Abstract
Background:
Ketamine-induced hemodynamic pressor response and psychomimetic effects should be attenuated by appropriate premedication. The present study was designed to evaluate the clinical efficacy and safety of dexmedetomidine premedication for balancing the ketamine-induced hemodynamic pressor response and psychomimetic effects.
Materials and Methods:
A total of 80 normotensive adult consented patients of ASA grade I and II of both genders, aged 21 to 55 years, who met the inclusion criteria for elective surgery under ketamine anesthesia were randomized for this prospective blind study and divided into two treatment groups of 40 patients each. Group I patients received premedication of midazolam and Group II patients received premedication of dexmedetomidine. Anesthetic and surgical techniques were standardized. Both groups were assessed for changes in heart rate and systolic blood pressure intraoperatively and psychomimetic effects with behavioral changes postoperatively.
Results:
Preoperatively, all patients were awake. Intraoperatively, the heart rate was 116.6±4.2 in group I versus 76.8±5.8 in group II (P value 0.0004) and systolic blood pressure was 153.07±16.05 in group I versus 139.17±19.9 in group II (P value 0.001). Post-anesthetic psychomimetic responses were not statistically significant between groups.
Conclusion:
The dexmedetomidine premedication effectively attenuated the ketamine induced hemodynamic pressor response and post-anesthetic delirium effects.
Keywords: Dexmedetomidine, hemodynamic pressor response, ketamine, midazolam, psychomimetic effects
INTRODUCTION
Ketamine, a phencyclidine analogue and a non-competitive antagonist of N-methyl-d-aspartate (NMDA) receptors, is introduced in clinical anesthesia as an induction agent for repetitive procedures, especially in children and in trapped casualties, as it induces analgesia, amnesia and unconsciousness which lasts for about 15 to 25 min.[1] It is a potent intravenous anesthetic, produces dissociative anesthetic state and is characterized by a rapid onset of action, preservation of airway reflexes and immediate recovery. Its clinical usefulness has been limited because of its intraoperative cardiostimulatory response and postoperative recovery with psychomimetic effects. Numerous drugs have been tried to attenuate these undesirable responses.[2]
The α2 adrenergic agonists, such as clonidine and dexmedetomidine, induce preoperative sedation, reduce anesthetic requirements and improve intraoperative hemodynamic stability with postoperative analgesia, which suggests that they might be suitable adjuncts to ketamine anesthesia.[3]
The present study was designed to assess the efficacy and safety of intravenous premedication with dexmedetomidine in relevance to ketamine anesthesia.
MATERIALS AND METHODS
After getting approval of the protocol from the Ethical Committee of Institution and written informed consent, this prospective blind randomized study was conducted on 80 normotensive adult patients of both genders, aged 21 to 55 years, with ASA physical status I and II, scheduled for elective surgery under ketamine anesthesia from February 2010 to January 2011. Patients with pre-existing cardiac disease, hypertension, increased intraocular and intracranial pressure, asthma and severe renal, hepatic or endocrinal dysfunction were excluded from the study. Other exclusion criteria included patients with known drug hypersensitivity, compromised airway, morbid obesity, those on antihypertensive or antidepressant drugs and those who refused consent. All the patients underwent pre-anesthetic evaluation with history, general and systemic examination and review of biochemical investigations before enrolment in the study. None of the patients had previous experience with general anesthesia.
They were randomized into two treatment groups of 40 patients each according to a computer-generated random table. Group I patients received premedication with midazolam (0.02 mg/kg) and Group II patients received dexmedetomidine (1 μg/kg), given slowly intravenously over 10 min. These doses are considered to provide adequate and comparable effects. The study solution was prepared and administered by the consultant anesthesiologist, not responsible for the study.
The protocol defined primary end points were intraoperative hemodynamic pressure response, oxygen saturation (SpO2) and ketamine requirement, quality of postoperative recovery and occurrence of ketamine-induced psychomimetic effects.
Anesthetic technique
In the operation theater, monitors were attached, and baseline heart rate, electrocardiogram (ECG), systemic arterial blood pressure, oxygen saturation (SpO2) and end-tidal carbon dioxide concentration were recorded. A crystalloid intravenous infusion of 6 to 8 ml/kg was started and patients were premedicated intravenously with metoclopramide (10 mg), glycopyrrolate (0.2 mg) and study solutions of midazolam (0.02 mg/kg) in group I and dexmedetomidine (1 μg/kg) in group II, given slowly over 10 min. After preoxygenation for 3 min with 100% oxygen, anesthesia was induced with intravenous ketamine (1% solution) at a dose of 2 mg/kg and injected slowly over 30 seconds. The hemodynamic parameters of heart rate and systolic arterial pressure were recorded before and after premedication, and after induction. Thereafter, the hemodynamic parameters were recorded every 5 min using a non-invasive blood pressure monitor till the end of surgery. The end-tidal carbon dioxide concentration was also monitored intraoperatively.
