Abstract
Introduction:
Daily interruption of sedation could minimize the problem of sedatives accumulation. Nevertheless, whatever is the sedation strategy; sedation, particularly deep levels, has been associated with high frequency of patient-ventilator asynchrony. Extending these findings, one would expect that no sedation protocol could reduce the frequency of patient-ventilator asynchrony.
Aim:
To assess the effect of no sedation protocol compared with daily interruption of sedation on patient-ventilator asynchrony in surgical intensive care patients.
Materials and Methods:
The study included 230 patients who expected to require mechanical ventilation for more than 48 h. They were randomized to receive either continuous sedation (1 mg/mL midazolam) to achieve a Ramsay score of 3-4 with daily interruption until awake (group D; n = 115), or no sedation (group N; n = 115). Both groups received bolus doses of morphine (2.5-5 mg) as needed to achieve a score of ≤2 on behavioral pain scale.
Results:
No sedation was associated with significantly lower ineffective triggering and asynchrony index but significantly higher double triggering. Patient's effort during triggering was significantly higher during no sedation. The respiratory rate increased and the PaCO2 decreased significantly in no sedation group.
Conclusion:
No sedation protocol reduces the asynchrony index and preserves the patient's effort during triggering.
Keywords: Asynchrony, daily interruption, patient-ventilator, sedation, sedation protocol
INTRODUCTION
Patient-ventilator asynchrony, defined as a mismatch between patient and ventilator inspiratory and expiratory times, is a common problem that can complicate ventilatory support.[1,2] Asynchrony causes patient's discomfort and increases work of breathing and oxygen consumption.[1] Asynchrony has also been found to be associated with longer duration of mechanical ventilation and lower rates of successful weaning due to wasted diaphragmatic energy expenditure.[3,4] Several types of patient-ventilator asynchrony exist and can be detected easily by visual inspection of flow-time and airway pressure-time waveforms of mechanically ventilated patients. Among these, ineffective triggering and double triggering being the most common types.[3] Sedation is commonly used for maintaining of patient-ventilator synchrony.
There are different strategies for administration of sedation; of these, daily interruption of sedative infusion is the standard practice in our surgical intensive care unit. Daily interruption of sedation reduces the amount and duration of sedation and minimizes the adverse consequences of sedatives accumulation; including prolonged mechanical ventilation, organ system failure and high reintubation rates.[5,6]
Recently, Strom et al.[7] reported that a protocol of no sedation, involving administration of intravenous morphine as bolus doses but no infusion of sedatives, significantly reduces the duration of mechanical ventilation compared with daily interruption of sedation. In a study of medical intensive care patients, Wit et al.[8] found deeper levels of sedation to be associated with high-frequency of asynchrony particularly ineffective triggering. Extending these findings, one would expect that no sedation protocol could reduce the frequency of patient-ventilator asynchrony.
We undertook the present prospective randomized study to assess the effect of no sedation protocol compared with daily interruption of sedation on patient-ventilator asynchrony in surgical intensive care patients.
MATERIALS AND METHODS
Patients who expected to require invasive mechanical ventilation for more than 48 h on admission to the surgical intensive care were included in the study unless they were less than 18 years, in need for PEEP>8 cm H2O, pregnant, ventilated through a tracheotomy, hemodynamically unstable, or underwent brain surgery. Patients with head or chest trauma, postcardiac arrest, chronic obstructive pulmonary disease (COPD), or BMI > 35 kg/m2 were, also, excluded. The study was approved by our Local Ethics Committee and a written informed consent was obtained from patients’ relatives.
Patients were ventilated with event ventilator (event medical Ltd, Gaiway, Ireland) using pressure assisted control ventilation (PACV). Ventilatory sittings were adjusted to obtain a tidal volume (VT) around 6-8 mL/kg, a respiratory rate (RR) <30/min. and an I:E ratio from 1-2 to 1-3. Pressure triggering sensitivity was set between −0.5 and −1 cm H2O. PEEP and FIO2 were adjusted to maintain PaO2> 90 mmHg. Patients were switched from PACV to pressure support ventilation (PSV) when they were able to trigger all breaths. Pressure support level was set to obtain a VT around 6-8 mL/kg and a RR < 30/min. Cycling from inspiration to expiration occurred at 25% of peak inspiratory flow. Patients with PaO2/FIO2> 150 were kept on PACV.
