Abstract
Background:
Hardware failure or malfunction after deep brain stimulation is an infrequent but costly occurrence with currently available systems.
Case Description:
The authors present the case of a 65-year-old female patient with predominantly tremoric Parkinson's disease who, 4 months after bilateral subthalamic nucleus stimulation with very good clinical results, began to display signs of recurrent disease and an increasingly smaller response to stimulation. Radiological studies, changes in electrode impedance and surgical findings and results established the diagnosis of Twiddler syndrome. Close patient follow-up, lack of a psychiatric history and physical examination findings were, however, contrary to the previously described causative mechanism.
Conclusion:
The clinical and radiological setup of Twiddler syndrome must be readily recognized. Its causative mechanism should remain under discussion, and intraoperative technical details may help to explain its occurrence.
Keywords: Deep brain stimulation, dysfunction, failure, hardware, surgery
INTRODUCTION
Nowadays, deep brain stimulation represents safe,[2] cost-effective[5] standard of care in medically refractory and debilitating conditions such as Parkinson's disease, tremor, or dystonia.
CASE REPORT
The authors present the case of a 65-year-old female patient with a predominantly tremoric form of idiopathic Parkinson's disease, with symptoms developing throughout an 11-year period. No relevant psychiatric history could be found, namely personal or family history of obsessive-compulsive disorder, except for a mild and medically compensated form of reactive depression.
The patient underwent bilateral subthalamic nucleus (STN) stimulation surgery in October 2011, under fluoroscopic control, and no intercurrences were registered. As usual in our department,[13] the implanted pulse generator (IPG) was placed in a left subclavicular pouch and sutured to the underlying muscle fascia tissue. No proximal stitches, namely galeal fixation, were part of the procedure. Functional results were quite favorable (UPDRS III: Preoperative score of 47, postoperative score of 7) allowing for a significant reduction in medical therapy. The patient was discharged with no evidence of surgical complication, and the usual written suggestions to avoid extreme exertion and contact sports.
Repeated medical visits included surgical wound inspection, and no sign of skin manipulation could be found. Four months after surgery, the patient started to display signs of recurrent disease, with an increasingly smaller response to stimulation. Over the following 3 months, the clinical condition eventually resumed preoperative status - by May 2012, on medication, UPDRS III score was 43 regardless of stimulation, which raised suspicion toward hardware failure. X-ray studies of the system displayed “twisted” extensor leads and an evident disconnection between these and the distally migrated electrode lead connections [Figure 1]. Proximally to the slightly rotated IPG, the extensors seemed to “curl”, however, the electrode leads were spared [Figure 1]. A change in electrode impedance was also identified, with persistent abnormally high readings.
Figure 1.
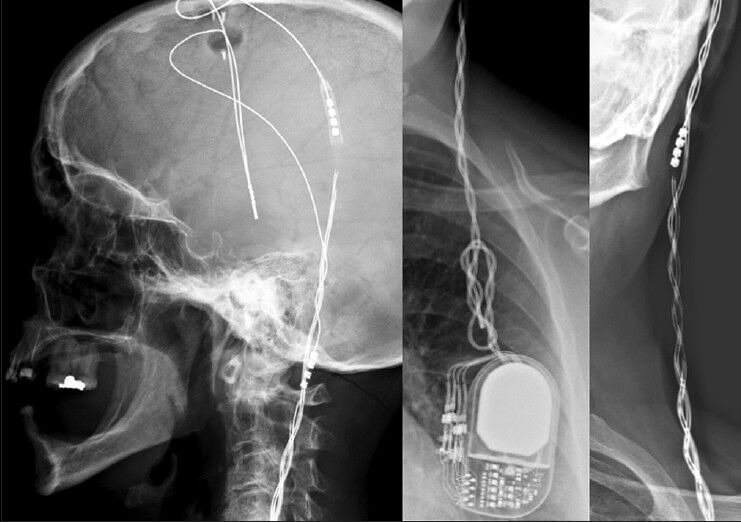
Preoperative characteristic radiological findings; please notice the evident distal migration of the lead connections, and how the proximal leads are spared from “curling”
The patient denied any active or passive hardware mobilization. She also denied feeling any tenderness throughout the extension path, except for a retroauricular “tightening” sensation. No other plausible physical causative factor could be identified, namely IPG rotation induced by upper limb movement.
She underwent revision surgery in June 2012, which clearly confirmed the radiologic findings: Cranially, the leads were no longer in contact inside the connector, and upon pectoral pouch opening extensor leads were clearly twisted and curling around the IPG [Figure 2]. A total of 17 full rotations were necessary for those to straighten fully. Electrode function was confirmed and the damaged extensor leads were replaced. Similar functional results as those previously obtained were achieved.
Figure 2.