Anesthesia was maintained with 40% O2 in N2O, using an anatomical face mask while the patient was breathing spontaneously. Tachycardia, hypertension, and clinically insufficient analgesia or anesthesia were controlled with 1 mg/kg dose of intravenous ketamine. The tachycardia was defined as heart rate faster than 100 beats/min, and hypertension was defined as systolic blood pressure (SBP) more than 180 mm Hg. Autonomic or somatic signs of insufficient anesthesia included lacrimation, sweating and flushing. Hypotension was treated primarily by increasing the intravenous infusion and additionally with vasoactive drugs. Bradycardia, defined as heart rate slower than 45 beats/min, was treated with intravenous atropine (0.01 mg/kg).
Anesthetic and surgical techniques were standardized for all patients. All groups were assessed for changes in heart rate and systolic arterial pressure intraoperatively and psychomimetic effects with behavioral changes postoperatively.
Postoperative follow-up
The patients were transferred to the post-anesthesia care unit (PACU) and monitored for at least 3 hours or until there were no signs of any drug-induced effects such as nausea, vomiting, hypotension/hypertension, tachycardia/bradycardia and emergence delirium. The patients were treated with ondansetron hydrochloride 0.1 mg/kg IV, atropine 0.01 mg/kg and vasoactive drugs, in case of these complications. Postoperative sedation and orientation in place and time, and digit symbol substitution test (DSST ) were also assessed.[4] At the end of PACU period, the occurrence of hallucinations, confusion, agitation or unpleasant dreams was recorded (yes or no) and assessed on a 3-grade scale (1-mild; 2-moderate; and 3-severe) based on patients′ subjective estimates.
Study population size and statistical analyses
The sample size was decided in consultation with a statistician and was based on initial pilot observations, which indicated that approximately 30 - 33 patients should be included in each group in order to ensure power 0.80 for detecting clinically meaningful reduction in heart rate, systemic arterial blood pressure and emergence delirium by 10 - 20%. Assuming a 5% dropout rate, the final sample size was set at 80 patients.
The results obtained in the study were tabulated and analyzed using Microsoft Excel, and SPSS software for windows. Hemodynamic variables were represented as mean±SD. Analyses for postoperative sedation and performance were applied to the measurements from 15 min or 30 min until 3 hours. Statistical significance in mean difference was calculated using Student's t-test and chi-square test, as appropriate. A P value of <0.05 was considered significant and <0.001 was considered highly significant .
RESULTS
A total of 80 patients, 40 in each group, were evaluated. Both groups were comparable with respect to the demographic profile and operational factors. No significant differences were found between groups with respect to age, gender, weight, ASA physical status and type of surgery [Table 1].
Table 1.
Patient demographic and baseline characteristics (Mean±SD)
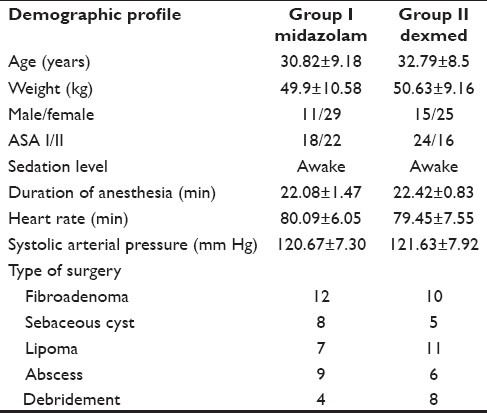
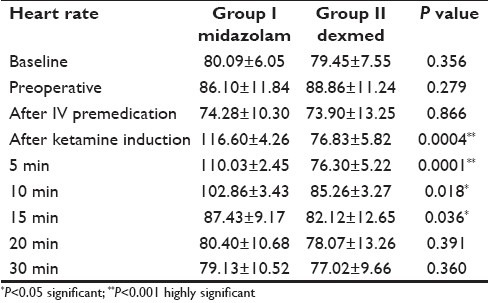
All the patients were fully awake when they arrived at the operation theater. Baseline systolic arterial pressure and heart rate were similar in both the groups [Table 1]. The ketamine induction resulted in tachycardia. The peak value of heart rate was 35% increase from base value in Group I, which gradually subsided. Dexmedetomidine administered group II showed stability in heart rate at the time of induction, along with statistical significance in heart rate during the study period (P<0.01) [Table 2].
