Abstract
Aqueous gel sieving chromatography on Sephadex G-10 of the Group IA cations (Li+, Na+, K+, Rb+, Cs+) plus NH4+ as the Cl- salts, in combination with previous results for the halide anions (F-, Cl-, Br-, I-) as the Na+ salts [Washabaugh, M.W. & Collins, K.D. (1986) J. Biol. Chem. 261, 12477-12485], leads to the following conclusions. (i) The small monovalent ions (Li+, Na+, F-) flow through the gel with water molecules attached, whereas the large monovalent ions (K+, Rb+, Cs+, Cl-, Br-, I-) adsorb to the nonpolar surface of the gel, a process requiring partial dehydration of the ion and implying that these ions bind the immediately adjacent water molecules weakly. (ii) The transition from strong to weak hydration occurs at a radius of about 1.78 A for the monovalent anions, compared with a radius of about 1.06 A for the monovalent cations (using ionic radii), indicating that the anions are more strongly hydrated than the cations for a given charge density. (iii) The anions show larger deviations from ideal behavior (an elution position corresponding to the anhydrous molecular weight) than do the cations and dominate the chromatographic behavior of the neutral salts. These results are interpreted to mean that weakly hydrated ions (chaotropes) are "pushed" onto weakly hydrated surfaces by strong water-water interactions and that the transition from strong ionic hydration to weak ionic hydration occurs where the strength of ion-water interactions approximately equals the strength of water-water interactions in bulk solution.
Full text
PDF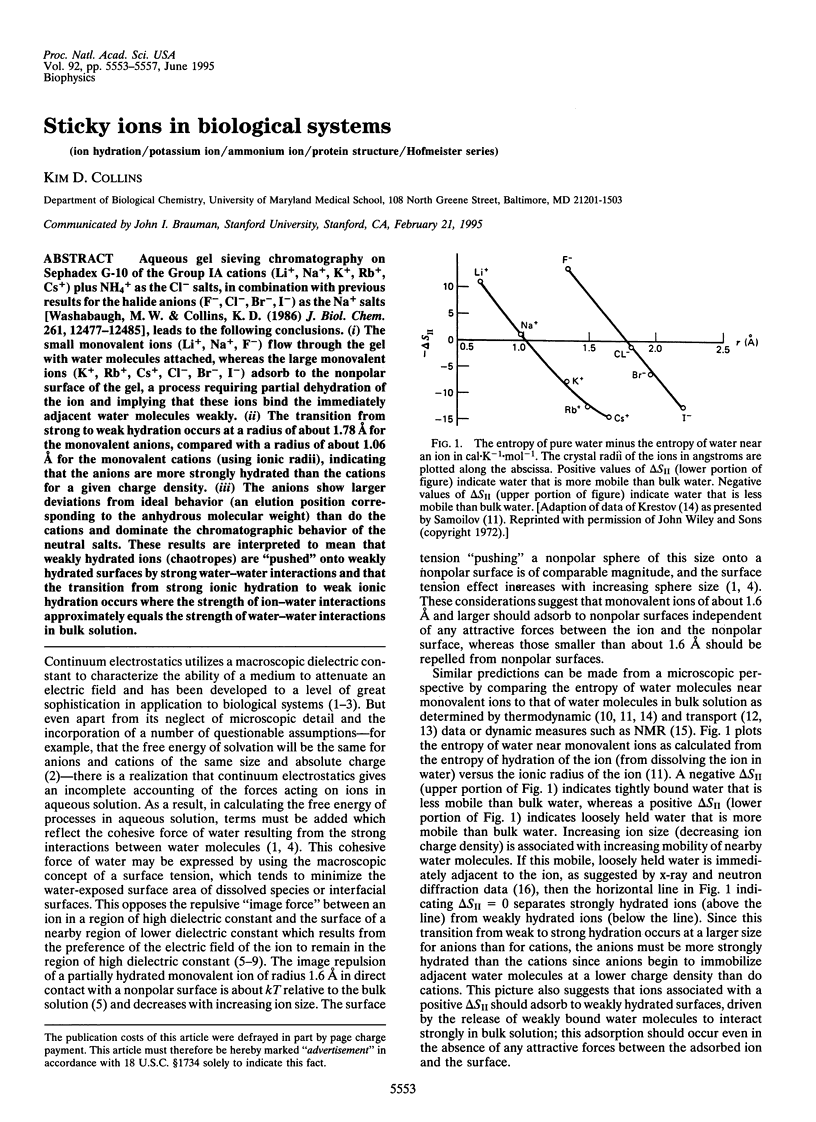


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREGMAN J. I. Cation exchange processes. Ann N Y Acad Sci. 1953 Nov 11;57(3):125–143. doi: 10.1111/j.1749-6632.1953.tb36392.x. [DOI] [PubMed] [Google Scholar]
- Brocchieri L., Karlin S. Geometry of interplanar residue contacts in protein structures. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9297–9301. doi: 10.1073/pnas.91.20.9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley S. K., Petsko G. A. Amino-aromatic interactions in proteins. FEBS Lett. 1986 Jul 28;203(2):139–143. doi: 10.1016/0014-5793(86)80730-x. [DOI] [PubMed] [Google Scholar]
- Collins K. D., Washabaugh M. W. The Hofmeister effect and the behaviour of water at interfaces. Q Rev Biophys. 1985 Nov;18(4):323–422. doi: 10.1017/s0033583500005369. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992 Mar;8(3):483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Varnum M. D., Osborne P. B., Christie M. J., Busch A. E., Adelman J. P., North R. A. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J Biol Chem. 1991 Apr 25;266(12):7583–7587. [PubMed] [Google Scholar]
- Kumpf R. A., Dougherty D. A. A mechanism for ion selectivity in potassium channels: computational studies of cation-pi interactions. Science. 1993 Sep 24;261(5129):1708–1710. doi: 10.1126/science.8378771. [DOI] [PubMed] [Google Scholar]
- Levitt M., Perutz M. F. Aromatic rings act as hydrogen bond acceptors. J Mol Biol. 1988 Jun 20;201(4):751–754. doi: 10.1016/0022-2836(88)90471-8. [DOI] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- Nair H. K., Seravalli J., Arbuckle T., Quinn D. M. Molecular recognition in acetylcholinesterase catalysis: free-energy correlations for substrate turnover and inhibition by trifluoro ketone transition-state analogs. Biochemistry. 1994 Jul 19;33(28):8566–8576. doi: 10.1021/bi00194a023. [DOI] [PubMed] [Google Scholar]
- Pérez-Cornejo P., Begenisich T. The multi-ion nature of the pore in Shaker K+ channels. Biophys J. 1994 Jun;66(6):1929–1938. doi: 10.1016/S0006-3495(94)80986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashin A. A., Bukatin M. A. A view of thermodynamics of hydration emerging from continuum studies. Biophys Chem. 1994 Aug;51(2-3):167–192. doi: 10.1016/0301-4622(94)00060-3. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Jr, Strottmann J. M., Stellwagen E. Prediction of neutral salt elution profiles for affinity chromatography. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2287–2291. doi: 10.1073/pnas.78.4.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydall J. R., Macdonald P. M. Investigation of anion binding to neutral lipid membranes using 2H NMR. Biochemistry. 1992 Feb 4;31(4):1092–1099. doi: 10.1021/bi00119a018. [DOI] [PubMed] [Google Scholar]
- Washabaugh M. W., Collins K. D. The systematic characterization by aqueous column chromatography of solutes which affect protein stability. J Biol Chem. 1986 Sep 25;261(27):12477–12485. [PubMed] [Google Scholar]



