Abstract
Dementia, most commonly caused by Alzheimer's disease (AD), affects approximately 35 million people worldwide, with the incidence expected to increase as the population ages. After decades of investigation, AD is now understood to be a complex disease that affects behavior and cognition through several mechanisms: Disrupted neuronal communication, abnormal regional tissue metabolism, and impaired cellular repair. Existing therapies have demonstrated limited efficacy, which has spurred the search for specific disease markers and predictors as well as innovative therapeutic options. Deep brain stimulation (DBS) of the memory circuits is one such option, with early studies suggesting that modulation of neural activity in these networks may improve cognitive function. Encapsulated cell biodelivery (ECB) is a device that delivers nerve growth factor to the cholinergic basal forebrain to potentially improve cognitive decline in AD patients. This review discusses the pathogenesis of AD, novel neuroimaging and biochemical markers, and the emerging role for neurosurgical applications such as DBS and ECB.
Keywords: Alzheimer's disease, deep brain stimulation, dementia, encapsulated cell biodelivery, limbic circuit, neurosurgery, stereotactic surgery
INTRODUCTION
Significant progress has been made in understanding the pathophysiology of Alzheimer's disease (AD) since Dr. Alois Alzheimer initially identified a patient with progressive loss of short-term memory associated with behavioral disturbances in 1901.[3,6] AD affects approximately 35 million people worldwide, and was associated with social and healthcare costs exceeding 600 billion USD in 2010. Unfortunately, the incidence of AD is expected to increase over the next decade, posing significant challenges for public health and allocation of health care resources.[3,30]
Today, AD is understood to be a complex disease that affects behavior and cognition through several mechanisms: Disrupted neuronal communication, abnormal regional tissue metabolism, and impaired cellular repair.[16,18,30,38] Anatomically, AD affects many structures of the brain; however, the main focus of damage is in the limbic system [Figures 1 and 2]. Understanding the anatomical nuances of this system opens the possibility for targeting the circuitry.[1,9,17,28,29]
Figure 1.
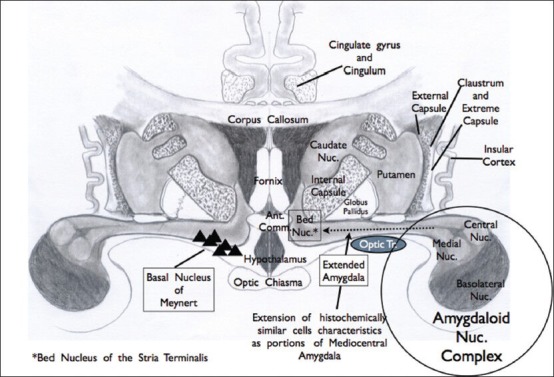
The “extended amygdala.” The right side of the diagram demonstrates the central and medial amygdaloid projection which has similar neuronal histological characteristics extending through the forebrain region and anterior perforating substance. This is referred to as the “extended amygdala” and is routed medially to the bed nucleus of the stria terminalis (BNST). On the left side of the diagram, the triangular cells represent the forebrain nucleus of Meynert, which is the focus of attention in Alzheimer's disease because of its known cholinergic component. This region was referred in the past as the substantia innominata. Immunohistology and specific staining have shown the complexity of this region, as it represents the ventral striatum and ventral pallium, as well as the neuronal pool noted above
Figure 2.
