Abstract
GABAergic interneurons represent a minority of all cortical neurons and yet they efficiently control neural network activities in all brain areas. In parallel, glial cell astrocytes exert a broad control of brain tissue homeostasis and metabolism, modulate synaptic transmission and contribute to brain information processing in a dynamic interaction with neurons that is finely regulated in time and space. As most studies have focused on glutamatergic neurons and excitatory transmission, our knowledge of functional interactions between GABAergic interneurons and astrocytes is largely defective. Here, we critically discuss the currently available literature that hints at a potential relevance of this specific signalling in brain function. Astrocytes can respond to GABA through different mechanisms that include GABA receptors and transporters. GABA-activated astrocytes can, in turn, modulate local neuronal activity by releasing gliotransmitters including glutamate and ATP. In addition, astrocyte activation by different signals can modulate GABAergic neurotransmission. Full clarification of the reciprocal signalling between different GABAergic interneurons and astrocytes will improve our understanding of brain network complexity and has the potential to unveil novel therapeutic strategies for brain disorders.
Keywords: astrocytes, GABA, interneurons
1. Background
Our knowledge of how the brain computes incoming sensory signals and governs our cognitive and motor functions has faced an exponential increase in the last decades. This was made possible thanks to a number of technological advances in molecular biology, brain imaging and optogenetics. It is now clear that the complexity of brain activity relies on fast dynamic interactions between different cell types that are finely and constantly regulated in time and space. The greatest challenge for neuroscientists is represented by the speed, the number and the heterogeneity of cellular signals that relentlessly occur in our brain. Over the last years our understanding of the functional role of two heterogeneous cell populations, i.e. GABAergic interneurons and astrocytic glial cells, has been marked by a significant improvement. Whether and how these cell types interact with each other and what functional significance such a signalling may have in the brain network remain, however, largely undefined.
Astrocytes are the major class of glial cells in the brain and play essential roles in brain homeostasis and metabolism. They are coupled in a syncytium through gap junctions and exert a homeostatic control of the extracellular space by regulating the pH, the water content and the extracellular concentration of different neurotransmitters and ions. In addition, astrocytes supply neurons with nutrients, trophic factors, cytokines and neuromodulators [1,2], contributing to the neurovascular coupling mechanism [3,4] and to the defensive reaction to tissue insult. Only recently the role of astrocytes has been extended to functions that were once considered an exclusive domain of neurons, such as short- and long-term modulation of synaptic transmission. This has led to the concept of the tripartite synapse in which the astrocytic fine processes, together with the pre- and postsynaptic neuronal elements, actively participate in the regulation of information transfer and integrative process [5,6]. Indeed, astrocytes express a wide variety of receptors for neurotransmitters, neuromodulators, cytokines and trophic factors that are used by these cells to sense the synaptic release of neurotransmitters as well as other extracellular signals [7]. In response to these signals, astrocytes can release molecules that regulate fundamental events in brain function by acting on neurons, glia and vascular cells. Molecules such as glutamate, ATP, d-serine, cytokines and GABA have been collectively termed gliotransmitters as they are similar to neurotransmitters and target similar receptors activated by neurotransmitters. Gliotransmitters have been shown to exert distinct modulatory actions on synaptic activity and network excitability through different mechanisms [7,8]. Evidence for a Ca2+-dependent exocytotic release mechanism has been provided for these gliotransmitters, but Ca2+-independent mechanisms have also been identified and characterized [9–11], including efflux through plasma membrane ion channels [12]. While we cannot draw a definitive conclusion, it seems conceivable that gliotransmitters are released from astrocytes through Ca2+-dependent and Ca2+-independent not mutually exclusive mechanisms.
According to these studies, the brain can be conceived as a network of interactive neurons and astrocytes. This novel view is, however, mainly based on results from in vitro and in vivo studies that focused on glutamatergic synaptic transmission. These studies revealed that astrocytes respond to glutamate with metabotropic glutamate receptor (mGluR)-mediated Ca2+ elevations and can signal back to neurons by releasing gliotransmitters. The excitatory amino acid glutamate is probably the most widely studied gliotransmitter. Glutamate of astrocytic origin was shown to potentiate excitatory synaptic transmission through presynaptic NMDA and mGlu receptor activation [13–15] and to favour neuronal synchronies by targeting postsynaptic NMDA receptors [16]. These are only some examples of the role of astrocytes in the modulation of neuronal activity (for review, see [7]). Whether the reciprocal signalling revealed at glutamatergic synapses can be extended to GABAergic synapses is unclear. Although several works reported that astrocytes are sensitive to GABA, the literature on this topic is very limited [17].
In the last decade, our knowledge of GABAergic interneuron physiology has grown exponentially, in parallel with that on the astrocytes. GABAergic interneurons are only one quarter of all neurons and yet their control of cortical excitability is crucial [18]. Their role is not only to inhibit glutamatergic pyramidal neurons and maintain in the neural network a proper excitatory/inhibitory balance, but also to coordinate and synchronize neuronal activity generating specific oscillations [19–24] that characterize neuronal network function in different brain regions [25]. Interneurons represent a very heterogeneous class of cells (for reviews, see [26–31]). They differ in membrane and firing properties, in morphology and in biological markers expressed. This great heterogeneity allows a division of the labour that is now intensively investigated. For example, somatostatin (Sst) positive Martinotti cells preferentially target pyramidal neuron distal dendrites to control synaptic integration and plasticity [32–34]. In parallel, parvalbumin (Pv) expressing basket and chandelier cells innervate proximal dendrites, soma and the axonal initial segment in pyramidal neurons, exerting an efficient control of the firing discharge in these cells that guarantees temporal precision and synchronization at various frequencies [22,35]. Reciprocal inhibition between different interneuronal subtypes and autaptic self-inhibition are two important aspects of interneuron physiology that have been addressed only recently [36,37]. The strong glutamatergic innervation of GABAergic interneurons allows a continuous and efficient feed-forward inhibition in response to physiological inputs, including sensory stimuli [38–41]. Notably, cortical interneurons, and especially Pv interneurons, receive a strong glutamatergic input. Accordingly, glutamatergic transmission generated either locally or at distant regions is invariably accompanied by a stimulation of GABA release, making inhibition and excitation de facto inseparable [18].
