Abstract
Spinal cord stimulation (SCS) is thought to relieve chronic intractable pain by stimulating nerve fibers in the spinal cord. The resulting impulses in the fibers may inhibit the conduction of pain signals to the brain, according to the pain gate theory proposed by Melzack and Wall in 1965 and the sensation of pain is thus blocked. Although SCS may reduce pain, it will not eliminate it. After a period of concern about safety and efficacy, SCS is now regaining popularity among pain specialists for the treatment of chronic pain. The sympatholytic effect of SCS is one of its most interesting therapeutic properties. This effect is considered responsible for the effectiveness of SCS in peripheral ischemia, and at least some cases of complex regional pain syndrome. The sympatholytic effect has also been considered part of the management of other chronic pain states such as failed back surgery syndrome, phantom pain, diabetic neuropathy, and postherpetic neuralgia. In general, SCS is part of an overall treatment strategy and is used only after the more conservative treatments have failed. The concept of SCS has evolved rapidly following the technological advances that have produced leads with multiple contact electrodes and battery systems. The current prevalence of patients with chronic pain requiring treatment other than conventional medical management has significantly increased and so has been the need for SCS. With the cost benefit analysis showing significant support for SCS, it may be appropriate to offer this as an effective alternative treatment for these patients.
Keywords: Back pain, chronic pain, failed back surgery, spinal cord injury, spinal cord stimulator
INTRODUCTION
Spinal cord stimulation (SCS) was proposed by Shealy in 1967 as an alternative to the procedures of neurolesion and it consisted, in the beginning, in translaminar implantation of an intrathecal monopolar electrode.[1,2] The physiological mechanism by which SCS relieves pain is partially explained by the gate theory proposed by Melzack and Wall.[3]
SCS is thought to relieve chronic intractable pain by stimulating nerve fibers in the spinal cord. The resulting impulses in the fibers may inhibit the conduction of pain signals to the brain, according to the pain gate theory proposed by Melzack and Wall in 1965[3] and the sensation of pain is thus blocked. Although SCS may reduce pain it will not eliminate it—SCS masks the sensation of pain by producing tingling sensations or numbness (paresthesias).[4] After a period of concern about safety and efficacy, SCS is now regaining popularity among pain specialists for the treatment of chronic pain.[5]
The sympatholytic effect of SCS is one of its most interesting therapeutic properties. This effect is considered responsible for the effectiveness of SCS in peripheral ischemia,[6] and at least some cases of complex regional pain syndrome (CRPS).[7] The sympatholytic effect has also been considered part of the management of other chronic pain states such as failed back surgery syndrome (FBSS),[8] phantom pain, diabetic neuropathy,[9] and postherpetic neuralgia.[10–12]
In general, SCS is a part of an overall treatment strategy and is used only after the more conservative treatments have failed. Trial stimulation is required before permanent implantation of the device, and a thorough psychologic assessment is sometimes also performed before implantation.
Chronic pain is defined by its duration. The International Association for the Study of Pain (IASP) defines chronic pain as persisting beyond normal tissue healing time, assumed to be 3 months.[13] This definition comprises continuous pain; however, chronic pain has been otherwise defined as being either continuous or intermittent.[14]
In addition to its duration and lack of associated observed pathology, chronic pain is frequently identified by an unpredictable prognosis and may include varying amounts of disability, from none to severe. It is often accompanied by psychological problems, particularly depression and anxiety,[15] although any causal link between these is not fully understood. Neuropathic pain is defined by IASP as pain initiated or caused by a primary lesion or dysfunction in the peripheral or central nervous systems.[16] The mechanisms involved in neuropathic pain are complex and involve both peripheral and central pathophysiological phenomena. Types of chronic neuropathic pain include: failed back surgery syndrome (FBSS), complex regional pain syndrome (CRPS), phantom limb pain, central pain (e.g., poststroke pain), diabetic neuropathy and postherpetic neuralgia.
The condition FBSS is clinically defined as persistent or recurrent pain, mainly in the lower back and legs, after technically and anatomically successful lumbosacral spine surgery.[17] It is sometimes referred to as persistent pain following (technically satisfactory) surgery. FBSS comprises both neuropathic and nociceptive pain.
