Abstract
Patent foramen ovale (PFO) is defined as a valve-like opening at the level of foramen ovale or between septum primum and secundum without evidence of the anatomical defect. Paradoxical embolism (PDE) is an embolus passing through a defect PFO leading to end-organ dysfunction. PDE in septic shock is not yet reported in the literature. A 49-year male presented to the emergency department with shortness of breath since one day and pain in the left side of the chest. Chest x0 -ray revealed middle-left lobe pneumonia with pleural effusion; he was started on Co-amoxiclav, and admitted to the ward. After 6 h, his chest pain suddenly significantly increased difficulty in breathing and his oxygen saturation dropped. X-ray of the chest showed left pneumothorax, chest drain was inserted and he was intubated. He became hemodynamically unstable with maximum ventilatory support and noradrenalin. On day 4, he was found to have new pansystolic murmur in the tricuspid area. An echocardiogram revealed global hypokinesia, two mobile thrombi in the right atrial cavity, and PFO. It was noticed that his right toe had blackish discoloration. An angiogram showed occlusion of the right superficial femoral artery and immediately an embolectomy. On day 11, he was transferred to the ward. PDE needs a high index of suspicion in septic shock patients with ARDS. PDE requires PEEP adjustment, early anticoagulation, and thromboprophylaxis.
Keywords: Enoxaparin, heparin, paradoxical embolism, patent foramen ovale
INTRODUCTION
Patent foramen ovale (PFO) is an opening in the septum secundum, which allows blood flow from right atrium to the left side of the heart in the prenatal life; soon after birth, the PFO closes functionally and by first birthday it is closed permanently.[1] But one in five adult population will have Probe or pencil patent PFO; when pressure increased in the right atrium or flow is directed toward PFO due to the postoperative changes or the presence of the Eustachian valve can leads to significant hypoxia or paradoxical embolism (PDE) and may cause end-organ damage resulting in increased morbidity and morality. We report a case of PDE in a patient with acute respiratory distress and septic shock.
CASE REPORT
A 49-year male presented to the emergency department with complains of short of breath for 1 day, with pain in left chest and cough on and off since 3 weeks. He was febrile, tachycardic with stable blood pressure. Chest X-ray showed middle-left lobe pneumonia with pleural effusion. He had leucocytosis (20 × 103/dL). He was started on broad-spectrum antibiotic and analgesics and admitted in the ward. After 6 hours of admission, suddenly his chest pain significantly increased, had difficulty in breathing, and dropped his oxygen saturation (to 60%). He was supplemented with oxygen, x0 -ray chest showed left pneumothorax, [Figure 1] his saturation was not improving, inserted left chest drain and intubated. Gash of air with 500 mL of dirty colored fluid was drained; he was shifted to intensive care unit (ICU), his leucocytosis (32 × 103/dl) worsens and became hemodynamically unstable, and remained on maximum ventilation. Femoral central line was inserted and he was started on noradrenalin. His condition was deteriorated and showed picture of acute respiratory distress syndrome (ARDS). Antibiotic was changed to Meropenem, added enoxaparin to the therapy, and continued aggressive chest physiotherapy. On day 3, femoral central line was removed and inserted the subclavian central line. Day 4, he had new pansystolic murmur in the tricuspid area. Echocardiogram (transesophageal and transthorasic) revealed global hypokinesia with left ventricular ejection fraction of 35%, two mobile thrombus in the right atrial cavity, [Figure 2] right ventricular systolic pressure of 54 mmHg, and patent foramen of ovale [Figure 3].
Figure 1.