Given both the emerging modulatory role of astrocytes on neuronal network activities and the fast GABAergic response to local excitatory inputs, it is of interest to provide a framework that summarizes what we know about the reciprocal interactions between GABAergic interneurons and astrocytes. Currently available literature on astrocytic response to GABAergic signals refers mainly to two different measurable effects on astrocytes of GABA applications: a membrane depolarization and an intracellular Ca2+ rise. Besides these functional effects, GABA significantly increases GFAP content and astrocytic branching [42], suggesting a GABA role on astrocytic maturation. In addition, a number of studies revealed that GABA inhibits proinflammatory cytokine release from astrocytes, suggesting an involvement of GABA signalling also in the modulation of inflammatory astrocytic response [43].
In our review, we will critically discuss currently available data that could help us to answer the following central questions: (i) Are astrocytes responsive to GABA signals? (ii) Do GABA-activated astrocytes signal back to either interneurons and/or principal neurons? (iii) Do astrocytes activated by non-GABAergic signals affect GABAergic transmission?
2. GABAergic interneuron signalling to astrocytes
(a). GABA-mediated depolarization
The first evidence that astrocytes can sense GABA comes from electrophysiological experiments on cultured or acutely isolated astrocytes [44–47] and later on hippocampal, retinal and cerebellar slices [48–51]. These studies revealed that astrocytes express functional GABAA receptors that are similar in many, though not all, aspects to those expressed by neurons. The first difference is that activation of astrocytic GABAA receptors leads to a depolarizing current in mature astrocytes, as opposed to mature neurons, due to the Na+/K+/Cl− cotransporter (NKCC1) expression and activity that maintains a larger intracellular [Cl−] in astrocytes. The roles of GABA-mediated Cl− efflux and astrocytic depolarization are still under investigation. It was proposed that during intense GABAergic interneuron firing, Cl− efflux from astrocytes helps to maintain a certain [Cl−]o level that could counteract Cl− entry into neurons [52]. Thus, GABA would act on astrocytes to ultimately buffer [Cl−]o and preserve the inhibitory driving force of Cl− ions in neurons. A recent work supports this concept and shows that gap-junction coupling is necessary to maintain astrocytic [Cl−]o buffering capacity [53]. The authors revealed that blockade of gap junctions during intense stimulation of GABAergic transmission to CA1 neurons induced a collapse of the Cl− gradient in these neurons. While we have to keep in mind that neurons are more sensitive to internal than external [Cl−] changes, the Cl− efflux from astrocytes may significantly contribute to the control of [Cl−]o at the restricted extracellular space surrounding the GABAergic synapse.
Another difference of GABAA-mediated currents in astrocytes compared to those in neurons is the effect of some allosteric modulators. In particular, the benzodiazepine site inverse agonist methyl-4-ethyl-6,7-dimethoxy-β-carboline-3-carboxylate (DMCM) acts in astrocytes, but not in neurons, as pure agonist by increasing GABAA-mediated depolarizing currents [46,54]. This result hints at a different subunit composition of the GABAA receptor complex in astrocytes with respect to neurons.
(b). GABA-mediated Ca2+ response in astrocytes
The advent of Ca2+ imaging technique revealed an unexpected responsiveness of astrocytes to several neurotransmitters and molecules [55,56]. It was thus found that GABA evokes astrocytic Ca2+ events through different intracellular pathways mediated by both ionotropic GABAA and metabotropic GABAB receptors as well as GABA transporters (GATs). Some groups observed exclusively GABAA-mediated responses that, by depolarizing the astrocytic membrane as reported above, activate voltage-sensitive Ca2+ channels [47], while other groups reported GABA-evoked Ca2+ events in astrocytes that were mediated exclusively by GABAB receptors [57,58]. Furthermore, other studies in cultured astrocytes [59] and rat hippocampal slices [60] described GABA-evoked astrocytic Ca2+ oscillations mediated by both GABAA and GABAB receptor activation. In the latter work, the authors observed a conserved GABAA-mediated response during development, while GABAB-mediated response peaked during the second postnatal week and then progressively decreased. The mechanism of GABAB-mediated Ca2+ events was shown to involve G proteins and Ca2+ release from internal stores [60,61]. It is unclear, however, which G protein is responsible for the Ca2+ response, because GABAB receptors are known to be coupled to Gi/o proteins, at least in neurons [62], while Ca2+ release from internal stores usually requires Gq protein activation. Further experiments are needed to address this important aspect of astrocytic physiology. Beside this mechanism, once GABA activates Ca2+ oscillations in astrocytes it may, in turn, activate gliotransmission. A point of interest here is that GABA-induced Ca2+ oscillations in astrocytes are comparable to those induced by glutamate or others excitatory transmitters. Given that Ca2+ elevations represent a form of Ca2+-based excitation in astrocytes, it turns out that in the neuron–astrocyte network an inhibitory GABA signal has the potential to become an excitatory signal through astrocyte activation. This leads to a number of questions: (i) Do GABA and glutamate trigger a similar Ca2+ response in astrocytes? (ii) Do individual astrocytes respond to both neurotransmitters or do astrocyte subpopulations exist that respond exclusively to either GABA or glutamate? In the case that an individual astrocyte can respond to both neurotransmitters, what is the ultimate effect of a simultaneous activity of the two signal pathways? In other words, does this astrocyte integrate the signals, as was observed in hippocampal astrocytes activated by glutamate and acetylcholine [5,63]? Interestingly, Meier et al. [60] observed that challenging astrocytes with a subthreshold stimulation using a GABAB agonist (baclofen) increased the Ca2+ response to t-ACPD, i.e. an mGluR agonist. This observation suggests that a spatial and temporal summation of different receptor-mediated Ca2+ signals can occur in astrocytes. For example, GABAergic and glutamatergic inputs can occur very closely in time, allowing a summation of the intracellular Ca2+ response that may evoke gliotransmission or the modulation of important astrocytic functions.
In slices of the olfactory bulb, a region where astrocytes enwrap mainly GABAergic synapses, GATs have been shown to indirectly activate Ca2+ events in astrocytes from P2–7 mice [64]. In particular, Doengi et al. [64] found that GABA-evoked astrocytic Ca2+ events are fully prevented by GAT blockers, but only partially by GABAB antagonists and they are not affected at all by GABAA antagonists. GAT activation leads to intracellular Na+ increase (that is cotransported with GABA), which indirectly inhibits the Na+/Ca2+ exchanger. The proposed mechanism is that the consequent Ca2+ increase is sufficient to induce Ca2+ release from internal stores in an inositol triphosphate-dependent manner. The authors provide also evidence that GABA mediates a blood vessel constriction that is blocked by GAT inhibitors, providing evidence for an important functional effect of this phenomenon.