Complex regional pain syndrome (which has been called chronic reflex sympathetic dystrophy, or reflex sympathetic dystrophy syndrome, or causalgia) is divided into two types. IASP has defined CRPS type I as usually following an initiating noxious event or period of immobilization and satisfying the three criteria:
continuing pain, allodynia (lowered pain threshold), or hyperalgesia (increased pain response);
edema (accumulation of tissue fluid), changes in skin blood flow, or abnormal sudomotor activity (nerves that stimulate sweat glands) in the region of pain; and
no existing conditions that would otherwise account for the degree of pain and dysfunction.[18]
CRPS type II follows nerve injury. IASP defines it as satisfying the three criteria:
continuing pain, allodynia, or hyperalgesia after nerve injury, usually but not necessarily limited to the distribution of the injured nerve;
edema, changes in skin blood flow, or abnormal sudomotor activity in region of pain; and
no existing conditions that would otherwise account for the degree of pain and dysfunction.[19]
Critical limb ischemia (CLI) has been defined by the trans-atlantic inter-society consensus on the management of peripheral arterial disease (TASC) as a manifestation of peripheral arterial disease that describes patients with typical chronic ischemic rest pain or patients with ischemic skin lesions, either ulcers or gangrene, with symptoms for more than 2 weeks.[19] Peripheral arterial disease is classified according to Fontaine's stages or Rutherford's categories, ranging in severity from asymptomatic to ulceration/gangrene/major tissue loss. CLI is associated with reduced peripheral blood pressure.[20]
Angina may not always be of ischemic origin; it can be the result of syndrome X, in which the coronary vessels appear normal. Refractory angina is a chronic condition in which frequent angina attacks occur despite optimal drug therapy/surgery. Angina pain typically occurs during exercise. The New York Heart Association defines cardiac disease in terms of functional capacity and objective assessment, with functional capacity ranging from Class I – cardiac disease without resulting limitation of physical activity, to Class IV – inability to carry on any physical activity without discomfort.[21]
PREVALENCE
Published estimates of the prevalence of any chronic pain (that is, not restricted to neuropathic and ischemic pain) vary widely. Elliott et al.[22] reports a range from 2% to 45%, suggesting that some of this variation can be ascribed to poor instruments, inadequate study size and studies concentrating on specific diagnoses within chronic pain. Their own study in the Grampian region of the UK reported a prevalence of 50.4% among adults. Overall prevalence increased with age (from around 30% of those aged 25–34 years to around 60% in those older than 65 years). The two commonest causes of pain were back pain (16%) and arthritis (16%). Back pain varied little with age, while arthritis and angina (4.5% of sample) both increased consistently with age. Severe chronic pain was reported by 10.8% of respondents. Restricting to pain of neuropathic origin, the prevalence of chronic neuropathic pain has been estimated by the Neuropathic Pain Network (2004) to be 3 million people, or 7.5%, in the United Kingdom.[22] A study conducted in the United Kingdom suggested the prevalence of chronic neuropathic pain to be 8.2%.[23]
A study from Norway looked at chronic critical lower limb ischemia in a population aged from 40 to 69 years, and found the prevalence to be 0.24%, with some increase with increasing age.[24] A UK study of men aged 40--59 years found a prevalence of definite angina of 4.8%, and of possible angina for a further 3.1% of all men.[25]
IMPACT OF HEALTH PROBLEM
Chronic pain is an important cause of physical and emotional suffering, familial and social disruptions, disability and work absenteeism. Breivik et al.[26] conducted a European survey of chronic pain (including but not limited to neuropathic pain), in 15 European countries and Israel showing that 19% of adults suffer chronic pain of moderate to severe intensity. In interviews with 4839 patients, it was found that chronic pain had a severe impact on the following daily activities: sleeping, exercising, lifting, household chores, walking, attending social activities, working outside the home, maintaining an independent lifestyle, driving, and maintaining relationships with family and friends. For instance, 32% of the respondents were no longer able to work outside their homes while 34% of the respondents were less able to attend social activities, and 65% were less able or unable to sleep. Breivik et al. also reported that of 300 respondents in the UK, 32% suffered severe pain (8, 9, or 10 on the 1–10 Numeric Rating Scale). As a result of pain, 25% had lost their job, 16% had changed job responsibilities and 18% had changed jobs entirely. The ability to work of people who suffer chronic pain can have a direct impact on society's economy. In-depth interviews also found that 24% of respondents in the United Kingdom had been diagnosed with depression by a medical doctor, showing that pain may have a direct influence on the emotional status of patients.
In a cross-sectional survey (observational), McDermott et al.[27] reported the association of neuropathic pain severity using the health-related quality of life instrument EuroQoL-5D (EQ5D) (27). This study considered 602 patients with neuropathic pain in six European countries (France, Germany, Italy, the Netherlands, Spain, and the United Kingdom). Pain severity was measured by the brief pain inventory (BPI) pain severity scores (range 0–10) and was found to be associated significantly (P<0.001) with poorer EQ5D scores. Scores of 0–3, 4–6, and 7–10 represented mild, moderate, and severe pain ratings, respectively. The EQ5D scores were 0.67 for mild, 0.46 for moderate, and 0.16 for severe pain. These scores are lower than those for other diseases such as heart attack 0.7617 and moderate stroke 0.6818 showing that neuropathic pain can have a heavy impact on the patients’ quality of life.
MANAGEMENT OF CHRONIC PAIN
Pharmacological treatment is primarily the use of analgesics, but can include other medication relevant to the conditions such as nonsteroidal anti-inflammatory drugs and anticonvulsants. Where other therapies have failed, intrathecal drug delivery is considered in some centers. Other therapies include physical therapy, and transcutaneous electrical nerve stimulation. Antidepressants are provided, as depression is often comorbid with chronic pain although treatment of one condition may not necessarily improve the other. Psychological therapies, including cognitive behavioral therapy and supported self-management, are delivered. The British Pain Society (BPS) recommends pain clinics and pain management programs, and has found that patients with chronic pain have often been to a number of secondary-care specialists before being referred to pain clinics.[28]
There are other possibilities for treatment specific to condition. For neuropathic pain, pharmacotherapy is the favored treatment, but nerve blocks may be considered. Patients with FBSS may undergo reoperation. For ischemic conditions, the preferred treatment is revascularization, for angina this includes coronary artery bypass grafting and percutaneous myocardial revascularization, for CLI it includes percutaneous angioplasty or distal grafting. However, not all patients with chronic ischemic pain are eligible for this, for example coronary artery bypass grafting is not considered suitable for refractory angina.