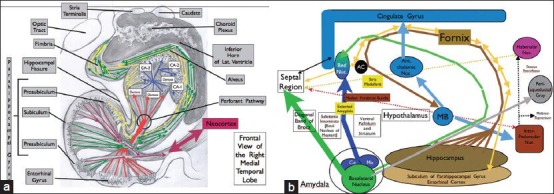
(a and b) Main structures of the human limbic system. Human brain showing the parahippocampal gyrus, hippocampus, dentate, subiculum, entorhinal cortex, and their major connections important in behavior and memory, and circuits involved in the symptoms of Alzheimer's disease. The hippocampus is subdivided into four sections CA1–4 (CA1–CA3 demonstrated in a). Hippocampus outputs are through the fornix and project to the mammillary bodies via the post-commissural fornix, to the septal nuclei, to the preoptic nucleus of the hypothalamus, to the ventral striatum, and to portions of the frontal lobe through the precommissural fornix, now used as target of DBS for Alzheimer's disease (see text). The fornix is bidirectional; therefore, its stimulation or suppression affects the hypothalamus, septal cortex, thalamic nuclei (anterior thalamic and dorsomedial nuclei), and cingulate gyrus, to mention a few, projecting back to the hippocampus and entorhinal cortex. These inputs can project to the dentate gyrus of the hippocampus via the perforant pathway, synapsing on granule cells. Granule cells then connect to pyramidal neurons in the CA3 region and project to CA1 pyramidal cells. These latter cells with the direct extension of the entorhinal and subicular neuronal pool give rise primarily to the fornix output. Thus, the input from the entorhinal cortex to the dentato-hippocampal complex travels to the fornix and after modulations from the hypothalamic nuclei, thalamic nuclei, septal cortex, neocortex, and cingulate gyrus, it can directly or indirectly return back to the entorhinal–hippocampus complex via the fornix, a bidirectional pathway. Notice the robust connection of this memory and behavior complex with the neocortex (a, neocortex bundle, red arrow). Lastly, there may be a small amount of bilateral hippocampal communication via the hippocampal commissure (psalterium). (b) Represents the schematic connections
Extensive cellular and molecular studies in human tissue and animal models have not yet revealed the underlying cause of AD, but much progress has been made in identifying environmental and genetic risk factors as well as histopathologic markers.[30,33] The well-known accumulation of β amyloid and aggregation of tau protein are thought to be implicated in the pathogenesis of AD, along with inflammatory microenvironments, impaired response to oxidative stress, and subsequent neuronal degeneration. Genetic loci known to predispose to AD include those on chromosomes 21, 14, 1, and 19, which code for amyloid precursor protein, presenilin 1, presenilin 2, and apolipoprotein E4/E4, respectively. These discoveries have been critical in improving our understanding of the pathophysiology of this disease.[7,29]
Current Food and Drug Administration (FDA)-approved medical therapies directed at improving the function of remaining neurons in the limbic system include cholinesterase inhibitors (ChEIs) which potentiate acetylcholine in the synaptic cleft and N-methyl-d-aspartate (NMDA) receptor antagonists intended to mitigate glutamatergic excitotoxicity. Experimental antiamyloid therapies, attempts to reverse tau deposition, and treatments targeted at minimizing oxidative stresses are also being studied.[3]
Recently, targeting of memory circuits via deep brain stimulation (DBS) has been attempted [Figure 2].[21] DBS has been used with great success in neurodegenerative and neuropsychological applications such as Parkinson's disease, obsessive compulsive disorder (OCD), and depression. AD as a novel application of this surgical technique has gained the interest of many experts. The result of first phase 1 trial of DBS of the fornix was published in 2010 by Laxton et al. The authors used positron emission tomography (PET) imaging to demonstrate that this therapy was effective in reversing impaired glucose utilization in the temporal and parietal lobes.[19]
Encapsulated cell biodelivery (ECB) is an implanted device that delivers nerve growth factor (NGF) to the cholinergic basal forebrain. Several animal studies have shown that delivery of NFG in basal forebrain could arrest or even reverse the neuronal degeneration process.[11,22,23,36] In 2012, Wahlberg et al. evaluated the safety and clinical efficacy of ECB for use in humans.[36] Currently, a phase II trial is ongoing for evaluating this approach.