Patients were randomized using closed sealed opaque envelopes to receive either daily interruption of sedation (group D; control group) or no sedation protocol (group N). The ICU team was responsible for patient care, ventilator settings, and management of sedation.
During the first 24 h from the start of mechanical ventilation, all patients received continuous infusion of midazolam (1 mg/mL) to achieve a Ramsay score[9] of 3-4 and IV boluses of morphine (2.5-5 mg) as needed to achieve a score of ≤ 2 on the behavioral pain scale (BPS).[10] The Ramsay score and BPS were investigated every 2-3 h to adjust the midazolam infusion and check for the need of morphine, respectively. After that (1) in group D: the morphine boluses was maintained; and the midazolam infusion was stopped daily, then restarted at 50% of the previous dose when patients were awake or agitation prevented successful waking, and titrated to achieve a Ramsay score of 3-4. Patients were considered “awake” if they were able to do three of the following actions on request:[7] open the eyes, follow the investigator by eyes, squeeze the hand, or stick out the tongue. (2) In group N; the midazolam infusion was stopped and they were kept on the morphine boluses only. If they were uncomfortable at any time, causes for discomfort (e.g., hypoxia, tube obstruction, inappropriate position, high machine alarms, bright lights, pain, and delirium) were investigated and treated; and they were verbally reassured as frequent as possible. Intravenous haloperidol boluses (1-5 mg) were given when delirium was suspected. If these actions failed to comfort the patients, they were sedated with propofol infusion (10 mg/mL) to achieve a Ramsay score of 3-4 for 6 h. Then, a new attempt to manage the patient without sedation was tried.
Patients were excluded from the study after enrollment if (1) they died or weaned during the study; (2) they were in need for muscle relaxants; (3) three consecutive trails of management without sedation in group N were failed due to agitation and fighting ventilator. In latter two situations, the patient was kept sedated with daily interruption of sedation.
Patients’ characteristics including age, sex, weight, acute physiology, and chronic health evaluation II (APACHE II) score, sequential organ failure assessment (SOFA), total morphine dose during the period, and reasons for mechanical ventilation were recorded. After the first 24 h from the start of mechanical ventilation, each patient underwent three observation periods per day, 15 min each, separated by 6-8 h for three consecutive days. During these periods, ventilator settings were not modified; and the ventilator airway pressure-time and flow-time waveforms were captured, downloaded, enlarged, and printed for counting the following asynchrony variables:[3] (1) ineffective triggering, defined as a simultaneous decrease in airway pressure and an increase in airflow without assisted cycle. (2) Double triggering, defined as two breaths separated by an expiratory time <50% of mean inspiratory time (Ti). (3) Short cycled breath, defined as a breath cycle with Ti < 50% of mean Ti. (4) Long-cycled breath, defined as a breath cycle with Ti> double mean Ti. (5) Asynchrony index, defined as sum of ineffectively triggered, double triggered, short-cycled, and long-cycled breaths divided by sum of triggered and ineffectively triggered breaths. Mean Ti equaled the average of 30 randomly selected breaths during each observation period. The inspiratory time was measured from breath initiation to cessation of the ventilator flow.
The printed waveforms of each period were given a code number and were analyzed by two of the study investigators who were blinded to group assignment of waveforms. The average of each asynchrony variables per patient was recorded for statistical analysis.
The primary outcome endpoint of the study was the effect of each sedation strategy on the asynchrony index. Secondary endpoints included patient's effort during triggering, ventilatory parameters (RR, VT, peak airway pressure (Paw), mean airway pressure (Pmean), plateau pressure (Pplat), PEEP and pressure support), and arterial blood gases (ABGs). The values of each parameter were averaged and recorded. Patient's effort was assessed by (1) the Paw drop below baseline 0.1 s after initiation of patient's effort (P0.1) and the maximal drop of Paw during the triggering phase (Ptr).[11]
Statistics
Sample size calculation was based on the study of Wit et al.[8] The power of the study was estimated at 95% confidence interval and P = 0.05. A total 188 patients were required. We included 230 patients after calculation of a dropout rate of 15%. Results were analyzed using SPSS V.11.5. Data were expressed as mean ± SD, or number (percent) where appropriate. Unpaired student's t-test and Mann–Whitney U test were used where appropriate for comparison of quantitative data between groups. Qualitative data were assessed using Fisher's exact test. A P value < 0.05 was considered statistically significant.