CURRENT SERVICE COST
In a European survey, Breivik et al.[26] reported that 13% of the respondents in the UK suffered from chronic pain. Although this study considers a very small sample of the UK population, if this figure is applied to 2006 population estimates, it equates to approximately 6.9 million people in England and Wales who suffer chronic pain.[29] In the prevalence estimates reported by Taylor[30] the neuropathic back and leg pain prevalence in the UK is 5800 per 100 000 population. Therefore, approximately 405 115 people in England and Wales suffer from neuropathic back and leg pain, costing approximately £2 billion a year (from a societal perspective). An estimate of approximately 4051 patients a year would be suitable for spinal cord stimulation (SCS) treatment if just 1% of the estimated chronic pain population were considered to be suitable for SCS in England and Wales. According to the British Heart Foundation Statistics Database[31] the prevalence of angina is approximately 1.1 million people, representing a cost estimate of £221 million in the UK. Estimates suggest that 5–10% of people who suffer from angina will develop refractory angina.[32] This represents an estimated cost of refractory angina in the UK of between £11 million and £22 million. In the year 2000 the estimated cost of CLI in the UK was over £200 million a year.[33]
Guidelines from the European Federation of Neurological Societies (EFNS) make an evidence based recommendation for the use of SCS in FBSS and CRPS type I.[34] They also suggest the need for comparative trials in other indications, although there are reports of positive findings from case series for SCS in CRPS type II, peripheral nerve injury, diabetic neuropathy, post-herpetic neuralgia, amputation pain and partial spinal cord injury.[34]
Detailed guidelines produced by the BPS and the Society of British Neurological Surgeons recommend that SCS should be delivered, with other therapies, through a multidisciplinary pain management team including clinicians experienced in SCS, with ongoing surveillance and support.[28]
These guidelines stress the need for informed consent from patients, and state that SCS is contraindicated in patents with a bleeding disorder, systemic or local sepsis, or a demand pacemaker or implanted defibrillator. Guidelines from the USA suggest that SCS is suitable for patients of either sex and any age (excluding children for whom safety has not been established) although evidence is not firmly established that SCS has equal efficacy across sex and age groups.[35]
DESCRIPTION OF TECHNOLOGY UNDER ASSESSMENT
Spinal cord stimulation has been used since 1967. The precise mechanism of pain modulation is not fully understood. One theory is that it involves direct and indirect inhibition of pain signal transmission, and to have autonomic effects, the technique may inhibit chronic pain by stimulating large diameter afferent nerve fibers in the spinal cord. Pain is masked by the production of numbness/tingling (paraesthesia). It has been speculated that for ischemic pain SCS gives an additional benefit of redistributing microcirculatory blood flow.[36]
Expected benefits of SCS are reduction in pain, improved quality of life and possible reduction in pain medication usage. Reduction in pain may improve sleep and also increase alertness by allowing reductions in drug intake. Improved function (including general activities of daily living and possibly also return to work), may be sought for some conditions, although for some conditions such as FBSS, return to work is considered unlikely. Spinal cord stimulation modifies the perception of neuropathic and ischemic pain by stimulating the dorsal columns of the spinal cord. It is not effective for nociceptive pain.[28] SCS is reversible.
In general, SCS is part of an overall treatment strategy and is used only after the more conservative treatments have failed. However, for indications well-supported by evidence, the BPS suggests that SCS may be considered when simple first-line therapies have failed. The implantation must be performed in an operating theatre suitable for implant surgery [Figures 1, 2(a,b), and 3]. As a long-term therapy for a chronic condition, it also requires appropriate infrastructure and funding for ongoing surveillance and maintenance (e.g., replacing the pulse generator, revising the leads). Positive findings from case series have been reported for SCS in FBSS, CRPS I and II, peripheral nerve injury, diabetic neuropathy, postherpetic neuralgia, stump or phantom limb pain, partial spinal cord injury, chronic low back pain, chronic back and leg pain, ischemic limb pain and angina pain.[34,37–40]
Figure 1.
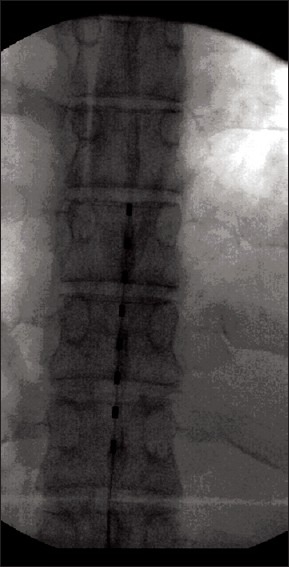
Single octrode stimulation lead over lumbosacral region. X-ray AP view
Figure 2 (a-b).