Using advances in functional imaging and cognitive neuroscience allows us to investigate the brain and central nervous system (CNS) disorders from an integrated neural network perspective. In this review, we discuss what is known about AD from the fundamental level of genetics and the abnormal biochemical gene products used as biomarkers for AD, as well as the surgical treatments.[11,22] We will then discuss the results of DBS used to treat AD, ECB implant, and the role of functional imaging in fostering the understanding of the disease process, potentially helping identify new and better targets for DBS and other surgical strategies to curb the progress of AD.[23,36]
IMAGING AND LABORATORY MARKERS IN AD
It has been suggested that the clinical diagnosis of AD be supplemented by neuroimaging assessments. Magnetic resonance imaging (MRI) and computed tomography (CT) usually demonstrate diffuse cortical atrophy in advanced AD. Newer imaging techniques are being developed to identify the disease at earlier stages.[7,38,39] Initial attempts at this goal focused on standard structural imaging techniques in the hope of identifying imaging markers that could recognize AD in the pre-clinical phase.[24] More recently, several newer imaging and laboratory techniques have been used to identify early AD and are discussed below.[38]
Early identification and classification of abnormal brain changes in patients at risk of AD is important for accurate diagnosis and prognosis, as well as taking therapeutic initiatives. Previously, our knowledge about brain regions affected in various types of dementia was directed by structural imaging modalities such as CT and MRI which offered nonspecific clues. For example, the characteristic mesial temporal lobe atrophy is found not only in AD, but also in other types of dementias. This finding is also more common in later stages of the disease. While mechanisms underlying the progression of cognitive decline in AD have not been fully described, advances in both structural and functional imaging have highlighted the key regions involved early in the disease course, fostering our understanding of the disease and helping to define the possibility and role of neuromodulation.
The default mode network (DMN) corresponds to intrinsic functional regions of the brain which are active only at rest, as seen with imaging techniques such as PET and functional MRI (fMRI).[8] The changes that occur in certain subareas of the DMN during the course of AD have been the subject of intense analysis. A common finding in resting state fMRI in AD patients is decreased functional activity in the posterior subregion of the DMN which comprises the precuneus and posterior cingulate cortex.[13,15] Simultaneously, the anterior subregion shows hyperactivity, theorized to be a compensatory mechanism that occurs earlier in the course of the disease. As the disease progresses, the connectivity across all network nodes is depressed, resulting in an overall deterioration of brain functions.[15] PET imaging employs the use of a radiolabeled marker such as oxygen-15 (15-O) or fluorodeoxyglucose (FDG) as a surrogate of regional changes in cerebral blood flow.
Most recently, Wang et al.[37] used fMRI to define the structure of the DMN in patients considered to be at risk for AD. Again, the posterior parietal and occipital cortices including the bilateral precuneus and postcentral gyrus were the locations of greatest difference between healthy controls and affected patients. The authors found three types of dysfunction in these brain regions: (1) Increased connectivity time between nodes in the DMN; (2) decreased strength of individual nodes; and (3) impaired communication between different functional modules (which are made up of nodes). These findings were highly sensitive and specific for distinguishing affected patients from healthy individuals.
Diffusion tensor imaging (DTI) is a structural neuroimaging modality that tracks the direction of diffusion of water molecules. Based on the degree of diffusivity of water in different tissues, the integrity of white matter tracts can be determined.[26] Hahn et al.[15] combined DTI with fMRI to provide a structural explanation for the functional deterioration as previously described. In their analysis, the authors found significantly decreased white matter connectivity within the posterior DMN, specifically the posterior parietal cortex. Furthermore, this decline in structural and functional connections within the posterior DMN was not explained by atrophy.
In addition to radiolabeled oxygen, another type of PET imaging was developed specifically for use in detecting AD. Pittsburgh compound B (c-PIB) has been used in PET imaging as a marker of amyloid deposition. Unfortunately, amyloid deposition in the initial stage of AD has not been associated with cognitive decline. Besides, reduction in the levels of Aβ (amyloid-beta) through amyloid immunization has not been associated with improved cognition.
Beyond neuroimaging, early AD markers have been sought in the cerebrospinal fluid. Decreased cerebrospinal fluid (CSF) Aβ42 levels and increased tau levels have both been associated with increased brain atrophy in multiple regions. Others have associated such atrophy with CSF apolipoprotein E (ApoE) levels. These CSF markers may thus be of some utility in the early detection of AD.