RESULTS
The study included 230 patients. They were randomly assigned in a 1:1 ratio to either group D (daily interruption of sedation; n = 115) or group N (no sedation; n = 115). Nineteen patients in group D and 20 patients in group N were excluded after enrollment, leaving 96 and 95 in group D and N, respectively. Exclusion was due to death (seven (6.1%) patients in group D and four (3.5%) patients in group N); requirement for muscle relaxant in group D (12 (10.4%) patients), or failure of the no sedation protocol (16 (13.9%) patients; of them 11 (68.8%) patients required muscle relaxants). In group D, 29 (30.2%) of enrolled patients failed the first awakening trail; of them 14 (48.2%) patients failed the second trail. In group N, 29 (30.5%) of enrolled patients developed agitation and required propofol infusion (zero to two times/day). Delirium occurred in 9 (9.3%) patients in group D and 21 (22.1%) patients in group N. The total doses of haloperidol (mg/day) used for management of these patients were comparable in both groups (P = 0.854).
Patients in group D underwent 864 observation periods (296 (34.3%) of them while they were awake and 502 (58.1%) during PSV). While in group N, 855 recording periods were performed (131 (15.3%) of them during propofol infusion and 571 (66.7%) during PSV). The Patients’ characteristics for the included patients were comparable except for the total dose of morphine during the study period that was significantly higher in group N (P = 0.011) [Table 1].
Table 1.
Patients’ characteristics
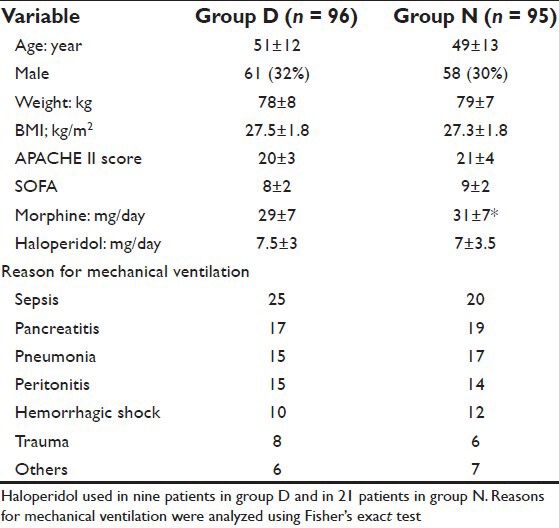
Table 2 showed that daily interruption of sedation was associated with significantly higher rate of asynchrony as assessed by the asynchrony index (P = 0.025). Ineffective triggering and double triggering were the most common types of asynchrony. Ineffective triggering occurred in 86 (89.5%) patients (82% of asynchrony events) in group D; and 79 (83.2%) patients (64% of events) in group N. While double triggering occurred in 26 (27.1%) patients (16% of events) in group D; and 61 (64.2%) patients (34% of events) in group N. Ineffective triggering was significantly higher in daily interruption of sedation group than in no sedation group (P = 0.001) [Figure 1]. On the contrary, double triggering was significantly higher in no sedation group (P = 0.00) [Figure 2]. Short-cycled and long-cycled breaths were comparable in both groups (P = 0.376 and 0.998, respectively). Patients’ effort as assessed by the P0.1 and Ptr was significantly higher in no sedation group (P = 0.001 and 0.005, respectively) [Figures 1 and 2]. The patients’ RR increased significantly in absence of sedation (P = 0.002). The VT and the mean Ti were comparable (P = 0.565 and 0.157, respectively). Paw, Pmean, Pplat, PEEP, and pressure support values were comparable in both group (P = 0.348, 0.220, 0.511, 0.308, and 0.274, respectively). The PaCO2 decreased significantly in group N compared to group D (P = 0.001). PH, PaO2, and HCO3 did not show significant changes (P = 0.575, 0.072, and 0.915, respectively).
Table 2.