3. GABA-activated astrocytes signal back to the neural network
What is the functional effect of GABA astrocyte activation on the neuronal network? A main consequence of cytosolic Ca2+ elevations in astrocytes is the release of gliotransmitters. The frequency, amplitude, kinetics and spatial extension of the Ca2+ change appear to be crucial factors that determine the responsiveness of astrocytes to neuronal signals. The functional consequences of GABA signalling to astrocytes are, however, undefined and it is not conclusively proved that GABA-mediated Ca2+ elevations trigger the release of gliotransmitters. Furthermore, both the nature and the ultimate modulatory action on the neuronal network of gliotransmitters released from astrocytes upon their activation by inhibitory signals are also unclear. Our current understanding of astrocyte-mediated modulation at inhibitory synapses is, thus, very defective, at least with respect to our knowledge of the gliotransmitter effects at excitatory synapses. Considering this important issue, we should not, however, under-evaluate a few studies that provided important clues for the potential of GABA-activated astrocytes to regulate network activities. For example, in hippocampal slices, Kang et al. [57] reported that stimulation of an intense interneuron firing induced Ca2+ oscillations in astrocytes and, in parallel, increased the probability of evoked unitary inhibitory postsynaptic currents (IPSCs). A similar increase was observed after a direct activation of individual astrocytes by mechanical stimuli or by stimulation with the selective GABAB receptor agonist baclofen. Notably, the effect was blocked by inserting the Ca2+ chelator BAPTA in the astrocytic syncytium. The authors suggested that GABA activation of astrocytes leads to a release of glutamate onto the presynaptic elements that increases inhibitory synaptic transmission onto pyramidal neurons. This study provided the first evidence that synaptically released GABA activates astrocytes that, in turn, modulate synaptic activity in the hippocampus. A role of GABA-activated astrocytes in modulating heterosynaptic depression in the hippocampus has been revealed by Serrano et al. [58]. These authors found that tetanization- or NMDA-induced heterosynaptic depression also evoked astrocytic Ca2+ oscillations, and that inhibition of astrocytic Ca2+ responses by BAPTA abolished the heterosynaptic depression indicating that astrocyte activation was necessary for this form of synaptic plasticity. More relevant for the issue that we discuss here, the authors reported that activation of astrocytes was dependent on GABAB receptors because GABAB receptor blockade prevented both Ca2+ responses in astrocytes and heterosynaptic depression in neurons, whereas stimulation with baclofen evoked both events. Finally, the authors found that ATP released from GABA-activated astrocytes was rapidly degraded to adenosine that inhibited glutamate release through presynaptic A1 receptor activation. The crucial role of astrocytes in mediating another form of hippocampal plasticity, i.e. the transient heterosynaptic depression, has been demonstrated by Andersson et al. [65]. In this case, the authors suggested that GABA-activated astrocytes release glutamate that induces this form of synaptic depression by acting on group II/III mGluRs.
Although these studies provide compelling evidence for a contribution of GABA-activated astrocytes in the modulation of synaptic activity, our knowledge of GABA-mediated gliotransmission remains unsatisfactory. It is likely that future studies will unveil that GABA-activated astrocytes are involved in other forms of synaptic modulations (figure 1).
Figure 1.
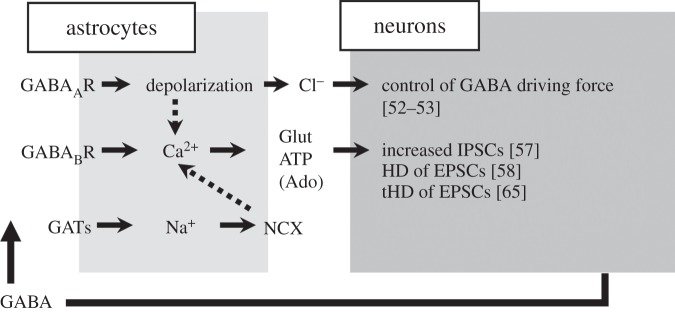
GABA-activated astrocytes modulate neuronal activity. Summary of the astrocytic response to GABA and the consequent signalling to neurons; dotted arrows refer to contradictory observations or limited brain regions (see §§1 and 2). Glut, glutamate; Ado, adenosine; HD, heterosynaptic depression; tHD, transient heterosynaptic depression; IPSCs, inhibitory postsynaptic currents; EPSCs, excitatory postsynaptic currents; GATs, GABA transporters; NCX, Na+ Ca2+ exchanger.
4. Astrocytes activated by non-GABAergic signals modulate GABAergic transmission
Astrocytes can modulate GABAergic transmission through different mechanisms. GATs expressed on astrocytic and neuronal membrane have an important functional significance in the control of the extracellular GABA concentration that sets the tone of GABAergic inhibition in local neural circuits [66–68]. In the neocortex, GAT-1 and GAT-3 are the most abundantly expressed, with GAT-1 mainly expressed in GABAergic interneurons [69,70] and less on astrocytes, while GAT-3 is located exclusively on astrocytic processes in the proximity of synapses [71]. Recent works show that astrocytic GAT-3 is important to control extracellular [GABA] also in vivo, particularly during periods of intense neuronal activity [72], and to shape GABAB postsynaptic currents in the thalamus [73]. Notably, in this brain region, GAT-1 is selectively expressed on astrocytes [74,75], as opposed to other regions, and its role is crucial in regulating GABA tonic inhibition and thalamocortical seizures that characterize absence epilepsy [76,77].
The efficacy of inhibitory synapses in the hippocampus was recently revealed to be finely regulated by the dynamics of GAT-3 expression in astrocytes [78]. These authors found that transient receptor potential A1 channels (TRPA1) mediate frequent and localized Ca2+ events in astrocytes that contribute to set the resting [Ca2+]i. Blocking TRPA1 channels reduced resting [Ca2+]i and the Ca2+-dependent membrane insertion of GAT-3. The consequent increase in extracellular GABA concentration desensitized GABAA receptors, leading to a reduction of IPSCs on hippocampal interneurons. This work shows the importance of astrocytic TRPA1 channels in regulating the inhibitory signalling in the hippocampus. It also shows the importance of resting Ca2+ levels for effective GAT trafficking to the cell membrane.