Several important questions still remain. Among all these candidate markers, is there one, if any, that reliably identifies early stages of AD? Moreover, will the sensitivity and specificity of these markers being evaluated in recent studies reveal important evidence toward more specific criteria to glimpse the disease progress timeline? Combination of several imaging modalities may be advantageous for early detection as well. Overall, however, numerous studies lend support to the consensus that AD is a progressive disease with functional abnormalities preceding structural findings, beginning with the precuneus and posterior parietal lobules.[8,37]
DBS IN AD
DBS is a proven therapy utilized for several neurological and psychiatric indications. It was initially found to be beneficial in reducing essential and lately Parkinsonian tremor and other cardinal symptoms of the disease. This result was then extended to treat multiple other conditions, including primary dystonia and OCD, and is in clinical trials for intractable pain, obesity, epilepsy, and restless legs syndrome.[5,8,14,18] Based on the effect of DBS on the neural circuitry linking in these systems-level disorders, its potential utility has been translated to AD.
Some investigators have examined the hypothesis that modulation and modification of memory circuits may be possible through implanted electrodes.[2] Rodent studies have examined the role of DBS in improving memory, and found that stimulation of the fornix can induce hippocampal neurogenesis, as detectable on histology using brain-derived neurotrophic factor (BDNF) staining.[2,13] Other animal studies have shown that electrical stimulation of the Papez circuit can reverse memory impairment while also stimulating hippocampal neurogenesis.[2,14]
Based on these promising animal results and improved cognitive scores in one patient undergoing DBS for obesity, Dr. Laxton and colleagues recently conducted an open-label study of DBS for AD.[20] Six patients with early AD were treated with DBS of the fornix bilaterally, over 1 year, monitoring both neuropsychological assessments and FDG-PET scans. Clinical evaluation with the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-COG) and the Mini Mental State Examination (MMSE) suggested a decrease in the rate of cognitive decline. The follow-up PET studies after 1 year demonstrated an increase in cerebral glucose metabolism that was subsequently associated with improved cognitive status. This result is rather encouraging, but it must be interpreted with caution as the study was a small phase I open-label trial conducted without placebo control.[20]
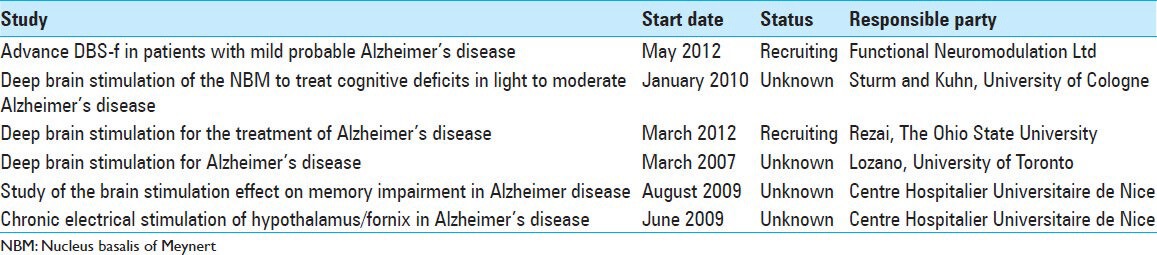
Currently, there are six clinical trials for DBS in AD that are listed by The National Institutes Health of Clinical Trial registry (http://www.clinicaltrials.gov) [Table 1]. Notably, one of these is a double-blind (stimulation on/off) design, which should prove to be very informative in evaluating the effectiveness of DBS as a therapeutic technique in AD.
Table 1.