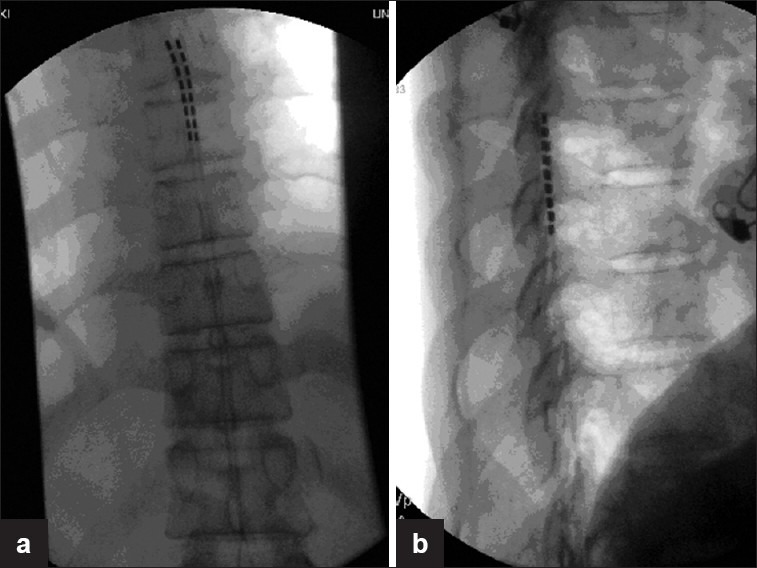
AP and lateral views of thoracic spine x ray showing Two electrodes in midline. The lateral view shows leads position dorsal to the dural sac
Figure 3.
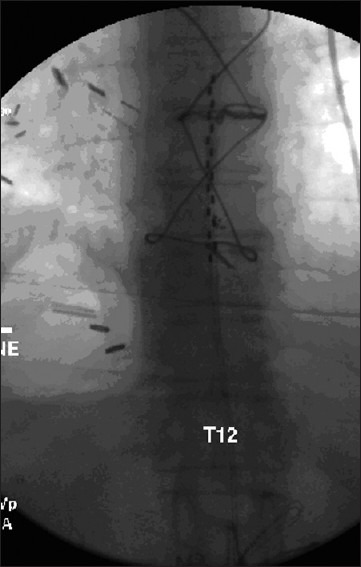
AP view of two stimulation leads in midline position. Note the juxta position of the contact points
There is no clear evidence indicating if test stimulation can predict how successful pain relief provided by SCS will be long-term. The EFNS suggests that test stimulation is not a guarantee of long-term success, but can identify patients who do not like the sensation or cannot achieve appropriate stimulation.[34]
Opinion is divided about the usefulness of test stimulation as a predictor of treatment effectiveness or as a means of setting parameters for level of stimulation. There are two types of test stimulation, one of which involves completely removing the device after test stimulation then later implanting SCS in patients for whom the test was successful. The other type uses a component from the test stimulation as part of the permanent implant.
A typical SCS device has four components:
implanted leads with a variable number of electrode contacts near the spinal cord;
an extension cable that connects the electrode(s) to the pulse generator;
an electrical pulse generator or receiver device which is surgically implanted under the skin in the abdomen, in the buttock area or in the lateral chest wall; and
a hand-held remote controller which the patient uses to turn the stimulator on or off, selecting different programs, and to adjust the level of stimulation, within limits as prescribed by the physician.
Rechargeable systems also include a charger. The implantation procedure involves placing leads in the epidural space, and implanting a subcutaneous generator and controller, which allow alteration of parameters such as pulse width, duration and intensity of stimulation. Repetitive electrical impulses are then delivered to the spinal cord. Pulse generation is achieved with an implantable pulse generator. An alternative form of pulse generation is the radiofrequency receiver. The choice of SCS device depends on individual patient requirements (e.g., pain patterns, power, and coverage needs) and preference as well as the physician's preference.
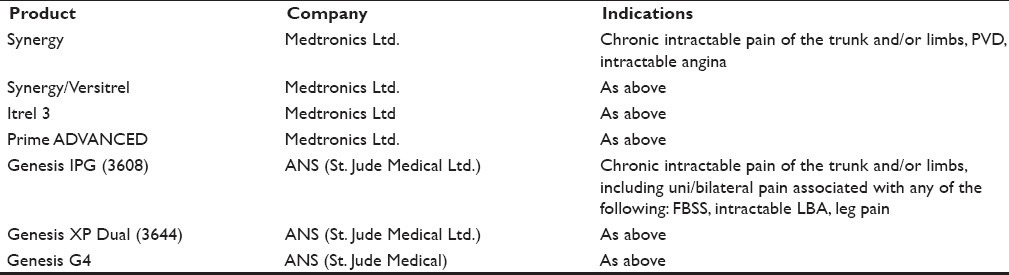
The IPG nonrechargeable systems have shown their superiority over the CMM strategies in all the reviews so far reported and certainly have a role to play even in the current scenario of pain management [Table 1].
Table 1.