Under certain conditions, GATs can reverse their function to release GABA in the extracellular space. In two separate studies, Heja et al. [79,80] found that astrocytes convert excitation to tonic inhibition of neurons in hippocampal slices and in in vivo experiments. In the presence of reduced Mg2+ to increase network activity, they show that glutamate uptake is coupled to the reversal of GAT2/GAT3 that induces a GABA tonic current. The proposed mechanism is that in the presence of elevated glutamatergic activity, excitatory amino acid transporters that co-transport Na+, as well as glutamate, inside the cell (1 glutamate−, 3Na+ and 1 H+ inside/1 K+ outside) lead to an intracellular Na+ increase. As also GATs use the Na+ gradient for uptake of GABA, this intracellular Na+ increase may be sufficient to reverse GAT transport and start extrusion of GABA. These studies provide evidence that this glutamate-induced GABA release via EAAT/GAT transporters is also present in vivo. This mechanism represents a compensatory feedback that may be protective under excessive excitatory events. Indeed, the authors show that epileptic-like discharges in slices are prolonged in the presence of GAT blockers, suggesting that their activity during epileptic-like activity is reversed and increases network inhibition [80].
Several recent works showed that astrocytes not only control [GABA]o through GATs but they can also directly affect [GABA]o by releasing GABA as a gliotransmitter. GABA in the brain is mainly synthesized in neurons by glutamic acid decarboxylases activity (GAD-65 and -67) [81]. Astrocytic GABA content is believed to be mainly due to GATs that capture the neurotransmitter from the external space. However, several studies have reported an astrocytic expression of GAD-67 and -65 [43,82,83]. For example, Lee et al. [43] found in human adult tissue a GAD-65 expression in astrocytes that was comparable to that in inhibitory interneurons. In addition, GABA can also be synthesized in astrocytes starting from the polyamine putrescine [80,84–86]. In astrocytes, GABA can be degraded by GABA-α-ketoglutaric acid aminotransferase (GABA-T) to glutamine, which is then released and subsequently captured by neurons. Most relevant to the focus of this review is the finding that GABA itself is released by astrocytes in many brain regions, including the olfactory bulb [87], the ventro-basal thalamus [77,88] and the hippocampus [89]. In the olfactory bulb, Kozlov and co-workers reported the first evidence that astrocytic GABA can induce slow outward currents (SOCs) in neurons. SOCs share common features with the slow inward currents (SICs) evoked by astrocytic glutamate that were observed in neurons from different brain regions [16,90–93]. Similarly to SICs, SOCs are tetrodotoxin insensitive, occur at low frequency and have significantly slower rise and decay time with respect to synaptic currents. Notably, astrocytes in the olfactory bulb were able to release both GABA and glutamate to inhibit or activate synchronously groups of specific cell populations, revealing a complex astrocytic modulation of local network activity [87]. A similar dual action of astrocytes was observed in the hippocampus [89]. All in all, these results raise a number of questions on the ultimate effects of astrocytic signalling in local networks. Do glutamatergic SICs and GABAergic SOCs derive from the same activated astrocytes or do they come from different ones? As to the GABA and glutamate release, Le Meur et al. [89] suggest that different astrocytes were probably involved because simultaneous SICs and SOCs were extremely rare. It is possible, however, that the same astrocyte may release both GABA and glutamate, but from distinct releasing sites in contact with different synapses. The mechanism of astrocytic GABA release is unclear. The fact that both in ventro-basal thalamus and hippocampal slices SOCs were increased in number upon hypo-osmotic challenge suggests a release mechanism sensitive to cell volume [88,89].
A different form of GABA release has been described in astrocytes from cerebellar slices [94,95]. In this region, GABA appears to be released by astrocytes through the bestrophin-1 channel, a large channel that may also allow glutamate efflux [96,97]. This astrocytic GABA release may contribute to GABA tonic inhibition of neurons that is particularly relevant in the cerebellum. As tonic inhibition can be crucial for neuronal excitability, this work opens a novel aspect in the astrocytic control of local circuit activity. Notably, GABA release has been described also in human astrocytes [98].
A recent study revealed an additional mechanism by which astrocytes regulate GABAergic inhibitory transmission. Lalo et al. [99] reported that exocytosis of ATP from astrocytes modulated both phasic and tonic inhibition in somato-sensory cortex. The authors showed that Ca2+ elevations in astrocytes evoked by TFFLR, a peptide agonist of the protease-activated receptor 1, lead to a vesicular ATP release that evoked P2X receptor-mediated currents in neurons. Ca2+ entry in neurons through P2X receptor openings lead, in turn, to a phosphorylation-dependent downregulation of GABAA receptors. In a transgenic mouse with impaired astrocytic ATP release, i.e. the dn-SNARE mouse, the IPSCs and tonic GABA currents were significantly larger. These data show that a Ca2+-dependent release of ATP from astrocytes can affect the responsiveness of neurons to synaptic and extrasynaptic GABAergic signals.
Finally, astrocytes can modulate inhibition in local circuits through a direct action on GABAergic interneurons. For example, by regulating, through the glutamate transporters, the occupancy of the mGluRs in oriens-lacunosum moleculare interneurons, astrocytes modulate the excitability of these hippocampal interneurons [100]. A Ca2+-dependent release of glutamate has also been reported to activate presynaptic kainate receptors at GABAergic synapses onto inhibitory interneurons, ultimately decreasing inhibitory transmission in the hippocampus [101]. An opposing effect was described for another gliotransmitter, such as ATP, that increased inhibitory synaptic transmission in the hippocampus through activation of P2Y1 receptors in interneurons [102].
Although our understanding of how GABAergic interneurons and astrocytes communicate in the neuronal network is largely undefined, these few studies hint at a richness of different mechanisms by which astrocytes can modulate GABAergic inhibition in local circuits (figure 2).
Figure 2.