Clinical trials involving deep brain stimulation for Alzheimer's disease
POTENTIAL TARGETS FOR DBS IN AD
Neuroimaging studies in AD have demonstrated widespread structural and metabolic abnormalities, principally in areas associated with memory functions, as described above.[24] As recent data suggest systems-level defects characterized by alterations in memory circuits, it is reasonable to expect that external stimulation of subareas within the circuitry will ameliorate symptoms. Ultimately, restoration of functional connectivity and induction of hippocampal neurogenesis may be triggered by DBS as well. In this section, we summarize possible DBS targets based upon a review of the literature.[6,25,31,39]
These sites are presented in Figures 1 and 2. The anterior thalamic nucleus (AN) is an important relay in the Papez circuit. Previous studies in rats have shown that AN stimulation at relatively high current disrupted the acquisition of contextual fear conditioning and impaired performance on a spatial alternating task. Stimulation of this area also modulates regional cerebral metabolism as detected by FDG-microPET.[12]
Other investigators argue that DBS of the nucleus basalis of Meynert (NBM) should improve or stabilize memory and cognitive functioning in patients with AD. The hypothesized mechanism involves a facilitation of neural oscillations and the synthesis of NGFs.[13]
Medial temporal structures, including the hippocampus and the entorhinal cortex [Figure 2], have long been known to be important in memory formation and recall.[32] Previous studies in refractory epileptic patients have shown that entorhinal stimulation enhanced the memory of spatial information when applied during learning. These findings suggest that as the major relay of cortical afferent input into the hippocampus, the entorhinal cortex may be the preferable location of DBS electrode placement for memory enhancement.[32,35]
The CA1 region of the hippocampus is another interesting target [Figure 2]. An experimental mouse model study showed that application of high-frequency stimulation to isolated hippocampal slices significantly increased synaptic plasticity in the CA1 region and promoted a twofold increase of non-amyloidogenic α-secretase activity, when compared to low-frequency stimulated controls from TgCRND8 mice. In other studies, DBS treatment has been shown to facilitate acquisition of object recognition memory in TgCRND8 mice, relative to their own baseline score before treatment. These results suggest that DBS of the CA1 region may enhance short-term memory in AD, mainly knowing that this region is reachable for implant in humans using advanced imaging and stereotactic techniques.
NGF IN BASAL FOREBRAIN FOR AD
NGF is a member of neurotrophin family that plays a role in cholinergic synaptic remodeling in the adult CNS. NGF-producing cells are present in basal forebrain neurons. Neurotrophins influence the survival and death of neuronal and non-neuronal cells. ECB is a combination of gene therapy and device technology that aims the development of a new treatment modality. There is a line of thought supporting the hypothesis that progressive degeneration of forebrain neurons is due to loss of neurotrophic factors available in this region. The exact process leading to neuronal loss is not understood, but deficits in NGF have been proved. Animal studies have shown that NGF has a positive effect in neuronal survival and can even promote cholinergic re-innervation of the frontal cortex. Whether or not NGF injections translate into clinical effects during the course of the disease is yet to be established.[4,34]
Eriksdotter-Jonhagen et al. used intraventricular infusion of NGF in three patients and observed electroencephalographic (EEG) normalization and glucose metabolism increase in the brain. However, patients developed spinal pain and other side effects, obviating further use of this delivery method of NGF in clinical trials.[10] Wahlberg et al. demonstrated that surgical implantation and removal of EGF-NGF in basal forebrain in AD patients is safe, well tolerated, and feasible.[36] The EGF-NGF may become an important therapeutic option within the neurosurgery arsenal. However, NGFs’ effects on cognitive function improvement and AD progression delay remain to be proven in phase II and III trials.
NEW TRENDS IN AD
The treatment options for AD followed until now lead to limited benefits. Research efforts that focused on antiamyloid therapies have been largely unsuccessful, as amyloid deposition appears to be a byproduct of the disease evolution, instead of being a causal factor. Currently, efforts are being directed toward development of new biochemical markers that can be used in neuroimaging, aiming to establish the objective criteria for early, or even preclinical, diagnosis of AD. Early diagnosis becomes important, as the therapies being tried likely could delay the progression of the disease. DBS and ECB implants that deliver NGF represent a novel and attractive strategy for treating this devastating illness and for possibly bringing insights into the pathophysiology of AD.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/9/385/140191
Contributor Information
Julio Leonardo Barbosa Pereira, Email: julio.pereira@me.com.