The studied variables
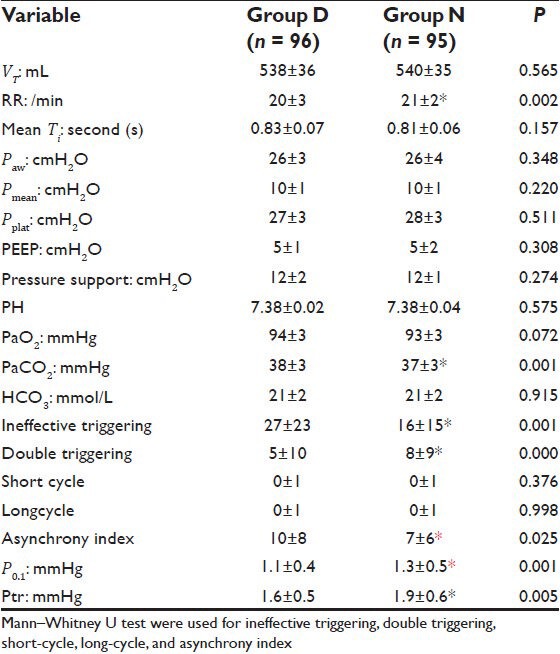
Figure 1.
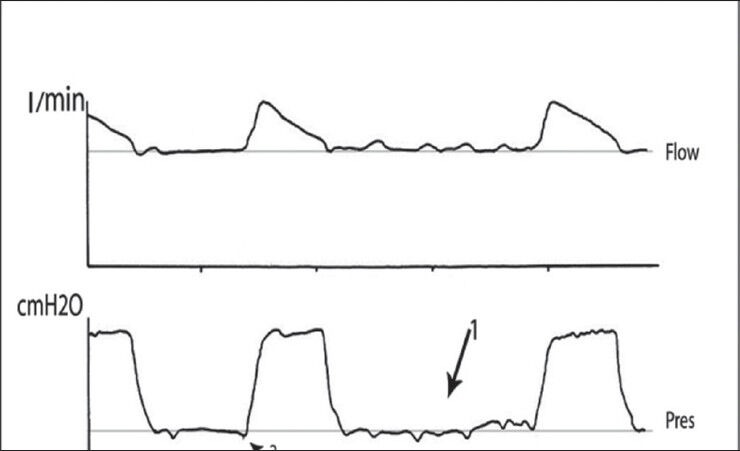
Flow and airway pressure waveforms in daily interruption of sedation group showing two effectively triggered breaths separated by four ineffectively triggered breaths during PSV (arrow 1) and reduced patient's effort (arrow 2)
Figure 2.
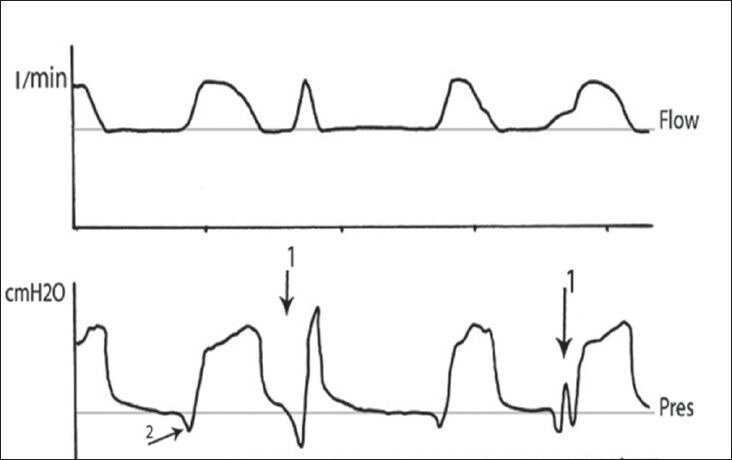
Flow and airway pressure waveforms in no sedation group showing double triggering during PSV (arrow 1) and increased patient's effort (arrow 2)
DISCUSSION
Sedation is commonly used to assist in adapting critically ill patient to mechanical ventilation though evidence to support this practice is insufficient.[8,12] Trigger asynchrony occurred in 97% of sedated patients[13] and in 10.9% of alert patients enough to provide informed consent for study participation.[4] However, these studies included patients at different stages of illness. In the former study, patients were in early stages of illness while in the latter study, they were admitted to a weaning center after prolonged mechanical ventilation (3-371 days).
In this study, we examined two comparable groups of patients at similar stages of illness. We found patients received no sedation protocol had significantly lower frequency of ineffective triggering and thus lower asynchrony index than patients received daily interruption of sedation. However, no sedation protocol was associated with a higher frequency of double triggering. This is in agreement with the results of Wit et al.[8] who studied the relationship between depth of sedation and patient-ventilator synchrony. They found “ineffective triggering increases linearly with deeper levels of sedation; for every one unit decrease in the Richmond Agitation-Sedation Scale, ineffective triggering index increased by 2.7%.”