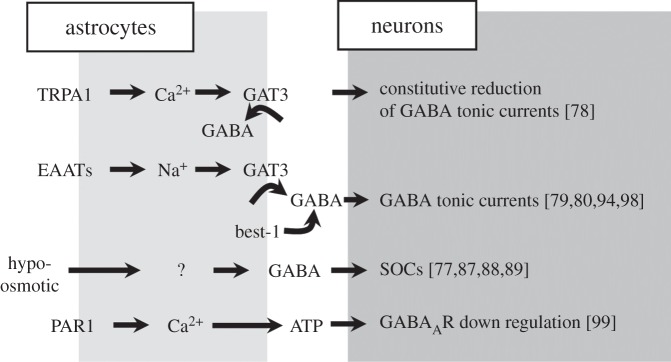
Astrocytes activated by different signals modulate GABAergic transmission. Summary of the astrocytic modulation of GABAergic transmission in response to different signals (see §3). TRPA1, transient receptor potential A1; GAT3, GABA transporter type 3; EAATs, excitatory amino acid transporters; Best-1, bestrophin-1; SOCs, slow outward currents; PAR1, protease-activated receptor type-1.
5. Open questions and conclusion
A large variety of neuronal signals, including the neurotransmitter GABA, are now recognized to trigger intracellular Ca2+ transients in astrocytes. Astrocytes are proposed to act as space and time integrators that decode the information deriving from different neuronal signals into dynamic Ca2+ signal changes that either remain spatially restricted to individual and multiple processes or recruit the entire astrocyte in a global Ca2+ response [7]. Based on this hypothesis, a number of questions regarding a possible reciprocal signalling between GABAergic interneurons and astrocytes need to be answered. First, we need to understand whether GABA released from a given class of interneurons establishes with astrocytes a specific signalling or whether all the different classes of interneurons can similarly activate astrocytes. Second, do all astrocytes respond to GABA or do different astrocyte subpopulations respond selectively to either GABA or other neurotransmitters? Third, how does the GABA-mediated response integrate with other neurotransmitter-mediated responses? This is a crucial issue since glutamategic and GABAergic signalling, as reported above, are intimately linked. Finally, has the same astrocyte the potential to release GABA as well as other gliotransmitters? Technological advances are now providing new powerful tools to address these questions and help us to fully understand the role of neuron–astrocyte communication in the brain. For example, the existence of a specific GABAergic signalling pathway between different interneuron classes and astrocytes can be investigated in mice that express the light-gated cation channel channelrhodopsin-2 selectively in a distinct class of interneurons. Similarly, the use of novel genetically encoded Ca2+ indicators will allow to study astrocytic Ca2+ responses with unprecedented time and space resolution with respect to that achieved after bulk loading with classical fluorescence Ca2+ indicators. This could also make it possible to study neuron–astrocyte crosstalk at fine astrocytic processes located at different subcellular sites, for example at dendritic versus somatic inhibitory synapses onto principal neurons.
Given the plethora of functions played by astrocytes in brain function, it is no surprise that their involvement in most neurological disorders is increasingly documented also from the very early stages of diseases such as epilepsy, Alzheimer's disease, Parkinson's disease, Hungtington's disease, amyotrophic lateral sclerosis, stroke and brain injury (for reviews, see [2,103–105]). Under pathological conditions, interneuron–astrocyte reciprocal interactions may also be affected. For example, it has been reported that astrocytes activated by an epileptogenic insult increase their synthetic machinery to produce neurosteroids, potent GABAA receptors modulators [106–108], that temporally prevent seizure generation and prolong the latent period in animal models of temporal lobe epilepsy [109].
In conclusion, we are only beginning to understand the dynamic interactions between distinct classes of GABAergic interneurons and astrocytes. Future studies are expected to greatly improve our knowledge in this field and have the potential to unveil novel mechanisms in brain physiology and pathology.
Acknowledgements
We thank Micaela Zonta for helpful assistance.
Funding statement
This work was supported by Telethon Foundation (grant nos. GGP10138 and GGP12265), Cariparo Foundation CNR Aging Project and FIRB RBAP11X42L.
References
- 1.Barres BA. 2008. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440. ( 10.1016/j.neuron.2008.10.013) [DOI] [PubMed] [Google Scholar]
- 2.Allaman I, Belanger M, Magistretti PJ. 2011. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 34, 76–87. ( 10.1016/j.tins.2010.12.001) [DOI] [PubMed] [Google Scholar]
- 3.Carmignoto G, Gomez-Gonzalo M. 2010. The contribution of astrocyte signalling to neurovascular coupling. Brain Res. Rev. 63, 138–148. ( 10.1016/j.brainresrev.2009.11.007) [DOI] [PubMed] [Google Scholar]
- 4.Kowianski P, Lietzau G, Steliga A, Waskow M, Morys J. 2013. The astrocytic contribution to neurovascular coupling: still more questions than answers? Neurosci. Res. 75, 171–183. ( 10.1016/j.neures.2013.01.014) [DOI] [PubMed] [Google Scholar]
- 5.Perea G, Navarrete M, Araque A. 2009. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 32, 421–431. ( 10.1016/j.tins.2009.05.001) [DOI] [PubMed] [Google Scholar]
- 6.Halassa MM, Fellin T, Haydon PG. 2007. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 13, 54–63. ( 10.1016/j.molmed.2006.12.005) [DOI] [PubMed] [Google Scholar]
- 7.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. 2014. Gliotransmitters travel in time and space. Neuron 81, 728–739. ( 10.1016/j.neuron.2014.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haydon PG, Carmignoto G. 2006. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 86, 1009–1031. ( 10.1152/physrev.00049.2005) [DOI] [PubMed] [Google Scholar]
- 9.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. 2004. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7, 613–620. ( 10.1038/nn1246) [DOI] [PubMed] [Google Scholar]
- 10.Montana V, Ni Y, Sunjara V, Hua X, Parpura V. 2004. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 24, 2633–2642. ( 10.1523/JNEUROSCI.3770-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, et al. 2004. Fusion-related release of glutamate from astrocytes. J. Biol. Chem. 279, 12 724–12 733. ( 10.1074/jbc.M312845200) [DOI] [PubMed] [Google Scholar]
- 12.Woo DH, et al. 2012. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151, 25–40. ( 10.1016/j.cell.2012.09.005) [DOI] [PubMed] [Google Scholar]
- 13.Jourdain P, et al. 2007. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331–339. ( 10.1038/nn1849) [DOI] [PubMed] [Google Scholar]