Angela Downes, Email: angelamd@gmail.com.
Alessandra Gorgulho, Email: a_gorgulho@yahoo.com.
Vishal Patel, Email: vnpatel@mednet.ucla.edu.
Dennis Malkasian, Email: dennis.malkasian@sbcglobal.net.
Antonio De Salles, Email: afdesalles@yahoo.com.
REFERENCES
- 1.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: A potential outcome measure in Alzheimer's disease treatment studies. Am J Psychiatry. 2002;159:738–45. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 2.Arrieta-Cruz I, Pavlides C, Pasinetti GM. Deep brain stimulation in the midline thalamic region facilitates short-term memory in a mouse model of Alzheimer's disease. Transl Neurosci. 2010;1:188–94. doi: 10.2478/v10134-010-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–31. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 4.Bishop KM, Hofer EK, Mehta A, Ramirez A, Sun L, Tuszynski M, et al. Therapeutic potential of CERE-110 (AAV2-NGF): Targeted, stable, and sustained NGF delivery and trophic activity on rodent basal forebrain cholinergic neurons. Exp Neurol. 2008;211:574–84. doi: 10.1016/j.expneurol.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL, et al. National estimates of the prevalence of Alzheimer's disease in the United States. Alzheimers Dement. 2011;7:61–73. doi: 10.1016/j.jalz.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol Aging. 2012;33:828e19–30. doi: 10.1016/j.neurobiolaging.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Salles AA, Frighetto L, Behnke E, Sinha S, Tseng L, Torres R, et al. Functional Neurosurgery in the MRI Environment. Minim Invasive Neurosurg. 2004;47:284–9. doi: 10.1055/s-2004-830094. [DOI] [PubMed] [Google Scholar]
- 10.Eriksdotter Jönhagen M, Nordberg A, Amberla K, Bäckman L, Ebendal T, Meyerson B, et al. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 1998;9:246–57. doi: 10.1159/000017069. [DOI] [PubMed] [Google Scholar]
- 11.Fjord-Larsen L, Kusk P, Tornøe J, Juliusson B, Torp M, Bjarkam CR, et al. Long-term delivery of nerve growth factor by encapsulated cell biodelivery in the Göttingen minipig basal forebrain. Mol Ther. 2010;18:2164–72. doi: 10.1038/mt.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao F, Guo Y, Zhang H, Wang S, Wang J, Wu JM, et al. Anterior thalamic nucleus stimulation modulates regional cerebral metabolism: An FDG-microPET study in rats. Neurobiol Dis. 2009;34:477–83. doi: 10.1016/j.nbd.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor MR imaging study. AJNR Am J Neuroradiol. 2007;28:226–35. [PMC free article] [PubMed] [Google Scholar]
- 14.Hamani C, Stone SS, Garten A, Lozano AM, Winocur G. Memory rescue and enhanced neurogenesis following electrical stimulation of the anterior thalamus in rats treated with corticosterone. Exp Neurol. 2011;232:100–4. doi: 10.1016/j.expneurol.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Hahn K, Myers N, Prigarin S, Rodenacker K, Kurz A, Förstl H, et al. Selectively and progressively disrupted structural connectivity of functional brain networks in Alzheimer's disease-Revealed by a novel framework to analyze edge distributions of networks detecting disruptions with strong statistical evidence. Neuroimage. 2013;81:96–109. doi: 10.1016/j.neuroimage.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Hardy J. Alzheimer's disease: The amyloid cascade hypothesis: An update and reappraisal. J Alzheimers Dis. 2006;9(3 Suppl):S151–3. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- 17.Hescham S, Lim LW, Jahanshahi A, Steinbusch HW, Prickaerts J, Blokland A, Temel Y. Deep brain stimulation of the forniceal area enhances memory functions in experimental dementia: The role of stimulation parameters. Brain Stimul. 2012;6:72–7. doi: 10.1016/j.brs.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: Implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacan G, De Salles AA, Gorgulho AA, Krahl SE, Frighetto L, Behnke EJ, et al. Modulation of food intake following deep brain stimulation of the ventromedial hypothalamus in the vervet monkey. Laboratory investigation. J Neurosurg. 2008;108:336–42. doi: 10.3171/JNS/2008/108/2/0336. [DOI] [PubMed] [Google Scholar]