Table 2.
Heart rate changes during ketamine anesthesia (Mean±SD)
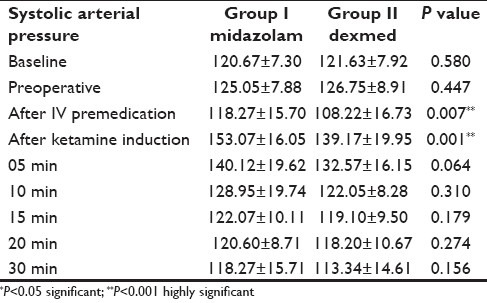
Preoperatively, difference in systemic arterial pressure was not statistically significant between the groups [Table 1]. In group I, the systolic arterial pressure had shown statistically significant increase of 27.5% above the baseline value (P<0.001) after ketamine induction and then it gradually subsided to base value. In dexmedetomidine group, the preinduction value of the systolic arterial pressure was lower (108.22 mm of Hg) than the baseline value (121.63 mm of Hg), and patients showed statistically significant stability during the study period, by reduction in the peak value to 15% of the base value P<0.007 [Table 2].
The hemodynamic parameters remained close to the baseline values in dexmedetomidine group II, whereas in the midazolam group I, approximately 25-35 mm Hg rise in systolic arterial pressure and 17-25 beats/min increase in heart rate was observed. Intraoperative systolic arterial pressure and heart rate values were stable in the dexmedetomidine group as compared to the midazolam group [Table 3].
Table 3.
Changes in systolic arterial pressure during ketamine anesthesia (Mean±SD)
Clinically significant respiratory depression was not seen in any of the patients. None of our patients had shown fall in oxygen saturation. Preoperatively, the lowest recorded SpO2 value was 97% in both the groups while it decreased to 95% in midazolam group I and 94% in dexmedetomidine group II.The intraoperative ketamine supplements were required in 18 patients (45%) of midazolam group as compared to only 4 patients (10%) of dexmedetomidine group. Only 2 patients (5%) of midazolam group and 8 patients (20%) of dexmedetomidine group had received IV atropine for intraoperative bradycardia (heart rate slower than 45 beats/min). The fluid infusion rate was increased in two patients of dexmedetomidine group II to treat hypotension. No patients had persistent or severe hypotension.
Recovery and postoperative follow-up
The time between discontinuation of nitrous oxide to opening of the eyes was 5±0.6 and 3±1.5 min in the midazolam and dexmedetomidine groups, respectively. The patients were transferred to the PACU and monitored for at least 3 hours. There was no difference among the groups with respect to recovery and awakening time. The Ramsay sedation score, scored from 1 (patient anxious and agitated or restless or both) to 6 (asleep: no response to light glabellar tap), was used to assess sedation.[5] Agitation and confusion was not observed by the staff in the PACU. Dexmedetomidine treated patients tended to be more sedated than patients in midazolam group with gradual recovery. In the PACU, systemic arterial pressure and heart rate remained stabilized at lower level in the dexmedetomidine group as compared to midazolam group. None of the patients required treatment for postoperative complication with atropine, vasoactive or antiemetic drugs.
All patients were interviewed at the end of the PACU follow-up for ketamine-induced psychomimetic effects of hallucinations and unpleasant dreams. These were evident in 10 patients (25%) of midazolam group, while only 2 patients (5%) of dexmedetomidine group reported experiencing such symptoms which were usually mild to moderate.
DISCUSSION
Ketamine, a phencyclidine analogue and a non-competitive antagonist of NMDA receptors, functionally “dissociates” the thalamus from the limbic cortex which is involved with the awareness of sensation. It is the only anesthetic available with analgesic, hypnotic and amnesic effects. In spite of several advantages, its anesthetic use has been limited due to its cardiostimulant response even in therapeutic doses. After an intravenous dose, the heart rate increases progressively up to 33% for 10–15 min and then subsides. The net effect is an increase in mean arterial pressure, heart rate and cardiac output as observed by Knox et al.[6] and Gupta et al.[7] Numerous drugs have been evaluated for attenuation of cardiovascular effects of ketamine. The present study was designed to evaluate the efficacy of intravenous premedication with midazolam and dexmedetomidine on ketamine-induced hemodynamic pressor response and psychomimetic effects.