- 14.Navarrete M, Araque A. 2010. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126. ( 10.1016/j.neuron.2010.08.043) [DOI] [PubMed] [Google Scholar]
- 15.Navarrete M, Perea G, de Sevilla DF, Gomez-Gonzalo M, Nunez A, Martin ED, Araque A. 2012. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 10, e1001259 ( 10.1371/journal.pbio.1001259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. 2004. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743. ( 10.1016/j.neuron.2004.08.011) [DOI] [PubMed] [Google Scholar]
- 17.Velez-Fort M, Audinat E, Angulo MC. 2011. Central Role of GABA in neuron–glia interactions. Neuroscientist 18, 237–250. ( 10.1177/1073858411403317) [DOI] [PubMed] [Google Scholar]
- 18.Isaacson JS, Scanziani M. 2011. How inhibition shapes cortical activity. Neuron 72, 231–243. ( 10.1016/j.neuron.2011.09.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. 2003. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421, 844–848. ( 10.1038/nature01374) [DOI] [PubMed] [Google Scholar]
- 20.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. 2009. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667. ( 10.1038/nature08002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohal VS, Zhang F, Yizhar O, Deisseroth K. 2009. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. ( 10.1038/nature07991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, Buzsaki G. 2013. Inhibition-induced theta resonance in cortical circuits. Neuron 80, 1263–1276. ( 10.1016/j.neuron.2013.09.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga C, Golshani P, Soltesz I. 2012. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc. Natl Acad. Sci. USA 109, E2726–E2734. ( 10.1073/pnas.1210929109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartos M, Vida I, Jonas P. 2007. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 8, 45–56. ( 10.1038/nrn2044) [DOI] [PubMed] [Google Scholar]
- 25.Buzsáki G, Draguhn A. 2004. Neuronal oscillations in cortical networks. Science 304, 1926–1929. ( 10.1126/science.1099745) [DOI] [PubMed] [Google Scholar]
- 26.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. 2004. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807. ( 10.1038/nrn1519) [DOI] [PubMed] [Google Scholar]
- 27.Ascoli GA, et al. 2008. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568. ( 10.1038/nrn2402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klausberger T, Somogyi P. 2008. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. ( 10.1126/science.1149381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. 2011. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61. ( 10.1002/dneu.20853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Defelipe J, et al. 2013. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 14, 202–216. ( 10.1038/nrn3444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kepecs A, Fishell G. 2014. Interneuron cell types are fit to function. Nature 505, 318–326. ( 10.1038/nature12983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi Y, Kubota Y. 1996. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J. Neurosci. 16, 2701–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovett-Barron M, et al. 2012. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat. Neurosci. 15, S421–S423. ( 10.1038/nn.3024) [DOI] [PubMed] [Google Scholar]
- 34.Palmer L, Murayama M, Larkum M. 2012. Inhibitory regulation of dendritic activity in vivo. Front. Neural Circuits 6, 26 ( 10.3389/fncir.2012.00026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freund TF, Katona I. 2007. Perisomatic inhibition. Neuron 56, 33–42. ( 10.1016/j.neuron.2007.09.012) [DOI] [PubMed] [Google Scholar]
- 36.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. 2013. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076. ( 10.1038/nn.3446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deleuze C, Pazienti A, Bacci A. 2014. Autaptic self-inhibition of cortical GABAergic neurons: synaptic narcissism or useful introspection? Curr. Opin. Neurobiol. 26C, 64–71. ( 10.1016/j.conb.2013.12.009) [DOI] [PubMed] [Google Scholar]
- 38.Murayama M, Perez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. 2009. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457, 1137–1141. ( 10.1038/nature07663) [DOI] [PubMed] [Google Scholar]
- 39.Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. 2011. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335. ( 10.1038/nature10674) [DOI] [PubMed] [Google Scholar]
- 40.Haider B, Hausser M, Carandini M. 2013. Inhibition dominates sensory responses in the awake cortex. Nature 493, 97–100. ( 10.1038/nature11665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris KD, Mrsic-Flogel TD. 2013. Cortical connectivity and sensory coding. Nature 503, 51–58. ( 10.1038/nature12654) [DOI] [PubMed] [Google Scholar]
- 42.Runquist M, Alonso G. 2003. GABAergic signaling mediates the morphological organization of astrocytes in the adult rat forebrain. Glia 41, 137–151. ( 10.1002/glia.10166) [DOI] [PubMed] [Google Scholar]
- 43.Lee M, Schwab C, McGeer PL. 2011. Astrocytes are GABAergic cells that modulate microglial activity. Glia 59, 152–165. ( 10.1002/glia.21087) [DOI] [PubMed] [Google Scholar]
- 44.Kettenmann H, Backus KH, Schachner M. 1984. Aspartate, glutamate and gamma-aminobutyric acid depolarize cultured astrocytes. Neurosci. Lett. 52, 25–29. ( 10.1016/0304-3940(84)90345-8) [DOI] [PubMed] [Google Scholar]
- 45.Kettenmann H, Schachner M. 1985. Pharmacological properties of γ-aminobutyric acid-, glutamate-, and aspartate-induced depolarizations in cultured astrocytes. J. Neurosci. 5, 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Backus KH, Kettenmann H, Schachner M. 1988. Effect of benzodiazepines and pentobarbital on the GABA-induced depolarization in cultured astrocytes. Glia 1, 132–140. ( 10.1002/glia.440010205) [DOI] [PubMed] [Google Scholar]
- 47.Fraser DD, Duffy S, Angelides KJ, Perez-Velazquez JL, Kettenmann H, MacVicar BA. 1995. GABAA/benzodiazepine receptors in acutely isolated hippocampal astrocytes. J. Neurosci. 15, 2720–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacVicar BA, Tse FW, Crichton SA, Kettenmann H. 1989. GABA-activated Cl− channels in astrocytes of hippocampal slices. J. Neurosci. 9, 3577–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark B, Mobbs P. 1992. Transmitter-operated channels in rabbit retinal astrocytes studied in situ by whole-cell patch clamping. J. Neurosci. 12, 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller T, Fritschy JM, Grosche J, Pratt GD, Mohler H, Kettenmann H. 1994. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. J. Neurosci. 14, 2503–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinhauser C, Jabs R, Kettenmann H. 1994. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus 4, 19–35. ( 10.1002/hipo.450040105) [DOI] [PubMed] [Google Scholar]