Spinal cord stimulation devices with implantable pulse generator and non-rechargeable internal battery
Limitations of such a nonrechargeable SCS system have included[1] the need for repeat surgical procedures in 2-5 years to replace the IPG owing to battery depletion[2] to prolong battery life, patients who require continuous or high-power stimulation may need to spend a portion of the day with the SCS system turned off, use a cycling mode, or on lower power settings, leading to reduced pain relief, and[3] the discomfort associated with the relatively large size of the devices for patients with a small physique.-[41] A rechargeable SCS system is projected to save up to $100 000 over a patient's lifetime. Fewer pulse generator replacements will also decrease patient discomfort and morbidity from procedural complications [Table 2].
Table 2.
Spinal cord stimulation devices with implantable pulse generator and rechargeable internal battery

The first fully implantable pulse generator (IPG) with a rechargeable battery was approved by the US Food and Drug Administration in 2004 (Precision SCS System, Boston Scientific Neuromodulation, Valencia, CA).[42]
EVIDENCE ASSESSMENT
Several reviews appeared about the interventional pain treatments, with the first review published in 1995.[43] Subsequently, Taylor et al.[38] concluded that the level of evidence for the efficacy of SCS in chronic back and leg pain secondary to FBSS was moderate. In another systematic study, Taylor[44] in evaluating neuropathic back and leg pain secondary to FBSS concluded that the evidence was of Grade B. A Cochrane review for SCS[45] concluded that evidence was limited for SCS for FBSS. Frey et al.[46] indicated the evidence to be Level II-1 or II-2 for clinical use on a long-term basis in relieving chronic intractable pain of FBSS.
Kumar et al. compared SCS with conventional medical management (CMM) in patients with neuropathic pain secondary to FBSS with predominant leg pain of neuropathic radicular origin. By 12 months, the protocol analysis showed 48% of the SCS group and 9% of the medical management group achieving at least 50% pain relief. By 24-month follow-up, 42 out of 52 randomized patients continuing SCS reported significantly improved leg pain relief, QOL, and functional capacity; and 13 patients (31%) required a device-related surgical revision.[47]
The authors concluded that compared with the CMM group, the spinal cord group experienced improved leg and back pain relief, QOL, and functional capacity, and greater treatment satisfaction.
North et al.[48] presented results of SCS versus repeated lumbosacral spine surgery for chronic pain in an RCT. Of the 99 patients from a consecutive series invited to participate in the study, 60 candidates consented to randomization and 50 proceeded to a treatment. Among 45 patients (90%) available for follow-up, SCS was more successful than reoperation (9 of 19 patients versus 3 of 26 patients, P≤0.01). The long-term success rates at 2.9±1.1 years were for SCS, 47% versus reoperation 12% (P≤0.01).
In a more extensive systematic review by the Health Technology Assessment group, clinical effectiveness was demonstrated for SCS over CMM in reducing pain for FBSS and CRPS type I, from good-quality trials. From approximately 6000 citations identified, 11 randomized controlled trials (RCTs) were included in the clinical effectiveness review: three of neuropathic pain and eight of ischemic pain. Trials were available for the neuropathic conditions failed back surgery syndrome (FBSS) and complex regional pain syndrome (CRPS) type I, and they suggested that SCS was more effective than CMM or reoperation in reducing pain.[49]
Frey et al.[46] in a systematic review of SCS for patients with FBSS indicated the level of evidence as II-1 or II-2 for long-term relief (>1 year) in managing patients with FBSS. In this systematic review, two randomized trials,[47,48,50] and eight observational studies were included.[51–58]
Some have criticized the study because reoperation is essentially a repeat of the same treatment, which in critics’ opinions produced a potential bias in favor of the new treatment.[50] However, long-term follow up showed 15 of 29 in the successful group for SCS, while it was only 3 of 16 in the reoperation group.
Long-term management of chronic non-cancer pain may be achieved with intrathecal infusion systems.[59] While there is a lack of conclusive evidence, Patel et al. due to the paucity of quality literature concluded that the level of evidence for intrathecal infusion systems was indicated as Level II-3 or Level III with longer than 1-year improvement considered as long-term response. There were no randomized trials meeting inclusion criteria for this systematic review.[60]
COST EFFECTIVENESS
Taylor et al. found that initial health care acquisition costs were offset by a reduction in post implant health care resource demands and costs. Mean 5-year costs were $29 123 in the intervention group compared to $38 029 in the control group for FBSS. Other investigators also showed similar findings illustrating cost effectiveness of spinal cord stimulation even though initial health care acquisition costs are higher than other treatments.[61] In 2004, Taylor et al.[62] performed a comprehensive, systematic literature review on the cost-effectiveness of SCS for chronic pain. They noted that the time period for recovering costs associated with the initial SCS implantation varied, and is associated with “relative efficacy of SCS, generator battery life, and the level of SCS usage by patients.” Kumar et al.[63] has shown that the initial high costs are recaptured after 21/2 years, and after this point CMM becomes more costly.