- 20.Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010;68:521–34. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 21.Lyketsos CG, Targum SD, Pendergrass JC, Lozano AM. Deep brain stimulation: A novel strategy for treating Alzheimer's Disease. Innov Clin Neurosci. 2012;9:10–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Nagahara AH, Bernot T, Moseanko R, Brignolo L, Blesch A, Conner JM, et al. Long-term reversal of cholinergic neuronal decline in aged non-human primates by lentiviral NGF gene delivery. Exp Neurol. 2009;215:153–9. doi: 10.1016/j.expneurol.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niewiadomska G, Mietelska-Porowska A, Mazurkiewicz M. The cholinergic system, nerve growth factor and the cytoskeleton. Behav Brain Res. 2011;221:515–26. doi: 10.1016/j.bbr.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Jack CR., Jr Imaging and biomarkers in early alzheimer's disease and mild cognitive impairment. Clin Pharmacol Ther. 2009;86:438–41. doi: 10.1038/clpt.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pouratian N, Zheng Z, Bari AA, Behnke E, Elias WJ, Desalles AA. Multi-institutional evaluation of deep brain stimulation targeting using probabilistic connectivity-based thalamic segmentation. J Neurosurg. 2011;115:995–1004. doi: 10.3171/2011.7.JNS11250. [DOI] [PubMed] [Google Scholar]
- 26.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. NeuroImage. 2007;37:1083–90. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 27.Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC Alzheimer's Disease Neuroimaging Initiative(ADNI) Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6:347–61. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith GS, de Leon MJ, George AE, Kluger A, Volkow ND, McRae T, et al. Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer's disease. Pathophysiologic implications. Arch Neurol. 1992;49:1142–50. doi: 10.1001/archneur.1992.00530350056020. [DOI] [PubMed] [Google Scholar]
- 29.Smith GS, Laxton AW, Tang-Wai DF, McAndrews MP, Diaconescu AO, Workman CI, et al. Increased cerebral metabolism after 1 year of deep brain stimulation in Alzheimer Disease. Arch Neurol. 2012;69:1141–8. doi: 10.1001/archneurol.2012.590. [DOI] [PubMed] [Google Scholar]
- 30.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, et al. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–10. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trojanowski JQ, Vandeerstichele H, Korecka M, Clark CM, Aisen PS, Petersen RC, et al. Update on the biomarker core of the Alzheimer's Disease neuroimaging initiative subjects. Alzheimers Dement. 2010;6:230–8. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–5. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 35.Vemuri P, Jones DT, Jack CR., Jr Resting state functional MRI in Alzheimer's Disease. Alzheimers Res Ther. 2012;4:2. doi: 10.1186/alzrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahlberg LU, Lind G, Almqvist PM, Kusk P, Tornoe J, Juliusson B, et al. Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: A technology platform for restorative neurosurgery. J Neurosurg. 2012;117:340–7. doi: 10.3171/2012.2.JNS11714. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Zuo X, Dai Z, Xia M, Zhao Z, Zhao X, et al. Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol Psychiatry. 2013;73:472–81. doi: 10.1016/j.biopsych.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Weiner MW, Aisen PS, Jack CR, Jr, Jagust WJ, Trojanowski JQ, Shaw L, et al. The Alzheimer's disease neuroimaging initiative: Progress report and future plans. Alzheimers Dement. 2010;6:202–11e7. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer's Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement. 2012;8(1 Suppl):S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]