- 52.Kettenmann H, Backus KH, Schachner M. 1987. γ-Aminobutyric acid opens Cl− channels in cultured astrocytes. Brain Res. 404, 1–9. ( 10.1016/0006-8993(87)91349-7) [DOI] [PubMed] [Google Scholar]
- 53.Egawa K, Yamada J, Furukawa T, Yanagawa Y, Fukuda A. 2013. Cl− homeodynamics in gap-junction-coupled astrocytic networks on activation of GABAergic synapses. J. Physiol. 591, 3901–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosewater K, Sontheimer H. 1994. Fibrous and protoplasmic astrocytes express GABAA receptors that differ in benzodiazepine pharmacology. Brain Res. 636, 73–80. ( 10.1016/0006-8993(94)90177-5) [DOI] [PubMed] [Google Scholar]
- 55.Verkhratsky A, Rodriguez JJ, Parpura V. 2012. Calcium signalling in astroglia. Mol. Cell. Endocrinol. 353, 45–56. ( 10.1016/j.mce.2011.08.039) [DOI] [PubMed] [Google Scholar]
- 56.Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. 2012. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN NEURO 4, 103–119. ( 10.1042/AN20110061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang J, Jiang L, Goldman SA, Nedergaard M. 1998. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat. Neurosci. 1, 683–692. ( 10.1038/3684) [DOI] [PubMed] [Google Scholar]
- 58.Serrano A, Haddjeri N, Lacaille JC, Robitaille R. 2006. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J. Neurosci. 26, 5370–5382. ( 10.1523/JNEUROSCI.5255-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson M, Eriksson PS, Ronnback L, Hansson E. 1993. GABA induces Ca2+ transients in astrocytes. Neuroscience 54, 605–614. ( 10.1016/0306-4522(93)90232-5) [DOI] [PubMed] [Google Scholar]
- 60.Meier SD, Kafitz KW, Rose CR. 2008. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 56, 1127–1137. ( 10.1002/glia.20684) [DOI] [PubMed] [Google Scholar]
- 61.Navarrete M, Araque A. 2008. Endocannabinoids mediate neuron–astrocyte communication. Neuron 57, 883–893. ( 10.1016/j.neuron.2008.01.029) [DOI] [PubMed] [Google Scholar]
- 62.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. 2004. Molecular structure and physiological functions of GABAB receptors. Physiol. Rev. 84, 835–867. ( 10.1152/physrev.00036.2003) [DOI] [PubMed] [Google Scholar]
- 63.Perea G, Araque A. 2005. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci. 25, 2192–2203. ( 10.1523/JNEUROSCI.3965-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doengi M, Hirnet D, Coulon P, Pape HC, Deitmer JW, Lohr C. 2009. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc. Natl Acad. Sci. USA 106, 17 570–17 575. ( 10.1073/pnas.0809513106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andersson M, Blomstrand F, Hanse E. 2007. Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J. Physiol. 585, 843–852. ( 10.1113/jphysiol.2007.142737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nusser Z, Mody I. 2002. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J. Neurophysiol. 87, 2624–2628. [DOI] [PubMed] [Google Scholar]
- 67.Semyanov A, Walker MC, Kullmann DM, Silver RA. 2004. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 27, 262–269. ( 10.1016/j.tins.2004.03.005) [DOI] [PubMed] [Google Scholar]
- 68.Farrant M, Nusser Z. 2005. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 6, 215–229. ( 10.1038/nrn1625) [DOI] [PubMed] [Google Scholar]
- 69.Minelli A, Brecha NC, Karschin C, DeBiasi S, Conti F. 1995. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J. Neurosci. 15, 7734–7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conti F, Melone M, De Biasi S, Minelli A, Brecha NC, Ducati A. 1998. Neuronal and glial localization of GAT-1, a high-affinity γ-aminobutyric acid plasma membrane transporter, in human cerebral cortex: with a note on its distribution in monkey cortex. J. Comp. Neurol. 396, 51–63. () [DOI] [PubMed] [Google Scholar]
- 71.Melone M, Ciappelloni S, Conti F. 2013. A quantitative analysis of cellular and synaptic localization of GAT-1 and GAT-3 in rat neocortex. Brain Struct. Funct. ( 10.1007/s00429-013-0690-8) [DOI] [PubMed] [Google Scholar]
- 72.Kersanté F, Rowley SC, Pavlov I, Gutierrez-Mecinas M, Semyanov A, Reul JM, Walker MC, Linthorst AC. 2013. A functional role for both γ-aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J. Physiol. 591, 2429–2441. ( 10.1113/jphysiol.2012.246298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beenhakker MP, Huguenard JR. 2010. Astrocytes as gatekeepers of GABAB receptor function. J. Neurosci. 30, 15 262–15 276. ( 10.1523/JNEUROSCI.3243-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Biasi S, Vitellaro-Zuccarello L, Brecha NC. 1998. Immunoreactivity for the GABA transporter-1 and GABA transporter-3 is restricted to astrocytes in the rat thalamus. A light and electron-microscopic immunolocalization. Neuroscience 83, 815–828. ( 10.1016/S0306-4522(97)00414-4) [DOI] [PubMed] [Google Scholar]
- 75.Vitellaro-Zuccarello L, Calvaresi N, De Biasi S. 2003. Expression of GABA transporters, GAT-1 and GAT-3, in the cerebral cortex and thalamus of the rat during postnatal development. Cell Tissue Res. 313, 245–257. ( 10.1007/s00441-003-0746-9) [DOI] [PubMed] [Google Scholar]
- 76.Cope DW, Di Giovanni G, Fyson SJ, Orban G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V. 2009. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat. Med. 15, 1392–1398. ( 10.1038/nm.2058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pirttimaki T, Parri HR, Crunelli V. 2013. Astrocytic GABA transporter GAT-1 dysfunction in experimental absence seizures. J. Physiol. 591, 823–833. ( 10.1113/jphysiol.2012.242016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. 2012. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 15, 70–80. ( 10.1038/nn.3000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heja L, et al. 2009. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS ONE 4, e7153 (doi:101371/journal.pone.0007153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heja L, et al. 2012. Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol. 10, 26 ( 10.1186/1741-7007-10-26). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin DL, Rimvall K. 1993. Regulation of gamma-aminobutyric acid synthesis in the brain. J. Neurochem. 60, 395–407. ( 10.1111/j.1471-4159.1993.tb03165.x) [DOI] [PubMed] [Google Scholar]
