, Prokopova S, Simonova Z. 1999. Glial swelling and astrogliosis produce diffusion barriers in the rat spinal cord. Glia 25, 56–70. () [DOI] [PubMed] [Google Scholar]
- 42.Dmytrenko L, Cicanic M, Anderova M, Vorisek I, Ottersen OP, Sykova E, Vargova L. 2013. The impact of alpha-syntrophin deletion on the changes in tissue structure and extracellular diffusion associated with cell swelling under physiological and pathological conditions. PLoS ONE 8, e68044 ( 10.1371/journal.pone.0068044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solenov EI, Vetrivel L, Oshio K, Manley GT, Verkman AS. 2002. Optical measurement of swelling and water transport in spinal cord slices from aquaporin null mice. J. Neurosci. Methods 113, 85–90. ( 10.1016/S0165-0270(01)00481-2) [DOI] [PubMed] [Google Scholar]
- 44.Tao L, Masri D, Hrabetova S, Nicholson C. 2002. Light scattering in rat neocortical slices differs during spreading depression and ischemia. Brain Res. 952, 290–300. ( 10.1016/S0006-8993(02)03254-7) [DOI] [PubMed] [Google Scholar]
- 45.Mazel T, Richter F, Vargova L, Sykova E. 2002. Changes in extracellular space volume and geometry induced by cortical spreading depression in immature and adult rats. Physiol. Res. 51(Suppl. 1), S85–S93. [PubMed] [Google Scholar]
- 46.Zhou N, Gordon GR, Feighan D, MacVicar BA. 2010. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb. Cortex 20, 2614–2624. ( 10.1093/cercor/bhq018) [DOI] [PubMed] [Google Scholar]
- 47.Vorisek I, Sykova E. 1997. Ischemia-induced changes in the extracellular space diffusion parameters, K+, and pH in the developing rat cortex and corpus callosum. J. Cereb. Blood Flow Metab. 17, 191–203. ( 10.1097/00004647-199702000-00009) [DOI] [PubMed] [Google Scholar]
- 48.Hrabetova S, Hrabe J, Nicholson C. 2003. Dead-space microdomains hinder extracellular diffusion in rat neocortex during ischemia. J. Neurosci. 23, 8351–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amiry-Moghaddam M, Ottersen OP. 2003. The molecular basis of water transport in the brain. Nat. Rev. Neurosci. 4, 991–1001. ( 10.1038/nrn1252) [DOI] [PubMed] [Google Scholar]
- 50.Verkman AS. 2013. Aquaporins. Curr. Biol. 23, R52–R55. ( 10.1016/j.cub.2012.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amiry-Moghaddam M, et al. 2003. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc. Natl Acad. Sci. USA 100, 13 615–13 620. ( 10.1073/pnas.2336064100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, Amiry-Moghaddam M. 2011. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl Acad. Sci. USA 108, 2563–2568. ( 10.1073/pnas.1012867108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao X, Hrabetova S, Nicholson C, Manley GT. 2008. Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. J. Neurosci. 28, 5460–5464. ( 10.1523/JNEUROSCI.0257-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kilb W, Dierkes PW, Sykova E, Vargova L, Luhmann HJ. 2006. Hypoosmolar conditions reduce extracellular volume fraction and enhance epileptiform activity in the CA3 region of the immature rat hippocampus. J. Neurosci. Res. 84, 119–129. ( 10.1002/jnr.20871) [DOI] [PubMed] [Google Scholar]
- 55.Lehmenkuhler A, Sykova E, Svoboda J, Zilles K, Nicholson C. 1993. Extracellular space parameters in the rat neocortex and subcortical white matter during postnatal development determined by diffusion analysis. Neuroscience 55, 339–351. ( 10.1016/0306-4522(93)90503-8) [DOI] [PubMed] [Google Scholar]
- 56.Prokopova S, Vargova L, Sykova E. 1997. Heterogeneous and anisotropic diffusion in the developing rat spinal cord. Neuroreport 8, 3527–3532. ( 10.1097/00001756-199711100-00022) [DOI] [PubMed] [Google Scholar]
- 57.Roitbak T, Sykova E. 1999. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia 28, 40–48. () [DOI] [PubMed] [Google Scholar]
- 58.Soleman S, Filippov MA, Dityatev A, Fawcett JW. 2013. Targeting the neural extracellular matrix in neurological disorders. Neuroscience 253, 194–213. ( 10.1016/j.neuroscience.2013.08.050) [DOI] [PubMed] [Google Scholar]