A benzodiazepine (midazolam) premedication abolishes the post-anesthetic psychomimetic effects of ketamine by closing the NMDA receptors, while an α2 adrenoceptor agonist induces central sympatholysis by activating presynaptic autoreceptors. Thus, dexmedetomidine induces sedation via central nervous system receptors which differ from those induced by the benzodiazepines drugs.[8–11] The other possible benefits include decreased postoperative shivering, decreased anesthetic and analgesic requirements and to provide sedation and anxiolysis.
In the present study, at doses that induced comparable and clinically adequate sedation in our healthy patients, premedication with dexmedetomidine was more effective than midazolam for attenuation of ketamine-induced intraoperative hypertension, tachycardia and postoperative unpleasant dreams. Thus, the sedative effects of dexmedetomidine and midazolam appear to be qualitatively different. Several studies have demonstrated the clinical utility of combining midazolam with ketamine in mitigating ketamine-induced adverse effects for providing total intravenous anesthesia. Taittoven et al. compared clonidine and midazolam as premedication agents and observed no differences in oxygen consumption, anxiolysis, energy expenditure and carbon dioxide production. The clonidine premedication improved hemodynamic stability and protected against the pressure response to laryngoscopy and intubation. Thus, pharmacodynamic profile of clonidine appears to be suitable for combination with ketamine.[12]
In a recent study by Munro et al., 300 μg oral clonidine was found to be more effective than 1.5 mg/kg intravenous lidocaine in preventing the cardiostimulatory effects of ketamine. There was statistically significant increase in heart rate and arterial blood pressure after ketamine induction. The partial attenuation could be due to the smooth muscle relaxation effect of lignocaine.[13,14]
The hemodynamic results of our study are in agreement with the results obtained for clonidine. Compared with clonidine, dexmedetomidine is about 10 times more selective toward the α2 adrenoceptor and acts as a full agonist in some pharmacologic tests. The effects of dexmedetomidine on sympathetic tone are opposite to those of ketamine, resulting in reduction in heart rate and blood pressure. Levanen et al. reported that premedication with 2.5 μg/kg dexmedetomidine is effective in attenuating the cardiostimulatory and post-anesthetic delirium effects of ketamine and reported its superiority over midazolam in a dose of 0.07 mg/kg.[15] The psychomimetic effects of ketamine appear to be linearly related to the venous plasma concentration, and thus, oral ketamine causes fewer side effects, perhaps because of the smaller plasma levels.[16,17]
Clinically significant respiratory depression was not seen in any of the patients. Intraoperatively, none of our patients had shown fall in oxygen saturation. No patient required a nasopharyngeal or oropharyngeal airway to maintain the patency of airway. This is similar to what was seen in a study conducted by two studies[15,16] or it may be due to appropriate speed of injecting ketamine. Recovery from anesthesia often results in pain and elevated catecholamine concentrations. At the same time, anesthesia residuals compromise breathing. The α2 agonists may prove beneficial in the postoperative period because of their sympatholytic and analgesic effects without causing respiratory depression.[18,19]
In the present study, dexmedetomidine increased perioperative bradycardia (defined as heart rate below 45 beats/min) and the subsequent use of atropine, suggesting that an anticholinergic drug should be given routinely before induction with ketamine. Ketamine increases salivary and tracheobronchial mucus secretion, further necessitating prophylactic administration of an antisialagogue.[1] Glycopyrrolate, which does not penetrate the blood brain barrier, would be a rational choice.