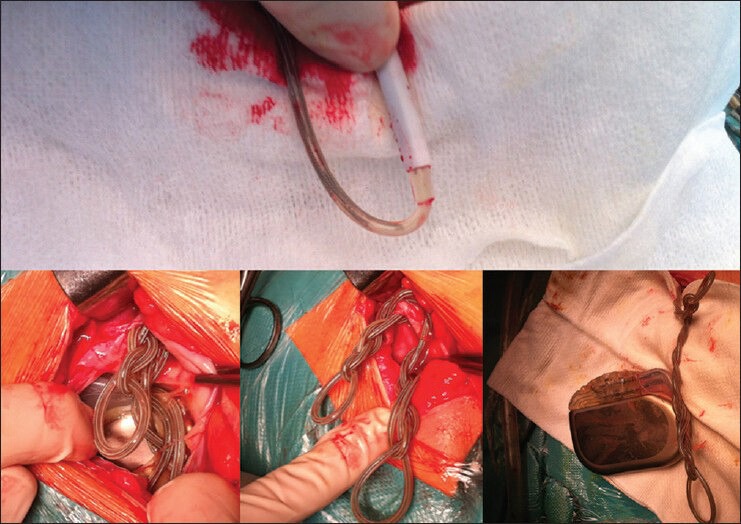
Intraoperative findings after exposure of the assembly: Distally migrated broken up connection leads and severely convoluted connection leads near the IPG
DISCUSSION
Twiddler's syndrome represents an uncommon form of hardware mobilization and subsequent dysfunction in which a patient (voluntarily or involuntarily) induces rotation of the IPG and/or the extensor leads in a way that provokes their detachment proximally. Its name derives from the active direct manipulation of hardware.
Initially described in Cardiology patients in the 1970s, it became most frequently cited as a cause of dysfunction in patients submitted to pacemaker[3,6] or cardioverter-defibrillator[10] placement surgery. A history of psychiatric disease (namely obsessive-compulsive disorder[12]), obesity, and higher age were described as risk factors.[1] Only recently has this entity been transposed to the neurosurgical field. This common ground between Cardiology and Neurology/Neurosurgery patients is easily explained by similar technology and surgical principles.
Few neurosurgical patient cases have been described and include different index pathologies.[1,7,9,14] The most frequent clinical setup is that of early[4] or late[8] clinical recurrence, with or without local pain or tenderness, after a successful primary surgical intervention. The absence of response or abnormal response to stimulation, coupled with altered electrode impedances, particularly in the presence of risk factors and/or evidence of IPG dislocation, should raise the suspicion of a hardware issue, and a radiological study becomes mandatory. Simple lead disconnections will most frequently be found, but the specific X-ray finding of “coiled” extensions, proximal disconnection and rotated IPG establishes the diagnosis.
Treatment for Twiddler's syndrome is a synonym with revision surgery,[4] which will allow for favorable outcome.[1,4,7,8,14] Direct revision of pectoral pouch suture may be enough,[1] but in other cases system damage may require lead (as in our case) or even electrode replacement.[8]
Even though a clear causative factor is sometimes found,[8] in our case we were left with no evidence of patient foul play. The progressive nature of the clinical worsening allowed for questions to be asked specifically toward the issue of hardware trauma and/or manipulation at that time, and evident concern on the patient's part underlined how foreign the matter seemed to her. She was under a regular gym plan at the time, which involved some upper torso movement, but she denied any unusual occurrence. Actually, the lack of evidence of voluntary or involuntary, active or passive manipulation is more frequent than the contrary in previously described cases,[1,7] which should be noted. Furthermore, in all but one patient,[4] no relevant psychiatric history is described. Considering most deep brain stimulation programs support the need for preoperative neuropsychological assessment, there is probably a greater knowledge about these patients’ psychological condition and motivation for treatment than in most other neurosurgical fields, which further substantiates these data.
Another previously described risk factor is obesity, which stands as a surrogate for greater subcutaneous space for IPG mobilization; in our case, although the extensors did curl around the IPG, there was limited space surrounding it and there was actually the need to widen the pouch to comfortably reintroduce the hardware. It is difficult to imagine such a high number of full IPG rotations (at least 17) to go unnoticed in a nonobese patient.
A greater emphasis should probably be put on surgery technical nuances. The fact that no damage could be proven upon the proximal electrodes (uncurled and functioning) suggests that the primary mechanism should be mostly distal to them. Even if this is our first occurrence in over 200 cases, our technical option to not fixate the proximal assembly does in practice allow for distal migration of the lead connection and greater physical stress during regular head and cervical movements. This might explain the passive rotation of the connector leads and eventual fracture, even if the bolts are tightly screwed. In our opinion, to protect the proximal assembly by galeal suture is safe and should always protect the intracranial leads, the most precious part of the system. Alternatively, subpectoral pocket placement has been described to try to prevent or solve Twiddler syndrome,[4,11] but reports questioning its usefulness[15] suggest that the issue may be found more proximally.
Increasing numbers in deep brain stimulation surgery will likely allow for Twiddler's syndrome to surface and be further studied as a relevant and benign (if expensive) cause of system failure and recurrent symptoms in a previously improved patient, and in our opinion its proposed etiology should not go unquestioned.