- 59.Dityatev A, Seidenbecher CI, Schachner M. 2010. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 33, 503–512. ( 10.1016/j.tins.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 60.Galtrey CM, Fawcett JW. 2007. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 54, 1–18. ( 10.1016/j.brainresrev.2006.09.006) [DOI] [PubMed] [Google Scholar]
- 61.Carulli D, et al. 2010. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133, 2331–2347. ( 10.1093/brain/awq145) [DOI] [PubMed] [Google Scholar]
- 62.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. 2002. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251. ( 10.1126/science.1072699) [DOI] [PubMed] [Google Scholar]
- 63.Happel MF, Niekisch H, Castiblanco Rivera LL, Ohl FW, Deliano M, Frischknecht R. 2014. Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc. Natl Acad. Sci. USA 111, 2800–2805. ( 10.1073/pnas.1310272111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morellini F, Sivukhina E, Stoenica L, Oulianova E, Bukalo O, Jakovcevski I, Dityatev A, Irintchev A, Schachner M. 2010. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb. Cortex 20, 2712–2727. ( 10.1093/cercor/bhq017) [DOI] [PubMed] [Google Scholar]
- 65.Suttkus A, Rohn S, Weigel S, Glockner P, Arendt T, Morawski M. 2014. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis. 5, e1119 ( 10.1038/cddis.2014.25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sykova E, Vorisek I, Antonova T, Mazel T, Meyer-Luehmann M, Jucker M, Hajek M, Ort M, Bures J. 2005. Changes in extracellular space size and geometry in APP23 transgenic mice: a model of Alzheimer's disease. Proc. Natl Acad. Sci. USA 102, 479–484. ( 10.1073/pnas.0408235102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zamecnik J, Homola A, Cicanic M, Kuncova K, Marusic P, Krsek P, Sykova E, Vargova L. 2012. The extracellular matrix and diffusion barriers in focal cortical dysplasias. Eur. J. Neurosci. 36, 2017–2024. ( 10.1111/j.1460-9568.2012.08107.x) [DOI] [PubMed] [Google Scholar]
- 68.Zamecnik J, Vargova L, Homola A, Kodet R, Sykova E. 2004. Extracellular matrix glycoproteins and diffusion barriers in human astrocytic tumours. Neuropathol. Appl. Neurobiol. 30, 338–350. ( 10.1046/j.0305-1846.2003.00541.x) [DOI] [PubMed] [Google Scholar]
- 69.Morgenstern DA, Asher RA, Fawcett JW. 2002. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 137, 313–332. ( 10.1016/S0079-6123(02)37024-9) [DOI] [PubMed] [Google Scholar]
- 70.Hu B, Kong LL, Matthews RT, Viapiano MS. 2008. The proteoglycan brevican binds to fibronectin after proteolytic cleavage and promotes glioma cell motility. J. Biol. Chem. 283, 24 848–24 859. ( 10.1074/jbc.M801433200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng PS, Wen J, Ang LC, Sheng W, Viloria-Petit A, Wang Y, Wu Y, Kerbel RS, Yang BB. 2004. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 18, 754–756. [DOI] [PubMed] [Google Scholar]
- 72.Sykova E, Vorisek I, Mazel T, Antonova T, Schachner M. 2005. Reduced extracellular space in the brain of tenascin-R- and HNK-1-sulphotransferase deficient mice. Eur. J. Neurosci. 22, 1873–1880. ( 10.1111/j.1460-9568.2005.04375.x) [DOI] [PubMed] [Google Scholar]
- 73.Bekku Y, et al. 2010. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J. Neurosci. 30, 3113–3123. ( 10.1523/JNEUROSCI.5598-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hrabetova S, Masri D, Tao L, Xiao F, Nicholson C. 2009. Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J. Physiol. 587, 4029–4049. ( 10.1113/jphysiol.2009.170092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thorne RG, Lakkaraju A, Rodriguez-Boulan E, Nicholson C. 2008. In vivo diffusion of lactoferrin in brain extracellular space is regulated by interactions with heparan sulfate. Proc. Natl Acad. Sci. USA 105, 8416–8421. ( 10.1073/pnas.0711345105) [DOI] [PMC free article] [PubMed] [Google Scholar]