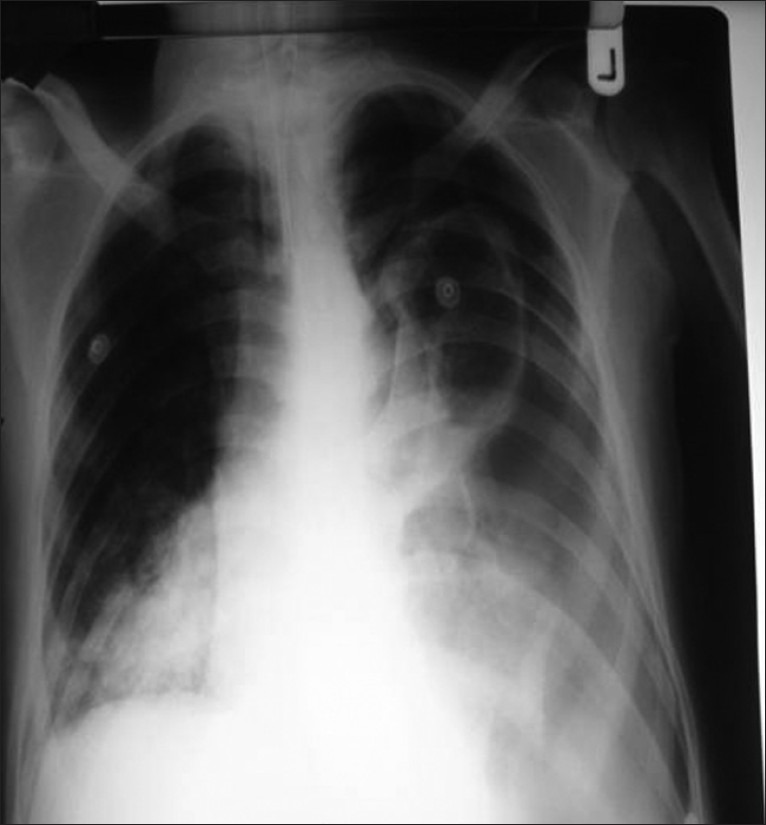
X-ray chest sohwing pneumothrorax
Figure 2.
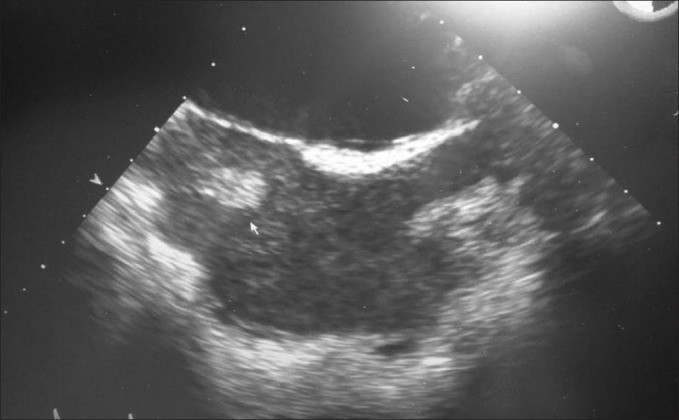
Echocardiogram showing thrombus
Figure 3.
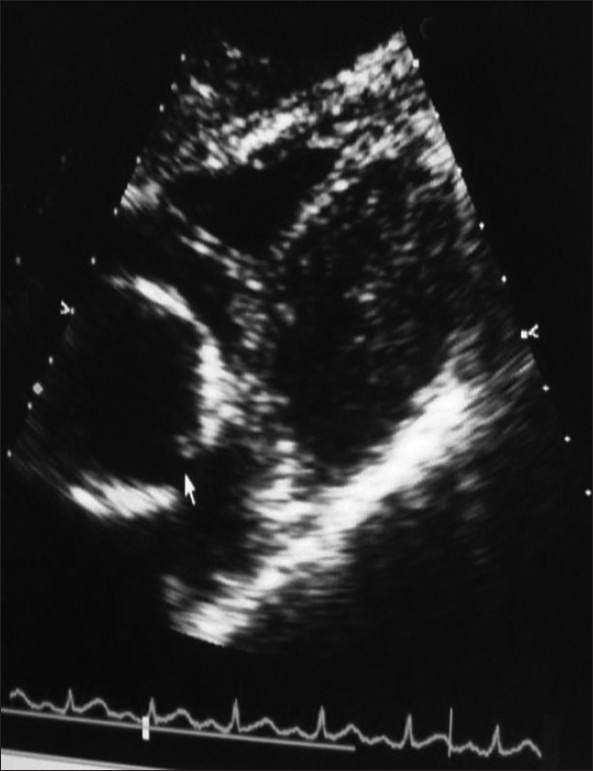
Echocardiogram showing PFO
Enoxaparin was stopped and he was started on heparin infusion. By the day 5, his hemodynamic and chest condition improved; he was weaned off from the vasopressor medication and inspired oxygen fraction was decreased to 40%. He was weaned from the ventilator and extubated on day 7. It was noticed that his right toe was having blackish discoloration and feeble right dorsalis pedis pulsations. Immediate angiogram showed complete occlusion of the right superficial femoral artery [Figure 4]. He had an immediate embolectomy. Heparin infusion was continued in the postoperative period and added warfarin to the treatment. He was transferred to the ward on day 11, from there discharged home to be followed in outpatient clinic.
