Abstract
Aim:
This study was to identify the safety and efficacy of adding epidural N-methyl-D-aspartate receptor antagonists (ketamine) to oral gabapentin for the treatment of post spinal cord injury-related chronic pain.
Materials and Methods:
Forty patients in the age range of 18–50 years with a diagnosis of neuropathic pain secondary to spinal cord injury were randomized into two equal groups. Group I received 0.2 mg/Kg of preservative-free ketamine (2 ml) single bolus epidural injection and gabapentin 300 mg three times daily. Group II received isotonic saline 0.9% (2 ml) single bolus epidural injection and gabapentin 300 mg three times daily. Pain scores were evaluated pre-injection, 7, 15, 30,45 and 60 days post injection. Patients were asked about any side-effects occurred during follow-up period.
Results:
At all time points examined, pain scores were significantly lower in both groups than pre-injection values (P < 0.0001). Pain scores were significantly lower in Group I than in Group II at 7, 15, 30 days after injection (P 0.02, < 0.0001, =0.0001 respectively), but no statistically significant difference was detected between groups at 45, 60 days post injection (P = 0.54, =0.25), there was no statistically significant difference regarding incidence of side-effects in both groups.
Conclusion:
Epidurally administrated ketamine seems to be a safe adjunct to gabapentin in post spinal cord injury-related chronic pain. However, its analgesic efficacy was limited to 30 days after injection.
Keywords: Epidural, gabapentine, ketamine, spinal injury
INTRODUCTION
Persistent severe neuropathic pain after spinal cord injury is a significant management problem for those involved in care of patients.[1] However, the mechanism of post-spinal cord injury neuropathic pain remains obscure. Recent clinical studies revealed that central sensitization of the nociceptive input is in part mediated by excitatory amino acids glutamate and aspartate which act at the N- methyl-D- aspartate (NMDA) receptors[2] and these have been speculated to contribute to a permanent hyperexcitability state in the pain pathway. During inflammation or injury there is increased glutamate and aspartate release in the spinal dorsal horn and increased synthesis of neurokinins in sensory ganglion as well as increase in their transport and release in the spinal cord that will stimulate central sensitization and wind up. Wind up can augment reaction of dorsal horn neurones up to 20-fold in magnitude and duration. This state of central hyperexcitability may continue after stoppage of the peripheral input and can be considered as temporal summation of excitatory postsynaptic potentials.[3,4] Gabapentin is commonly used for treatment of neuropathic pain.[5] Gabapentin has a high binding capacity for the α2δ subunit of the presynaptic voltage-gated calcium channels[6] which inhibits calcium influx and subsequent release of excitatory neurotransmitters in the pain pathways.
This study aimed to evaluate the effect of adding epidurally administrated NMDA receptor antagonists (ketamine) to oral anticonvulsant (gabapentin) for treatment of chronic neuropathic pain secondary to spinal cord injury.
MATERIALS AND METHODS
Approval was obtained from the institutional ethical committee and written informed consent from patients. This study was carried out on 40 patients aged 18–50 years with a diagnosis of post-spinal cord injury (lower dorsal and lumbar) pain as confirmed by magnetic resonance imaging (MRI) or computed tomography (CT). Duration of symptoms was more than six months in all patients. Patients were asked about any side-effects occurred during follow-up period. The process of inclusion into the study went on until the target number of patients was reached.
Patients with previous chronic anticoagulation therapy, coagulation disorders, infection in the back, bed sores, spine deformity, hepatic or renal impairment were excluded from the study. A prospective, randomized (sealed envelopes indicate the group of assignment), double-blind design was used, with both patients and post-injection caregivers and assessors blinded to management protocol.
All patients were premedicated with IM Midazolam 0.05 mg / Kg, and monitored with noninvasive arterial blood pressure, electrocardiography (ECG), and pulse oximetry. The patients were randomized into two equal groups. Group I received 0.2 mg/Kg of preservative -ree ketamine (2 ml) single bolus through epidural injection and gabapentin 300 mg three times per day. Group II received isotonic saline 0.9% (2 ml) single bolus epidural injection and gabapentin 300 mg three times per day. The epidural space was identified by loss of resistance to saline and confirmed radiologically (C- arm) by the characteristic longitudinal vacuolated spread of dye (Isovest 300) in the epidural space. Pain scores were obtained pre-injection, 7, 15, 30, 45 and 60 days post injection. Patients were also asked to report any side-effects related to ketamine (blurred vision, confusion, drowsiness, increased blood pressure or heart rate, mental or mood changes, nausea, nightmares, vomiting) or gabapentin (dizziness, drowsiness, weakness, tired feeling, lack of coordination, blurred vision, nausea, vomiting, stomach pain, loss of appetite).