In 2002, Kemler and Furne’e[64] published a full economic evaluation that included quality of life. They assessed both the costs and benefits of SCS plus physical therapy compared with physical therapy alone in patients with complex regional pain syndrome. This study calculated that the cost per quality-adjusted life year (QALY) gain was $22 581 over 1 year, suggesting that the therapy is cost-effective. Taylor et al. (above) examined the cost-effectiveness of SCS versus CMM in FBSS patients using decision-analytic modeling techniques. Mean 5-year costs were $29,123 in the intervention group compared to $38,029 in the control group for FBSS. Their model used detailed costs from a study of FBSS patients in Canada.-[65] The results show that SCS is both more effective and less costly than CMM over a patient's lifetime. They conclude that over the lifetime of patients, SCS is both cost saving to the healthcare system and more effective when compared to CMM, a finding that was robust across all sensitivity analysis. All of these studies were conducted at a time when only older, nonrechargeable SCS technologies were available. North et al.[66] performed cost-effectiveness and cost utility analysis based on a randomized, controlled trial with a 3.1 year follow-up. The mean per-patient cost was US$31 530 for SCS versus US$38 160 for reoperation (intention to treat).
SAFETY AND COMPLICATIONS
The most common adverse event reported in the literature is lead migration followed by lead fracture and infection at the incision site of implantable pulse generator or in the surgical pocket.[37,67]
Overall up to 34% of SCS patients may experience an adverse event,[68] though our review reported a higher incidence combining device-related and nondevice-related events.
SUMMARY
Spanning over four decades, spinal cord stimulators have come a long way in terms of technological advancements and the review systems for analysis of efficacy. There is level II evidence (both IIa and b) testifying the clinical safety as well as efficacy of SCS in the current literature; especially beneficial for FBSS and CRPS-1.
The data for spinal interventional techniques in the Medicare population from 1997 to 2006 shows an increase of 235%. The 22.2% yearly increase in expenditures in the Medicare population for IPM procedures has been even more significant from 2002 to 2006. Yet, during the same period, the United States’ population increased by 12% and the medicare population increased by 13% as a proportion of the population.[60]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg. 1967;46:489–91. [PubMed] [Google Scholar]
- 2.Shealy CN, Mortimer JT, Hagfors NR. Dorsal column electroanalgesia. J Neurosurg. 1970;32:560–4. doi: 10.3171/jns.1970.32.5.0560. [DOI] [PubMed] [Google Scholar]
- 3.Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 4.Alfano S, Darwin J, Picullel B. Spinal cord stimulation: Patient management guidelines for clinicians. Medtronic, undated. [Last accessed on 2011 March 18]. Available from: http://www.medtronic.com/neuro/paintherapies/pain_treatment_ladder/pdf/1_patient_management.pdf .
- 5.Segal R, Ott M, Levy E, Richard R. Prospective study of long-term results of totally implantable spinal cord stimulation (SCS) in a cohort of 78 patients. Neurosurgery. 1999;45:696. [Google Scholar]
- 6.Cook AW, Oygar A, Baggenstos P, Pacheco S, Kleriga E. Vascular disease of extremities.Electrical stimulation of spinal cord and posterior roots. N Y State J Med. 1976;76:366–8. [PubMed] [Google Scholar]
- 7.Kemler MA, Barendse GA, van Kleef M, de Vet HC, Rijks CP, Furnée CA, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343:618–24. doi: 10.1056/NEJM200008313430904. [DOI] [PubMed] [Google Scholar]
- 8.North RB, Kidd DH, Lee MS, Piantodosi S. A prospective, randomized study of spinal cord stimulation versus reoperation for failed back surgery syndrome: Initial results. Stereotact Funct Neurosurg. 1994;62:267–72. doi: 10.1159/000098631. [DOI] [PubMed] [Google Scholar]
- 9.Tesfaye S, Watt J, Benbow SJ, Pang KA, Miles J, MacFarlane IA. Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996;348:1698–701. doi: 10.1016/S0140-6736(96)02467-1. [DOI] [PubMed] [Google Scholar]
- 10.Meglio M, Cioni B, Rossi GF. Spinal cord stimulation in management of chronic pain.A 9-year experience. J Neurosurg. 1989;70:519–24. doi: 10.3171/jns.1989.70.4.0519. [DOI] [PubMed] [Google Scholar]
- 11.MeglioM , Cioni B, Prezioso A, Talamonti G. Spinal cord stimulation (SCS) in deafferentation pain. Pacing Clin Electrophysiol. 1989;12:709–12. doi: 10.1111/j.1540-8159.1989.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 12.Meglio M, Cioni B, Prezioso A, Talamonti G. Spinal cord stimulation (SCS) in the treatment of post-herpetic pain. Acta Neurochir Suppl (Wien) 1989;46:65–6. doi: 10.1007/978-3-7091-9029-6_15. [DOI] [PubMed] [Google Scholar]
- 13.Association for the Study of Pain. Classification of chronic pain. Pain. 1986;3:S1–226. [PubMed] [Google Scholar]
- 14.Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. Lancet. 1999;354:1248–52. doi: 10.1016/s0140-6736(99)03057-3. [DOI] [PubMed] [Google Scholar]
- 15.Ashburn MA, Staats PS. Management of chronic pain. Lancet. 1999;353:1865–9. doi: 10.1016/S0140-6736(99)04088-X. [DOI] [PubMed] [Google Scholar]
- 16.Merskey H, Bogduk N. Seattle: IASP Press; 1994. Classification of chronic pain: Descriptions of chronic pain syndromes and definition of pain terms. [Google Scholar]
- 17.Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: Results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31:S13–9. doi: 10.1016/j.jpainsymman.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Merskey H, Bogduk N. Seattle: IASP Press; 1994. Classification of chronic pain: Descriptions of chronic pain syndromes and definition of pain terms. [Google Scholar]
- 19.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial disease (TASC II) J Vasc Surg. 2007;33:S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 20.European Working Group on Critical Limb Ischemia. Second European consensus document on chronic critical leg ischemia. Circulation. 1991;84:1–26. [PubMed] [Google Scholar]
- 21.Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. 1994. The Criteria Committee of the New York Heart Association; pp. 253–6. [Google Scholar]
- 22.Neuropathic Pain Network. 2004. [Last accessed on 2011 March 18]. Available from: http://www.Neuropathicpainnetwork.org/english/index.asp .