Figure 4.
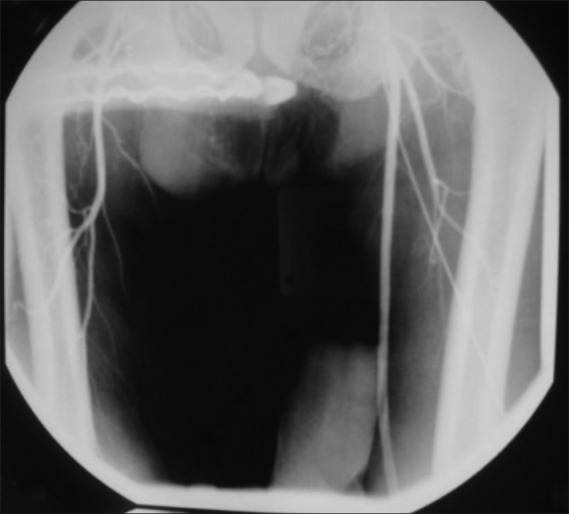
Femoral angiogram, showing femoral artery occlusion
DISCUSSION
PFO is a valve-like opening at the level of foramen ovale or between septum primum and secundum without evidence of the anatomical defect.[1] PDE is embolus (air, thrombus, or amniotic fluid) passes through a defect (PFO) from right side of the heart to the arterial circulation leading to end-organ dysfunction. The definitive diagnosis of PDE is done at autopsy or by direct visualization of thrombus passing through right to left shunt, but one should suspect the PDE when (i) arterial embolism occurs in the absences of proximal sources or left arterial embolism. (ii) Venous thromboembolism and demonstration of the intracardiac defect with right to left shunt. (iii) Evidence of transient or continuous increased right atrial pressure.[2]
Epidemiology
A PFO is found in 25% of the general population. The “pencil patent” PFO is present in 6% of the cases and a “probe patent” PFO is found in 29% of the patients, after a random 1000 autopsies.[3] There is no significant difference in the presence of PFO and gender. Usually the size of PFO varies from 1 to 19 mmHg and it is increasing with the age.[4] PDE is a rare phenomenon, it accounts for 2% of all systemic embolism.[5] If the right atrial pressure is increased, the chance of PDE will increased by 60%.[6]
Risk factors
There are no risk factors for the development of PFO. Factors that decide the perioperative and intensive care complications arising from the PFO are:
(i) size of PFO, (ii) pressure gradient between right atrium (RA) and left atrium (LA), and (iii) direction of the inferior Vena caval blood flow.
High risk PFO for complications are those that are associated with atrial septal aneurysm, spontaneous intracardiac passage of bubbles on contrast echocardiography without any provocative measures, tunnel-like PFO.[7]
Pathophysiology of PFO complications
During the prenatal life as the pulmonary vascular resistance is high, PFO allows blood flow from RA to LA; however, after the birth with first breath LA pressure exceeds the RA pressure due to significant decrease in pulmonary resistance and increased systemic vascular resistance. Functional closure of PFO occurs within the first year of life.[8] In significant number of population there will be a flap-like valve that is formed between septum primum and secundum, and it keeps foramen ovale patent or occurrence of preferential flow from inferior vena cava toward the PFO as a result of postsurgical changes or the presence of prenatal circulation (Eustachian valve). When due to any etiological reason RA pressure increases, it leads to either hypoxia or venous thrombus will cross to left side of the heart causing PDE.
Diagnosis
PFO was a postmortem diagnosis in the past, but due to improvement in the imaging technologies PFO is increasingly recognized in the patients. Various imaging studies that can detect PFO are transoeophageal/transthorcic echocardiography (TEE/TTE respectively), and transcranial Doppler.[9]
TEE is the gold standard for the detection of PFO. It is 100% sensitive and 92% specific. But the limitation of TEE is that its limited resolution; hence, it will detect he large PFO and the passage of the contrast or color flow through the defect has to be demonstrated to confirm the diagnosis. Commonly during the echocardiography, agigitated saline is used to confirm the PFO. Transcranial Doppler is a blind technique and needs provocative measures.[9]
Perioperative procedure with pathological significance of PFO
Neurosurgery
Sitting position posterior fossa surgery in patients with PFO will enhance the risk of paradoxical embolism. In posterior fossa surgery and laminectomies incidence varies from 25–100%.[10] It is of a critical value to detect the PFO, preoperatively in patients undergoing this type of surgeries, and if possible to avoid the said positions.