Statistical analysis
Data were presented in the form of mean ± S.D. Comparison of data parameters regarding patient characteristics was performed using student t-test. Mann-Whitney-U test was used to compare between the two groups regarding pain scores. Power of significance was considered significant if P<0.05.
RESULTS
The study population consisted of 40 patients. Patient characteristics were similar in the two groups [Table 1].
Table 1.
Patient characteristics in both groups
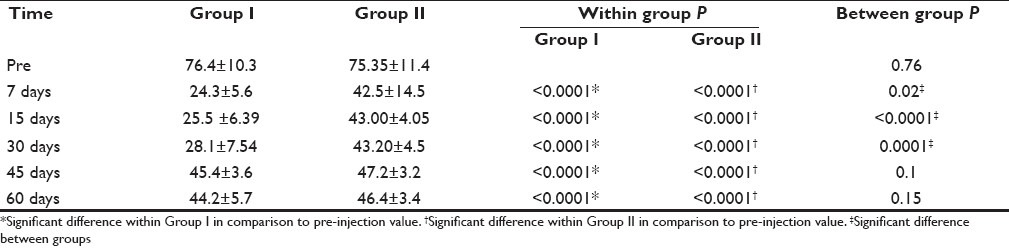
At all time periods examined [Table 2], pain scores were significantly lower in both groups than pre-injection (P<0.0001). The pain scores were significantly lower in Group I than in Group II at 7, 15, 30 days after injection (P 0.02, <0.0001,0.0001 respectively) but there was no statistically significant difference between groups at 45, 60 days (P=0.1, 0.15). No detectable side-effects were reported from ketamine epidural injection. Regarding gabapentin side-effects six patients experienced feeling of tiredness and lack of coordination which lasted for a few days after starting medication.
Table 2.
Visual analogue scale for pain before treatment, 7, 15, 30, 45 and 60 days post injection in Group I and Group II
DISCUSSION
In the present study, the analgesia induced by addition of epidural ketamine to gabapentin was better and sustained for one month after injection when compared to that in the group of gabapentin alone. The long potency was explained by blocking of wind-up of dorsal horn by NMDA receptor antagonists.[7] Interestingly,[8] a study carried out by insertion of epidural catheter in patients with low back pain and ketamine in a dose of 0.1 mg/kg followed by 30 mg lidocaine 1% applied three times daily (8-h interval) for three weeks, concluded that epidural ketamine compared to clonidine was effective for control of refractory chronic low back pain. On comparing post-injection to pre-injection VAS in that study, the VAS was maintained between 0-3 cm during epidural ketamine administration and for two to five weeks following epidural catheter removal, no side-effects for ketamine were recorded.
Another study[9] revealed that pain scores decreased after single slow intravenous push of ketamine at 250 mcg/Kg in patients with chronic neuropathic pain.
Intrathecal ketamine[10] was reported as a successful treatment for non-malignant pain. Also, another study[11] reported that although the evidence of ketamine for the treatment of chronic pain is moderate to weak, ketamine is successfully used in situations where standard analgesic options have failed. Block of NMDA receptors may lead to unwanted psychotomimetic and hemodynamic effects, which limits the use of high dose.[12] The side-effects during single, low-dose injections were mild and well tolerated. In the present study, no side-effects attributed to ketamine were reported.
Intravenous application of low-doses ketamine did not affect the maintenance of established long-term potentiation when used without opioids in a rat model.[13] This finding reflects the clinical situation where the use of NMDA receptor antagonist in the treatment of some forms of established chronic pain is unsatisfactory.-[14] On the contrary, another study[15] has revealed that mechanical allodynia is reduced after administration of intrathecal ketamine in a rat model, probably by preventing sensitization in the spinal cord. However, this effect continued for at least two weeks but diminished after one month.
The author's previous published study examining the effect of adding a multi-day low-dose ketamine intravenous infusion to oral gabapentin for treating chronic post-spinal cord injury pain, reported the drug was safe and efficacious in reducing pain, however, the analgesic effect ceased two weeks after termination of infusion.[16] Meanwhile, in the present study, there was a longer potency (one month post injection) for epidural ketamine.