- 23.Torrance N, Smith BH, Bennett M, Lee AJ. The epidemiology of chronic neuropathic pain in the community.Results from a general population survey. J Pain. 2006;7:281–9. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Jensen SA, Vatten LJ, Myhre HO. The prevalence of chronic critical lower limb ischaemia in a population of 20,000 subjects 40–69 years of age. Eur J Vasc Endovasc Surg. 2006;32:60–5. doi: 10.1016/j.ejvs.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Shaper AG, Cook DG, Walker M, Macfarlane PW. Prevalence of ischaemic heart disease in middle aged British men. Br Heart J. 1984;51:595–605. doi: 10.1136/hrt.51.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breivik H, Collet B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 27.McDermott A, Toelle TR, Rowbotham DJ, Schefer CP, Dukes E. The burden of neuropathic pain: Results from a cross-sectional survey. Eur J Pain. 2006;10:127–35. doi: 10.1016/j.ejpain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Spinal cord stimulation for the management of pain: recommendations for best clinical practice. London: British Pain Society; 2005. British Pain Society and Society of British Neurological Surgeons. [Google Scholar]
- 29.National Statistics. [Last Accessed on 2008 January 15]. Available from: http://www.statistics.gov.uk/cci/nugget.asp?id=6 .
- 30.Taylor RS. Epidemiology of refractory neuropathic pain. Pain Pract. 2006;6:22–6. doi: 10.1111/j.1533-2500.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- 31.British Heart Foundation. [last Accessed on 2008 January 15]. Available from: http://datapage.asp?id=122 .
- 32.Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, et al. The problem of chronic refractory angina: report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J. 2002;23:355–70. doi: 10.1053/euhj.2001.2706. [DOI] [PubMed] [Google Scholar]
- 33.Beard JD. ABC of arterial and venous disease: chronic lower limb ischaemia. BMJ. 2000;320:854–7. doi: 10.1136/bmj.320.7238.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruccu G, Simpson BA, Taylor RS. 56 EFNS guidelines on spinal cord stimulation for neuropathic pain. Eur J Pain. 2007;11:22. [Google Scholar]
- 35.North R, Shipley J, Prager J, Barolat G, Barulich M, Bedder M, et al. Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med. 2007;8:S200–S75. doi: 10.1111/j.1526-4637.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 36.Middleton P, Simpson B, Maddern G. ASERNIP-S Report No. 43. Adelaide, South Australia: ASERNIP-S; 2003. Spinal cord stimulation/neurostimulation: an accelerated systematic review. [Google Scholar]
- 37.Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20- year literature review. J Neurosurg. 2004;100:254–67. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- 38.Taylor RS, Van Buyten J, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: A systematic review and analysis of prognostic factors. Spine (Phila Pa 1976) 2005;30:152–60. doi: 10.1097/01.brs.0000149199.68381.fe. [DOI] [PubMed] [Google Scholar]
- 39.Grabow TS, Tella PK, Raja SN. Spinal cord stimulation for complex regional pain syndrome: An evidence-based medicine review of the literature. Clin J Pain. 2003;19:371–83. doi: 10.1097/00002508-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91–101. doi: 10.1016/j.ejpain.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Hornberger J, Kumar K, Verhulst E, Clark MA, Hernandez J. Rechargeable Spinal Cord Stimulation Versus Nonrechargeable System for Patients With Failed Back Surgery Syndrome: A Cost-Consequences Analysis. Clin J Pain. 2008;24:244–52. doi: 10.1097/AJP.0b013e318160216a. [DOI] [PubMed] [Google Scholar]
- 42.Oakley JC, Krames ES, Prager JP, Foster AM, Weiner R, Rashbaum RR, et al. A new spinal cord stimulation system effectively relieves chronic, intractable pain: A multicenter prospective clinical study. Neuromodulation. 2007;10:262–78. doi: 10.1111/j.1525-1403.2007.00115.x. [DOI] [PubMed] [Google Scholar]
- 43.Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: a systematic literature synthesis. Neurosurgery. 1995;37:1088–95. doi: 10.1227/00006123-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: Results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31:S13–9. doi: 10.1016/j.jpainsymman.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Mailis-Gagnon A, Furlan AD, Sandoval JA, Taylor R. Spinal cord stimulation for chronic pain. Cochrane Database Syst Rev. 2004;3:CD003783. doi: 10.1002/14651858.CD003783.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Frey ME, Manchikanti L, Benyamin RM, Schultz DM, Smith HS, Cohen SP. Spinal cord stimulation for patients with failed back surgery syndrome: A systematic review. Pain Physician. 2009;12:379–97. [PubMed] [Google Scholar]