It has to be recognized that our patient population and surgical procedure did not represent the normal clinical circumstances for the use of ketamine. More relevant patient populations should be studied before any final conclusions or recommendations are made concerning this combination. The experience of dexmedetomidine in elderly patients is limited; the possible anti-ischemic properties of α2 agonist could be beneficial as they reduce the incidence of the myocardial ischemic episodes in patients with known or suspected coronary artery disease undergoing non-cardiac surgery.[20,21]
CONCLUSION
Dexmedetomidine premedication has effectively and safely attenuated the ketamine-induced hemodynamic pressor response and psychomimetic effects. Due to its tendency to cause bradycardia, routine use of an anticholinergic drug should be considered. The sympatholytic and antinociceptive effects of dexmedetomidine may prove beneficial for high-risk surgical patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.White PF, Way WL, Trevor AJ. Ketamine- its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–36. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Lilburn JK, Moore J, Dundee JW. Attempts to attenuate the cardiovascular effects of ketamine. Anaesth. 1978;33:499–505. doi: 10.1111/j.1365-2044.1978.tb08385.x. [DOI] [PubMed] [Google Scholar]
- 3.Maze M, Tranquilli W. Alpha-2 adrenergic agonists: defining the role in clinical anesthesia. Anesthesiology. 1991;74:581–605. [PubMed] [Google Scholar]
- 4.Hindmarch I. Psychomotor function and psychoactive drugs. Br J Clin Pharmacol. 1980;10:189–209. doi: 10.1111/j.1365-2125.1980.tb01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stawicki SP. Sedation scales: Very useful, very under used.OPU 12. Scientist. 2007;1:10–2. [Google Scholar]
- 6.Knox JW, Boviil JG, Clarke RS, Dundee JW. Clinical studies of induction agents, ketamine. Br J Anaesth. 1970;42:875–85. doi: 10.1093/bja/42.10.875. [DOI] [PubMed] [Google Scholar]
- 7.Gupta PK, Sehgal R, Gupta M, Kawatra R. Attenuation of cardiovascular responses to ketamine: Comparative evaluation of lignocaine and clonidine. Indian J Anaesth. 2002;46:457–9. [Google Scholar]
- 8.Doak GJ, Duke PC. Oral clonidine premedication attenuates the hemodynamic effects associated with ketamine anaesthetic induction in humans. Can J Anaesth. 1993;40:612–8. doi: 10.1007/BF03009697. [DOI] [PubMed] [Google Scholar]
- 9.Ghignone M, Calvillo O, Quintin L. Anesthesia and hypertension: the effect of clonidine on perioperative hemodynamics and isoflurane requirements. Anaesthesiology. 1987;67:3–10. [PubMed] [Google Scholar]
- 10.Lowenthal DT, Matzek KM, Macgregor TR. Clinical pharmacokinetics of clonidine. Clin Pharmacokinet. 1988;14:282–316. doi: 10.2165/00003088-198814050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Tanskanen PE, Kytta JV, Randell TT, Aantaa RE. Dexmedetomidine as an anesthetic adjuvant in patients undergoing intracranial tumour surgery: A doubleblind, randomized and placebocontrolled study. Br J Anesth. 2006;97:658–65. doi: 10.1093/bja/ael220. [DOI] [PubMed] [Google Scholar]
- 12.Taittonen M, Kirvela O, Aantaa R, Kanto J. Cardiovascular and metabolic responses to clonidine and midazolam premedication. Eur J Anaesthesiol. 1997;14:190–6. doi: 10.1046/j.1365-2346.1997.00103.x. [DOI] [PubMed] [Google Scholar]
- 13.Munro HM, Sleigh JW, Paxton LD. The cardiovascular response to ketamine: The effects of clonidine and Lignocaine. Acta Anaesthesiol Scand. 1993;37:75–8. doi: 10.1111/j.1399-6576.1993.tb03602.x. [DOI] [PubMed] [Google Scholar]
- 14.Sikka R, Mohindra BK, Sodhi GS. Attenuation of cardiovascular responses to ketamine: Comparative study with clonidine and lignocaine. J Anaesth Clin Pharmacol. 2008;24:455–7. [Google Scholar]
- 15.Levannen J, Makela LM, Scheinin H. Dexmedetomidine premedication attenuates ketamine induced cardio stimulatory effects of post anaesthetic delirium. Anesthesiology. 1995;82:1117–25. doi: 10.1097/00000542-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Nishikawa T. Oral clonidine premedication attenuates the hypertensive response to ketamine. Br J Anaesth. 1994;73:758–62. doi: 10.1093/bja/73.6.758. [DOI] [PubMed] [Google Scholar]
- 17.Handa F, Tanaka M, Nishikawa T, Toyooka H. Effect of oral clonidine premedication on side effects of intravenous ketamine anesthesia, a randomized double blind placebo-controlled study. J Clin Anesth. 2000;12:19–24. doi: 10.1016/s0952-8180(99)00131-2. [DOI] [PubMed] [Google Scholar]
- 18.McVey JD, Tobias JD. Dexmedetomidine and ketamine for sedation during spinal anesthesia in children. J Clin Anesth. 2010;22:538–45. doi: 10.1016/j.jclinane.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Mester R, Easley RB, Brady KM, Chilson K, Tobias D. Monitored anesthesia care with a combination of ketamine and dexmedetomidine during cardiac catheterization. Am J Ther. 2008;15:24–30. doi: 10.1097/MJT.0b013e3180a72255. [DOI] [PubMed] [Google Scholar]
- 20.Talke PO, Mangano DT. Alpha 2-adrengic agonist and perioperative ischaemia. Anaesth Pharmacol Rev. 1993;1:310–5. [Google Scholar]
- 21.Chalikonda SA. Alpha 2-adrenergic agonists and their role in the prevention of perioperative adverse cardiac events. AANA J. 2009;77:103–8. [PubMed] [Google Scholar]


