Abstract
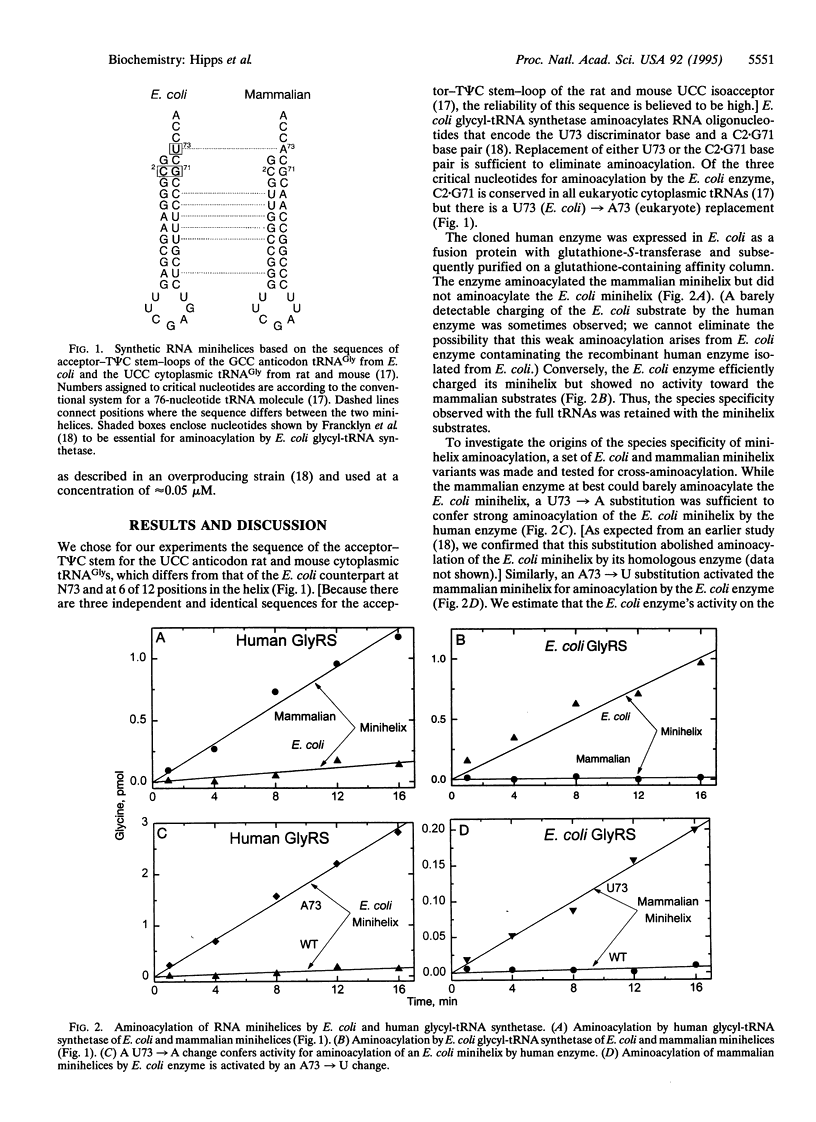
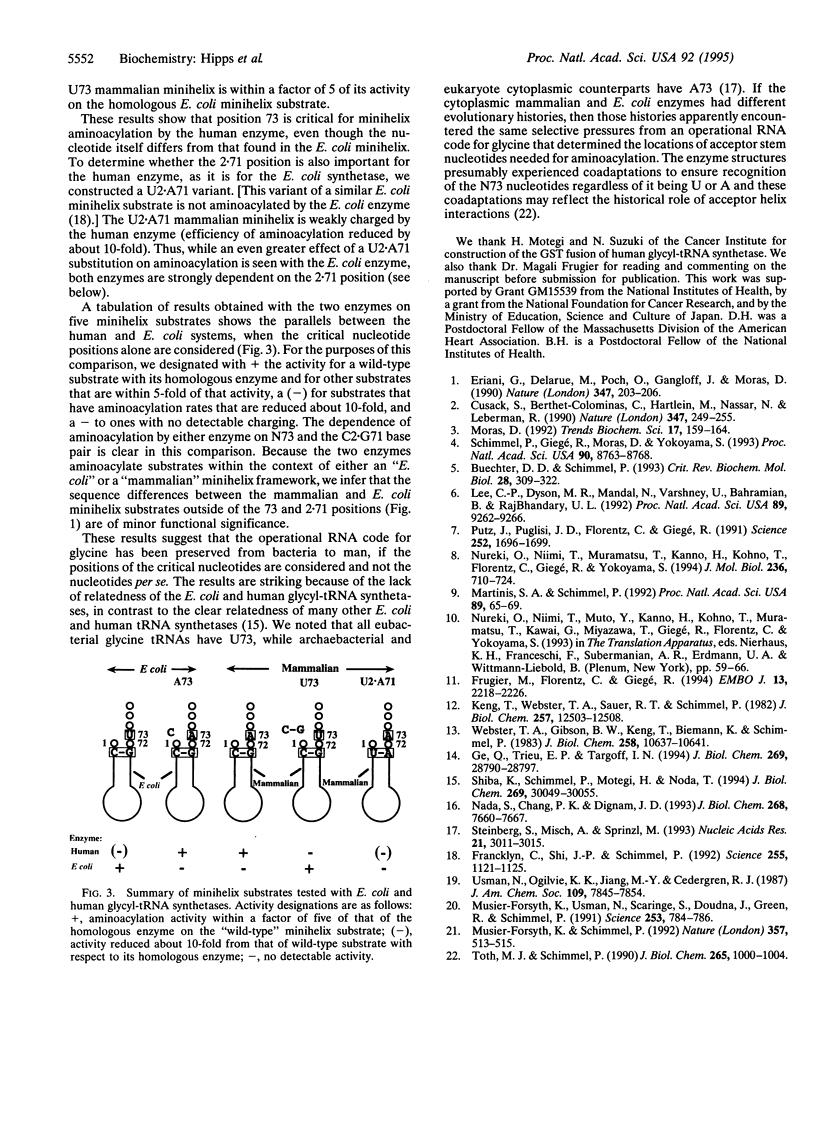
The genetic code is based on aminoacylation reactions where specific amino acids are attached to tRNAs bearing anticodon trinucleotides. However, the anticodon-independent specific aminoacylation of RNA minihelix substrates by bacterial and yeast tRNA synthetases suggested an operational RNA code for amino acids whereby specific RNA sequences/structures in tRNA acceptor stems correspond to specific amino acids. Because of the possible significance of the operational RNA code for the development of the genetic code, we investigated aminoacylation of synthetic RNA minihelices with a human enzyme to understand the sequences needed for that aminoacylation compared with those needed for a microbial system. We show here that the species-specific aminoacylation of glycine tRNAs is recapitulated by a species-specific aminoacylation of minihelices. Although the mammalian and Escherichia coli minihelices differ at 6 of 12 base pairs, two of the three nucleotides essential for aminoacylation by the E. coli enzyme are conserved in the mammalian minihelix. The two conserved nucleotides were shown to be also important for aminoacylation of the mammalian minihelix by the human enzyme. A simple interchange of the differing nucleotide enabled the human enzyme to now charge the bacterial substrate and not the mammalian minihelix. Conversely, this interchange made the bacterial enzyme specific for the mammalian substrate. Thus, the positional locations (if not the actual nucleotides) for the operational RNA code for glycine appear conserved from bacteria to mammals.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buechter D. D., Schimmel P. Aminoacylation of RNA minihelices: implications for tRNA synthetase structural design and evolution. Crit Rev Biochem Mol Biol. 1993;28(4):309–322. doi: 10.3109/10409239309078438. [DOI] [PubMed] [Google Scholar]