- 47.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63:762–70. doi: 10.1227/01.NEU.0000325731.46702.D9. [DOI] [PubMed] [Google Scholar]
- 48.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: A randomized, controlled trial. Neurosurgery. 2005;56:98–107. doi: 10.1227/01.neu.0000144839.65524.e0. [DOI] [PubMed] [Google Scholar]
- 49.Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J. Spinal cord stimulation for chronic pain of neuropathic or ischemic origin: systematic review and economic evaluation. Health Technology Assessment. 2009;13:1–154. doi: 10.3310/hta13170. [DOI] [PubMed] [Google Scholar]
- 50.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomized controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179–88. doi: 10.1016/j.pain.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 51.Van Buyten JP, Van Zundert J, Vueghs P, Vanduffel L. Efficacy of spinal cord stimulation: 10 years of experience in a pain centre in Belgium. Eur J Pain. 2001;5:299–307. doi: 10.1053/eujp.2001.0249. [DOI] [PubMed] [Google Scholar]
- 52.De La Porte C, Van de Kelft E. Spinal cord stimulation in failed back surgery syndrome. Pain. 1993;52:55–61. doi: 10.1016/0304-3959(93)90113-4. [DOI] [PubMed] [Google Scholar]
- 53.Devulder J, De Laat M, Van Bastelaere M, Rolly G. Spinal cord stimulation: A valuable treatment for chronic failed back surgery patients. J Pain Symptom Manage. 1997;13:296–301. doi: 10.1016/s0885-3924(96)00322-3. [DOI] [PubMed] [Google Scholar]
- 54.North RB, Ewend MG, Lawton MT, Kidd DH, Piantadosi S. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery. 1991;28:692–9. [PubMed] [Google Scholar]
- 55.Dario A. Treatment of failed back surgery syndrome. Neuromodulation. 2001;4:105–10. doi: 10.1046/j.1525-1403.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- 56.De La Porte C, Siegfried J. Lumbosacral spinal fibrosis (spinal arachnoiditis).Its diagnosis and treatment by spinal cord stimulation. Spine. 1983;8:593–603. [PubMed] [Google Scholar]
- 57.Burchiel KJ, Anderson VC, Brown FD, Fessler RG, Friedman WA, Pelofsky S, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine. 1996;21:2786–94. doi: 10.1097/00007632-199612010-00015. [DOI] [PubMed] [Google Scholar]
- 58.Ohnmeiss DD, Rashbaum RF, Bogdanffy GM. Prospective outcome evaluation of spinal cord stimulation in patients with intractable leg pain. Spine. 1996;21:1344–50. doi: 10.1097/00007632-199606010-00013. [DOI] [PubMed] [Google Scholar]
- 59.Patel VB, Manchikanti L, Singh V, Schultz DM, Hayek SM, Smith HS. Systematic review of intrathecal infusion systems for long-term management of chronic non-cancer pain. Pain Physician. 2009;12:345–60. [PubMed] [Google Scholar]
- 60.Manchikanti L, Singh V, Pampati V, Smith HS, Hirsch JA. Analysis of growth of interventional techniques in managing chronic pain in the medicare population: A 10-year evaluation from 1997 to 2006. Pain Physician. 2009;12:9–34. [PubMed] [Google Scholar]
- 61.Manca A, Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial) Eur J Pain. 2008;12:1047–58. doi: 10.1016/j.ejpain.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Taylor RS, Taylor RJ, Van Buyten JP, Buchser E, North R, Bayliss S, et al. The cost effectiveness of spinal cord stimulation in the treatment of pain: A systematic review of the literature. J Pain Symptom Manage. 2004;27:370–8. doi: 10.1016/j.jpainsymman.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Kumar K, Wilson JR, Taylor RS, Gupta S. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine. 2006;5:191–203. doi: 10.3171/spi.2006.5.3.191. [DOI] [PubMed] [Google Scholar]
- 64.Kemler MA, Furne´e CA. Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology. 2002;59:1203–9. doi: 10.1212/01.wnl.0000028686.74056.e3. [DOI] [PubMed] [Google Scholar]
- 65.Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: Cost-effectiveness analysis. Neurosurgery. 2002;51:106–15. doi: 10.1097/00006123-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 66.North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: A cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery. 2007;61:361–8. doi: 10.1227/01.NEU.0000255522.42579.EA. [DOI] [PubMed] [Google Scholar]
- 67.Bagger JP, Jensen BS, Johannsen G. Long-term outcome of spinal cord electrical stimulation in patients with refractory chest pain. Clin Cardiol. 1998;21:286–8. doi: 10.1002/clc.4960210410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: A systemic review of effectiveness and complications. Pain. 2004;108:137–47. doi: 10.1016/j.pain.2003.12.016. [DOI] [PubMed] [Google Scholar]