Orthopedic surgery
As compared to the general surgery patients the orthopedic surgery patients are at increased risk of developing PDE; this may be related to the deep venous thrombosis associated with surgery and possibility of fat embolism.[11]
Thoracic surgery
Many cases reported in the literature of hypoxia in up-right position (orthodeoxia) or when patient stands up (platypnea), particularly in patients with pneumonectomy, where the right atrial pressure is normal and because of the altered anatomical relationship between inferior vena cava flow with preferential flow through the PFO.[12]
Cardiac surgery
Post-CABG patients with PFO will have a worsening right to left shunt, most probably due to small pulmonary artery emboli, right ventricular (RV) infarction, loculated pericardial infarction causing right ventricular collapse, or transient right ventricular dysfunction. Patient with PFO planned for off pump coronary bypass graft surgery will have increased RV pressure on elevation of the heart and leading to hypoxia.[13]
Transplant surgery
Silent PFO in the transplanted heart results in hypoxia, due to post-transplant increased in pulmonary vascular resistance will provoke right to left shunt through PFO. Donor heart should be meticulously reassessed for the presence of PFO.
Endoscopic surgery
Pneumoperitoneum for laparoscopic surgeries due to intraabdominal hypertension lead to right to left shunt.[14]
Pathological significance of PFO in intensive care patients
Central venous catheterization
Patients with PFO are at the high risk of PDE (air) during insertion or removal of the central venous catheter.[15]
Mechanical ventilation
During the emergence from anesthesia, reaction to the endotreaheal tube and coughing causes temporary positive pressure between RA and LA in patients with PFO, leading to the hypoxia. In a comparative study for the effect of PEEP on shunt in patients with PFO, it is found that the shunt fraction increases with adding PEEP in patients with PFO where as it decreases in patients without PFO. it is also explained that PEEP induced increase in the pulmonary vascular resistance might also contributing to the hypoxia in patients with PFO.[16]
Acute respiratory distress syndrome
In these patients as a result of increased pulmonary artery pressure and positive pressure mechanical ventilation will cause increased in right atrial pressure leading to right to left shunt and hypoxia. In a recent study, it is reported that moderate to large PFO shunting occurred in 19.2% of the patient with acute respiratory distress syndrome (ARDS). They also demonstrated that in ARDS patients with PFO will have poor response to PEEP, greater use of adjunctive interventions, and a longer intensive care and hospital stay.[17]
Severe sepsis and septic shock
These patients are at increased risk of deep venous thrombosis (DVT) and recommended to have DVT prophylaxis.[18] Moreover with the presence of a femoral line will increase the risk of DVT.
Preoperative screening for PFO
Preoperative screening for PFO is justified only in those patients, in whom consequences may be devastating and a preventable strategy is feasible (posterior fossa surgery/ Cardiac transplant).[19]
Perioperative operative management of PFO related complications
The reversal of right to left shunt can be accomplished by the administration of positive inotropic medications, nitric oxide or both, in the setting of right ventricular failure or pulmonary hypertension. In patients with loculated pericardial effusion, draining the fluid is essential and patients with pulmonary embolism; thrombolysis will improve patient's condition.[10] Direct closer of PFO is treatment of choice during cardiac surgery and in patients with resistance hypoxia, platypnea orthodeoxia.[19]
Perioperative prevention of PFO related complications
In patients with the PFO and history of PDE, preoperative closer or perioperative anticoagulation should be considered, especially when the surgical procedure had a higher incidence of deep venous thrombosis.[19]
Treatment of PFO
Percutaneous device closure of PFO can be done with (i) cardioseal double umbrella device and (ii) amplaster septal occluder device.