- Cusack S., Berthet-Colominas C., Härtlein M., Nassar N., Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature. 1990 Sep 20;347(6290):249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Francklyn C., Shi J. P., Schimmel P. Overlapping nucleotide determinants for specific aminoacylation of RNA microhelices. Science. 1992 Feb 28;255(5048):1121–1125. doi: 10.1126/science.1546312. [DOI] [PubMed] [Google Scholar]
- Frugier M., Florentz C., Giegé R. Efficient aminoacylation of resected RNA helices by class II aspartyl-tRNA synthetase dependent on a single nucleotide. EMBO J. 1994 May 1;13(9):2218–2226. doi: 10.1002/j.1460-2075.1994.tb06499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q., Trieu E. P., Targoff I. N. Primary structure and functional expression of human Glycyl-tRNA synthetase, an autoantigen in myositis. J Biol Chem. 1994 Nov 18;269(46):28790–28797. [PubMed] [Google Scholar]
- Keng T., Webster T. A., Sauer R. T., Schimmel P. Gene for Escherichia coli glycyl-tRNA synthetase has tandem subunit coding regions in the same reading frame. J Biol Chem. 1982 Nov 10;257(21):12503–12508. [PubMed] [Google Scholar]
- Lee C. P., Dyson M. R., Mandal N., Varshney U., Bahramian B., RajBhandary U. L. Striking effects of coupling mutations in the acceptor stem on recognition of tRNAs by Escherichia coli Met-tRNA synthetase and Met-tRNA transformylase. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9262–9266. doi: 10.1073/pnas.89.19.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinis S. A., Schimmel P. Enzymatic aminoacylation of sequence-specific RNA minihelices and hybrid duplexes with methionine. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):65–69. doi: 10.1073/pnas.89.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musier-Forsyth K., Schimmel P. Functional contacts of a transfer RNA synthetase with 2'-hydroxyl groups in the RNA minor groove. Nature. 1992 Jun 11;357(6378):513–515. doi: 10.1038/357513a0. [DOI] [PubMed] [Google Scholar]