- 82.Ochi S, Lim JY, Rand MN, During MJ, Sakatani K, Kocsis JD. 1993. Transient presence of GABA in astrocytes of the developing optic nerve. Glia 9, 188–198. ( 10.1002/glia.440090304) [DOI] [PubMed] [Google Scholar]
- 83.Yoon BE, Woo J, Lee CJ. 2012. Astrocytes as GABA-ergic and GABA-ceptive cells. Neurochem. Res. 37, 2474–2479. ( 10.1007/s11064-012-0808-z) [DOI] [PubMed] [Google Scholar]
- 84.Seiler N, al-Therib MJ, Kataoka K. 1973. Formation of GABA from putrescine in the brain of fish (Salmo irideus Gibb.). J. Neurochem. 20, 699–708. ( 10.1111/j.1471-4159.1973.tb00030.x) [DOI] [PubMed] [Google Scholar]
- 85.Seiler N, Askar A. 1971. A micro method for the quantitative estimation of putrescine in tissues. J. Chromatogr. 62, 121–127. ( 10.1016/S0021-9673(01)96817-7) [DOI] [PubMed] [Google Scholar]
- 86.Kremzner LT, Hiller JM, Simon EJ. 1975. Metabolism of polyamines in mouse neuroblastoma cells in culture: formation of GABA and putreanine. J. Neurochem. 25, 889–894. ( 10.1111/j.1471-4159.1975.tb04423.x) [DOI] [PubMed] [Google Scholar]
- 87.Kozlov AS, Angulo MC, Audinat E, Charpak S. 2006. Target cell-specific modulation of neuronal activity by astrocytes. Proc. Natl Acad. Sci. USA 103, 10 058–10 063. ( 10.1073/pnas.0603741103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jimenez-Gonzalez C, Pirttimaki T, Cope DW, Parri HR. 2011. Non-neuronal, slow GABA signalling in the ventrobasal thalamus targets δ-subunit-containing GABAA receptors. Eur. J. Neurosci. 33, 1471–1482. ( 10.1111/j.1460-9568.2011.07645.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le Meur K, Mendizabal-Zubiaga J, Grandes P, Audinat E. 2012. GABA release by hippocampal astrocytes. Front. Comput. Neurosci. 6, 59 ( 10.3389/fncom.2012.00059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parri HR, Gould TM, Crunelli V. 2001. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat. Neurosci. 4, 803–812. ( 10.1038/90507) [DOI] [PubMed] [Google Scholar]
- 91.D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. 2007. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc. Natl Acad. Sci. USA 104, 1995–2000. ( 10.1073/pnas.0609408104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. 2008. Two forms of astrocyte calcium excitabilty have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J. Neurosci. 28, 6659–6663. ( 10.1523/JNEUROSCI.1717-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pirttimaki TM, Codadu NK, Awni A, Pratik P, Nagel DA, Hill EJ, Dineley KT, Parri HR. 2013. α7 Nicotinic receptor-mediated astrocytic gliotransmitter release: Aβ effects in a preclinical Alzheimer's mouse model. PLoS ONE 8, e81828 ( 10.1371/journal.pone.0081828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. 2010. Channel-mediated tonic GABA release from glia. Science 330, 790–796. ( 10.1126/science.1184334) [DOI] [PubMed] [Google Scholar]
- 95.Yoon BE, Jo S, Woo J, Lee JH, Kim T, Kim D, Lee CJ. 2011. The amount of astrocytic GABA positively correlates with the degree of tonic inhibition in hippocampal CA1 and cerebellum. Mol. Brain 4, 42 ( 10.1186/1756-6606-4-42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oh SJ, Han KS, Park H, Woo DH, Kim HY, Traynelis SF, Lee CJ. 2012. Protease activated receptor 1-induced glutamate release in cultured astrocytes is mediated by Bestrophin-1 channel but not by vesicular exocytosis. Mol. Brain 5, 38 ( 10.1186/1756-6606-5-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park H, Han KS, Oh SJ, Jo S, Woo J, Yoon BE, Lee CJ. 2013. High glutamate permeability and distal localization of Best1 channel in CA1 hippocampal astrocyte. Mol. Brain 6, 54 ( 10.1186/1756-6606-6-54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee M, McGeer EG, McGeer PL. 2011. Mechanisms of GABA release from human astrocytes. Glia 59, 1600–1611. ( 10.1002/glia.21202) [DOI] [PubMed] [Google Scholar]
- 99.Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y. 2014. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 12, e1001747 ( 10.1371/journal.pbio.1001747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. 2004. Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J. Neurosci. 24, 4551–4559. ( 10.1523/JNEUROSCI.5217-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Q-S, Xu Q, Arcuino G, Kang J, Nedergaard M. 2004. Astrocyte-mediated activation of neuronal kainate receptors. Proc. Natl Acad. Sci. USA 101, 3172–3177. ( 10.1073/pnas.0306731101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bowser DN, Khakh BS. 2004. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J. Neurosci. 24, 8606–8620. ( 10.1523/JNEUROSCI.2660-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seifert G, Schilling K, Steinhauser C. 2006. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat. Rev. Neurosci. 7, 194–206. ( 10.1038/nrn1870) [DOI] [PubMed] [Google Scholar]
- 104.Rossi D, Volterra A. 2009. Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res. Bull. 80, 224–232. ( 10.1016/j.brainresbull.2009.07.012) [DOI] [PubMed] [Google Scholar]
- 105.Losi G, Cammarota M, Carmignoto G. 2012. The role of astroglia in the epileptic brain. Front. Pharmacol. 3, 132 ( 10.3389/fphar.2012.00132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. 1990. Neurosteroids act on recombinant human GABAA receptors. Neuron 4, 759–765. ( 10.1016/0896-6273(90)90202-Q) [DOI] [PubMed] [Google Scholar]
- 107.Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. 2003. Neurosteroid modulation of GABAA receptors. Prog. Neurobiol. 71, 67–80. ( 10.1016/j.pneurobio.2003.09.001) [DOI] [PubMed] [Google Scholar]
- 108.Reddy DS. 2010. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 186, 113–137. ( 10.1016/B978-0-444-53630-3.00008-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biagini G, Longo D, Baldelli E, Zoli M, Rogawski MA, Bertazzoni G, Avoli M. 2009. Neurosteroids and epileptogenesis in the pilocarpine model: evidence for a relationship between P450scc induction and length of the latent period. Epilepsia 50(Suppl. 1), 53–58. ( 10.1111/j.1528-1167.2008.01971.x) [DOI] [PMC free article] [PubMed] [Google Scholar]