Ineffective triggering and double triggering were the most common types of patient-ventilator asynchrony in our study as reported previously.[3,8] Ineffective triggering occurs when patient's effort fails to drop airway pressure below ventilator trigger sensitivity. Therefore, ineffective triggering results mainly from improper ventilatory settings (e.g., inappropriate trigger sensitivity) or abnormal pulmonary mechanics.[4,8] In this study, we used a low trigger sensitivity (from −5 to −1 cmH2O) in both groups to exclude its effects, though proper sitting of trigger sensitivity failed to eliminate ineffective triggering in a previous trail.[4]
Abnormal pulmonary mechanics cause ineffective triggering by two important mechanisms: inability to reduce the airway pressure below the level of intrinsic PEEP or inability to overcome expiratory recoil (e.g., severe muscle weakness and flail chest).[14] Thille et al.[3] investigated factors associated with patient-ventilator asynchrony during assisted mechanical ventilation in 62 critically ill patients, of them 16 (26%) had COPD. They found that an excess of pressure support and tidal volume may prolong mechanical inspiration and precipitates intrinsic PEEP due to shortening of expiratory time; and thus increases ineffective triggering. This is true in patients with COPD who already have dynamic hyperinflation and may be affected by the short expiratory time.[2,4,14,15] In this study, we did not measure the intrinsic PEEP, nevertheless high intrinsic PEEP is unlikely the cause of ineffective triggering difference as the plateau pressure, a marker of intrinsic PEEP, was comparable between groups. Moreover, we excluded patients with COPD and both groups were comparable concerning pressure support, tidal volume, mean inspiratory time and mean airway pressure.
Alternatively, we demonstrated lower P0.1 and Ptr in patients who received daily interruption of sedation. Decreases in these parameters indicate decreased respiratory drive;[11] suggesting that the higher frequency of ineffective triggering in this group might result from decreased respiratory drive associated with deeper levels of sedation. Our results support those of Grasso et al.,[16] who found inspiratory muscle effort decreases incrementally with deep levels of sedation. On the other hand, patients received no sedation experienced a higher frequency of double triggering. These patients showed higher P0.1 and Ptr, higher respiratory rate and lower PaCO2; suggesting that they had a higher respiratory drive. This is in consistent with the previous studies related double triggering to the greater drive to breathe.[3,17]
Patient-ventilator asynchrony increases respiratory work that contributes to respiratory muscle injury or fatigue and delays weaning.[1,18] Moreover, asynchrony may introduce a bias during recording of respiratory rate.[4,8] The ventilator will not record ineffectively triggered breaths, while double triggered breaths falsely increase the respiratory rate. This bias can affect decisions concerned with ventilator sitting and weaning tolerance.
In this study, we observed that the morphine dose was higher in no sedation group; probably because of synergism between benzodiazepine and morphine that reduced its dose in daily interruption of the sedation group.[5] Morphine has sedative effect, and therefore no sedation group patients received some sedation. However, the morphine dose was considerably low to affect their respiratory drive.
The incidences of agitation and delirium were slightly higher in no sedation group than in daily interruption of sedation group emphasizing the importance of more frequent assessment for anxiety, agitation, and adaptation to ventilator during no sedation protocol.