- Musier-Forsyth K., Usman N., Scaringe S., Doudna J., Green R., Schimmel P. Specificity for aminoacylation of an RNA helix: an unpaired, exocyclic amino group in the minor groove. Science. 1991 Aug 16;253(5021):784–786. doi: 10.1126/science.1876835. [DOI] [PubMed] [Google Scholar]
- Nada S., Chang P. K., Dignam J. D. Primary structure of the gene for glycyl-tRNA synthetase from Bombyx mori. J Biol Chem. 1993 Apr 15;268(11):7660–7667. [PubMed] [Google Scholar]
- Nureki O., Niimi T., Muramatsu T., Kanno H., Kohno T., Florentz C., Giegé R., Yokoyama S. Molecular recognition of the identity-determinant set of isoleucine transfer RNA from Escherichia coli. J Mol Biol. 1994 Feb 25;236(3):710–724. doi: 10.1006/jmbi.1994.1184. [DOI] [PubMed] [Google Scholar]
- Pütz J., Puglisi J. D., Florentz C., Giegé R. Identity elements for specific aminoacylation of yeast tRNA(Asp) by cognate aspartyl-tRNA synthetase. Science. 1991 Jun 21;252(5013):1696–1699. doi: 10.1126/science.2047878. [DOI] [PubMed] [Google Scholar]
- Schimmel P., Giegé R., Moras D., Yokoyama S. An operational RNA code for amino acids and possible relationship to genetic code. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8763–8768. doi: 10.1073/pnas.90.19.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Schimmel P., Motegi H., Noda T. Human glycyl-tRNA synthetase. Wide divergence of primary structure from bacterial counterpart and species-specific aminoacylation. J Biol Chem. 1994 Nov 25;269(47):30049–30055. [PubMed] [Google Scholar]
- Steinberg S., Misch A., Sprinzl M. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1993 Jul 1;21(13):3011–3015. doi: 10.1093/nar/21.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M. J., Schimmel P. Deletions in the large (beta) subunit of a hetero-oligomeric aminoacyl-tRNA synthetase. J Biol Chem. 1990 Jan 15;265(2):1000–1004. [PubMed] [Google Scholar]
- Webster T. A., Gibson B. W., Keng T., Biemann K., Schimmel P. Primary structures of both subunits of Escherichia coli glycyl-tRNA synthetase. J Biol Chem. 1983 Sep 10;258(17):10637–10641. [PubMed] [Google Scholar]