There are many limitations in our study, the number of injections (single injection) may have been too low to produce an effect for a long term. We did not study the effect on pain which occurred before six months. This was a small clinical study in a highly selected patient population.
CONCLUSION
Though epidurally administrated ketamine may be a safe adjunct to gabapentin in post-spinal cord injury-related chronic pain, its effect was limited to one month after single injection.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Loubser PG, Akman NM. Effects of intrathecal baclofen chronic spinal cord injury pain. J Pain Symptom Manage. 1996;12:241–7. doi: 10.1016/0885-3924(96)00152-2. [DOI] [PubMed] [Google Scholar]
- 2.Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: A quantitative and qualitative systematic review. Anesth Analg. 2004;99:482–95. doi: 10.1213/01.ANE.0000118109.12855.07. [DOI] [PubMed] [Google Scholar]
- 3.Pockett S. Spinal cord synaptic plasticity and chronic pain. Anesth Analg. 1995;80:173–9. doi: 10.1097/00000539-199501000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Sandkuhler J. Learning and memory in pain pathways. Pain. 2000;88:113–8. doi: 10.1016/S0304-3959(00)00424-3. [DOI] [PubMed] [Google Scholar]
- 5.Dirks J, Fredensborg BB, Christensen D, Fomsgaard JS, Flyger H, Dahl JB. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology. 2002;97:560–4. doi: 10.1097/00000542-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, et al. Injury type-specific calcium channel alpha2delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmcol Exp Ther. 2002;303:1199–205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- 7.Warnke T, Stubhaug A, Jorum E. Ketamine, an NMDA receptor antagonist suppresses spatial and temporal properties of burn induced hyperalgesia in man: A double blind, cross over comparison with morphine and placebo. Pain. 1999;76:357–63. doi: 10.1016/s0304-3959(97)00006-7. [DOI] [PubMed] [Google Scholar]
- 8.Lauretti GR, Rodrigues AM, Reis MP. Epidural Ketamine versus epidural clonidine as therapeutics for refractory chronic low back pain. Reg. Anesth Pain Med. 2001;26:88. [Google Scholar]
- 9.Backonja M, Arndt G, Gombar KA, Check B, Zimmermann M. Response of chronic neuropathic pain syndromes to Ketamine: A preliminary study. Pain. 1994;58:433. doi: 10.1016/0304-3959(94)90149-X. [DOI] [PubMed] [Google Scholar]
- 10.Sotar-Katzenschler S, Deusch E, Maier P, Spacek A, Kress HG. The long-term antinociceptive effect of intrathecal S(+) Ketamine in patient with established morphine tolerance. Anesth Analg. 2001;93:1032–4. doi: 10.1097/00000539-200110000-00047. [DOI] [PubMed] [Google Scholar]
- 11.Hewtti DJ. The use of NMDA – receptor antagonists in the treatment of chronic pain. Clin J Pain. 2000;16:73–79. doi: 10.1097/00002508-200006001-00013. [DOI] [PubMed] [Google Scholar]
- 12.Fisher K, Coderre TJ, Hagen NA. Targetting the N methyl D aspartate receptor for chronic pain mangement.Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000;20:358–73. doi: 10.1016/s0885-3924(00)00213-x. [DOI] [PubMed] [Google Scholar]
- 13.Benrath J, Brecntel C, Stark J, Sandkuhler J. Low dose of S (+) Ketamine prevents long term potentiation in pain pathways under strong opioid analgesia in the rat spinal cord in vivo. Br J Anaesth. 2005;95:518–35. doi: 10.1093/bja/aei215. [DOI] [PubMed] [Google Scholar]
- 14.Hocking G, Cousins MJ. Ketamine in chronic pain management: An evidence – based review. Anesth Analg. 2003;97:1730–9. doi: 10.1213/01.ANE.0000086618.28845.9B. [DOI] [PubMed] [Google Scholar]
- 15.Burton AW, Lee DH, Saab C, Chung JM. Preemptive intrathecal ketamine injection producse a long lasting decreases in neuropathic pain behaviours in a rat model. Reg Anesth Pain Med. 1999;24:208–13. doi: 10.1016/s1098-7339(99)90129-3. [DOI] [PubMed] [Google Scholar]
- 16.Amr YM. Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: A prospective, randomized, double blind trial. Pain Physician. 2010;13:245–9. [PubMed] [Google Scholar]