CONCLUSIONS
It is vital today to recognize the clinical setup and the peculiar radiological appearance of Twiddler's syndrome in order to avoid further system damage and improve time to surgical revision. Available patient descriptions, as is the case, suggest that more must be known about its causative mechanisms and risk factors. Intraoperative technical options may help to explain its occurrence.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/9/410/140201
Contributor Information
Pedro Alberto Silva, Email: pedroalbertosilva.neurocirurgia@gmail.com.
Clara Chamadoira, Email: clarachamadoira@gmail.com.
Henrique Costa, Email: henriq.costa@gmail.com.
Paulo Linhares, Email: paulojlinhares@yahoo.com.
Maria José Rosas, Email: rosa.mariajose@gmail.com.
Rui Vaz, Email: ruimcvaz@gmail.com.
REFERENCES
- 1.Astradsson A, Schweder PM, Joint C, Green AL, Aziz TZ. Twiddler's syndrome in a patient with a deep brain stimulation device for generalized dystonia. J Clin Neurosci. 2011;18:970–2. doi: 10.1016/j.jocn.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Baizabal Carvallo JF, Mostile G, Almaguer M, Davidson A, Simpson R, Jankovic J. Deep brain stimulation hardware complications in patients with movement disorders: Risk factors and clinical correlations. Stereotact Funct Neurosurg. 2012;90:300–6. doi: 10.1159/000338222. [DOI] [PubMed] [Google Scholar]
- 3.Berul CI, Hill SL, Estes NA., 3rd A teenager with pacemaker twiddler syndrome. J Pediatr. 1997;131:496–7. [PubMed] [Google Scholar]
- 4.Burdick AP, Okun MS, Haq IU, Ward HE, Bova F, Jacobson CE, et al. Prevalence of Twiddler's syndrome as a cause of deep brain stimulation hardware failure. Stereotact Funct Neurosurg. 2010;88:353–9. doi: 10.1159/000319039. [DOI] [PubMed] [Google Scholar]
- 5.Dams J, Siebert U, Bornschein B, Volkmann J, Deuschl G, Oertel WH, et al. Cost-effectiveness of deep brain stimulation in patients with Parkinson's disease. Mov Disord. 2013;28:763–71. doi: 10.1002/mds.25407. [DOI] [PubMed] [Google Scholar]
- 6.Fahraeus T, Hoijer CJ. Early pacemaker twiddler syndrome. Europace. 2003;5:279–81. doi: 10.1016/s1099-5129(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 7.Geissinger G, Neal JH. Spontaneous twiddler's syndrome in a patient with a deep brain stimulator. Surg Neurol. 2007;68:454–6. doi: 10.1016/j.surneu.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 8.Gelabert-Gonzalez M, Relova-Quinteiro JL, Castro-Garcia A. “Twiddler syndrome” in two patients with deep brain stimulation. Acta Neurochir (Wien) 2010;152:489–91. doi: 10.1007/s00701-009-0366-6. [DOI] [PubMed] [Google Scholar]
- 9.Grapsa J, Koa-Wing M, Fox KF. Fiddling with the pacemaker: Twiddler's syndrome in a parkinsonian patient. Perfusion. 2013;28:31–3. doi: 10.1177/0267659112461161. [DOI] [PubMed] [Google Scholar]
- 10.Gronefeld G, Kleine P, Israel CW, Hohnloser SH. “Twiddler syndrome” in a subpectorally implanted cardioverter defibrillator. J Cardiovasc Electrophysiol. 2002;13:94. doi: 10.1046/j.1540-8167.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 11.Israel Z, Spivak A. A tremulous twiddler. Stereotact Funct Neurosurg. 2008;86:297–9. doi: 10.1159/000155231. [DOI] [PubMed] [Google Scholar]
- 12.Jaafari N, Bachollet MS, Paillot C, Amiel A, Rotge JY, Lafay N, et al. Obsessive compulsive disorder in a patient with twiddler's syndrome. Pacing Clin Electrophysiol. 2009;32:399–402. doi: 10.1111/j.1540-8159.2008.02251.x. [DOI] [PubMed] [Google Scholar]
- 13.Linhares P, Carvalho B, Vaz R. One-step tunneling of DBS extensions-A technical note. Acta Neurochirur (Wien) 2013;155:837–40. doi: 10.1007/s00701-013-1667-3. [DOI] [PubMed] [Google Scholar]
- 14.Penn DL, Wu C, Skidmore C, Sperling MR, Sharan AD. Twiddler's syndrome in a patient with epilepsy treated with deep brain stimulation. Epilepsia. 2012;53:e119–21. doi: 10.1111/j.1528-1167.2012.03489.x. [DOI] [PubMed] [Google Scholar]
- 15.Udink Ten Cate FE, Adelmann R, Schmidt BE, Sreeram N. Use of an active fixation lead and a subpectoral pacemaker pocket may not avoid Twiddler's syndrome. Ann Pediatr Cardiol. 2012;5:203–4. doi: 10.4103/0974-2069.99629. [DOI] [PMC free article] [PubMed] [Google Scholar]