Surgical closure is indicated, when the size of PFO is more than 25 mm, or device failure or during the open heart surgery.[20]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sukermik M, Bennett-Guerrero E. The incidental finding of patent foramen ovale during cardiac surgery: Should it always be repaired. A core review? Anesth Analg. 2007;105:602–10. doi: 10.1213/01.ane.0000278735.06194.0c. [DOI] [PubMed] [Google Scholar]
- 2.Guo S, Ingram R, Missri J. paradoxical embolism, deep vein thrombosis, pulmonary embolism in patient with patent foramen ovale. J Med Case Reports. 2007;1:104. doi: 10.1186/1752-1947-1-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson TE. Paradoxical embolism. Q J Med. 1930;23:135–50. [Google Scholar]
- 4.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during first 10 decade of life. An autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 5.Foster PP, Boriek AM, Butler BD, Gerhardt ML, Bove AA. Patent foramen ovale and systemic paradoxical embolism: A bibliographic review. Aviat Space Environ Med. 2003;74:b1–64. [PubMed] [Google Scholar]
- 6.Henry G, Walker P, Kern I. Femoral artery embolus post-appendiceectomy in a 12 year old boy. Pediatr Surg Int. 1996;11:187–8. doi: 10.1007/BF00183764. [DOI] [PubMed] [Google Scholar]
- 7.Hasan A, Parvez A, Ajmal MR. Patent foramen ovale- Clinical significance. JIndian Acad Clin Med. 2004;5:339–44. [Google Scholar]
- 8.Hara H, Viramani R, Ladich E, Mackey-Bojack S, Titus J, Reisman M, et al. PFO current pathology, Pathophysiology and clinical status. J Am Coll Cardiol. 2005;46:1768–76. doi: 10.1016/j.jacc.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Di Tullio M, Sacco RL, Venketasubramanian N, Sherman D, Mohr JP, Homma S. Comparison of the diagnostic technique for the detection of patent foramen ovale in stroke patients. Stroke. 1993;24:1020–4. doi: 10.1161/01.str.24.7.1020. [DOI] [PubMed] [Google Scholar]
- 10.Mammoto T, Hayashi Y, Ohnishi Y, kuro M. Incidence of venous and paradoxical air embolism in neurosurgical patients in the sitting position: Detection by tranesophageal echocardiography. Acta Anaesthesiol Scand. 1998;42:643–7. doi: 10.1111/j.1399-6576.1998.tb05295.x. [DOI] [PubMed] [Google Scholar]
- 11.Della Valle CJ, Jazrawi LM, Di Cesare PE, Steiger DJ. Paradoxical cerebral embolism, complication of a major orthopedic operation. A report of two cases. J Bone Joint Surg Am. 1999;81:108–10. doi: 10.2106/00004623-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Smeenk FW, Postmus PE. Interartrial right to left shunt developing after pulmonary resection in absence of elevated right sided heart pressures. Chest. 2003;103:528–31. doi: 10.1378/chest.103.2.528. [DOI] [PubMed] [Google Scholar]
- 13.Akhter M, Layos TZ. Pitfalls of undetected Patent foramen ovale in off pump cases. Am Thorac Surg. 1999;67:546–8. doi: 10.1016/s0003-4975(98)01246-6. [DOI] [PubMed] [Google Scholar]
- 14.Iwase K, Takeo T, Wantanabe H, Tanaka Y, Kido T, Sunada S, et al. Right to left atrial shunt through patent foramen ovale during pneumoperitoneum for laparoscopic cholecystectomy. Surg Endosc. 1994;8:1110–2. doi: 10.1007/BF00705732. [DOI] [PubMed] [Google Scholar]
- 15.Zuha R, Price T, Powles R, Treleaven J. Paradoxical emboli after central venous catheter removal. Ann Oncol. 2000;11:885–6. doi: 10.1023/a:1008303601457. [DOI] [PubMed] [Google Scholar]
- 16.Cujec B, Polasek P, Mayers I, Johnson D. Positive end expiratory pressure increases the right to left shunt in mechanically ventilated patients with patent foramen ovale. Ann Intern Med. 1993;119:887–94. doi: 10.7326/0003-4819-119-9-199311010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Mekontso Dessap A, Boissier F, Leon R, Carreira S, Roche Campo F, Lemaire F, et al. Prevalence and prognosis of shunting across patent foramen ovale during acute respiratory distress syndrome. Crit Care Med. 2010;38:1786–92. doi: 10.1097/CCM.0b013e3181eaa9c8. [DOI] [PubMed] [Google Scholar]
- 18.Delinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36:1394–6. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 19.Sukernik MR, Mets B, Bennett-Guerreno E. Patent foramen ovale and its significance in perioperative period. Anesth Analg. 2001;93:1137–46. doi: 10.1097/00000539-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Brawn MV, Fankender D. Transcather closure of PFO in patients with cerebral ischemia. J Am Coll Cardiol. 2002;39:2019–25. doi: 10.1016/s0735-1097(02)01904-6. [DOI] [PubMed] [Google Scholar]