Our study limited by being unblinded because of its practical nature. However, we tried to minimize this problem by masking the group assignment of the printed airway pressure and flow waveforms during their analysis. Second, we assessed patients during two modes of mechanical ventilation. Thille et al.[3] showed that PSV may reduce the incidence of double triggering than ACV because of the partial dependency of the inspiratory time on the patient's ventilatory demand during PSV, while it is preset on the ventilator during ACV. In addition, the inspiratory time during PSV tend to be longer than the patient's neural inspiratory time.[19] On the contrary, Varon et al.[13] reported no association between trigger asynchrony and ventilator mode. Lastly, we focused on major pattern of asynchrony that can be easily detected by analyzing the flow and airway pressure waveforms. Consequently, we missed other asynchronies such as expiratory asynchrony. However, the previous studies[3,4,20] support the reliability of noninvasive asynchrony detection based on analysis of the flow and airway pressure waveforms. Concerning expiratory asynchrony, it is surprising to see perfect expiratory synchrony between patient and ventilator and it can be an important problem in obstructive patients who were excluded from the study.[21]
In conclusion, we found no sedation protocol associated with lower asynchrony index in surgical intensive care patients as it preserves the patient's effort during triggering. Nevertheless, in view of the study limitations, further studies including different groups of patients using different modes of ventilation and invasive methods for detection of patient-ventilator asynchrony are required to study this promising finding extensively.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sassoon CS, Foster GT. Patient-ventilator asynchrony. Curr Opin Crit Care. 2001;7:28–33. doi: 10.1097/00075198-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Tobin MJ, Jubran A, Laghi F. Patient-ventilator interaction. Am J Respir Crit Care Med. 2001;163:1059–63. doi: 10.1164/ajrccm.163.5.2005125. [DOI] [PubMed] [Google Scholar]
- 3.Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during mechanical ventilation: Prevalence and risk factors. Intensive Care Med. 2006;32:1515–22. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 4.Chao DC, Scheinhorn DJ, Stearn-Hassenpflug M. Patient-ventilator trigger asynchrony in prolonged mechanical ventilation. Chest. 1997;112:1592–9. doi: 10.1378/chest.112.6.1592. [DOI] [PubMed] [Google Scholar]
- 5.Kress JP, Pohlman A, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 6.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–8. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 7.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomized trail. Lancet. 2010;375:475–80. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 8.Wit MD, Pedram S, Best AM, Epstein SK. Observational study of patient-ventilator asynchrony and relationship to sedation level. J Crit Care. 2009;24:74–80. doi: 10.1016/j.jcrc.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stawicki SP. Sedation scales: Very useful, very underused. OPUUS 12 Scientist. 2007;1:10–2. [Google Scholar]
- 10.Aïssaoui Y, Zeggwagh AA, Zekraoui A, Abidi K, Abouqal R. Validation of a Behavioral Pain Scale in critically ill, sedated, and mechanically ventilated patients. AnesthAnalg. 2005;101:1470–6. doi: 10.1213/01.ANE.0000182331.68722.FF. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Huang YT, MacIntyre NR. Patient-ventilator synchrony during pressure-targeted versus flow-targeted small tidal volume assisted ventilation. J Crit Care. 2007;22:252–7. doi: 10.1016/j.jcrc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Rhoney DH, Murry KR. National survey of the use of sedating drugs, neuromuscular blocking agents, and reversal agents in the intensive care unit. J Intensive Care Med. 2003;18:139–45. doi: 10.1177/0885066603251200. [DOI] [PubMed] [Google Scholar]
- 13.Varon J, Fromm R, Rodarte J. Prevalence of patient ventilator asynchrony in critically ill patients (abstract) Chest. 1994;106:141S. [Google Scholar]
- 14.Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patient effort, and dyspnea. Am J RespirCrit Care Med. 1997;155:1940–8. doi: 10.1164/ajrccm.155.6.9196100. [DOI] [PubMed] [Google Scholar]
- 15.Vitacca M, Bianchi L, Zanotti E, Vianello A, Barbano L, Porta R, et al. Assessment of physiologic variables and subjective comfort under different levels of pressure support ventilation. Chest. 2004;126:851–9. doi: 10.1378/chest.126.3.851. [DOI] [PubMed] [Google Scholar]
- 16.Grasso S, Fanelli V, Cafarelli A, Dalfino L, Ingenito G, Ancona G, et al. Patient ventilator interaction during PSV at different levels of sedation in ALI patients. Intensive Care Med. 2004;30:A13. [Google Scholar]
- 17.Tokioka H, Tanaka T, Ishizu T, Fukushima T, Iwaki T, Nakamura Y, et al. The effect of breath termination criterion on breathing patterns and the work of breathing during pressure support ventilation. AnesthAnalg. 2001;92:161–5. doi: 10.1097/00000539-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 18.Hunter KD, Faulkner JA. Pliometric contraction-induced injury of mouse skeletal muscle: Effect of initial length. J Appl Physiol. 1997;82:278–83. doi: 10.1152/jappl.1997.82.1.278. [DOI] [PubMed] [Google Scholar]
- 19.Beck J, Gottfried SB, Navalesi P, Skrobik Y, Comtois N, Rossini M, et al. Electrical activity of the diaphragm during pressuresupport ventilation in acute respiratory failure. Am J RespirCrit Care Med. 2001;164:419–24. doi: 10.1164/ajrccm.164.3.2009018. [DOI] [PubMed] [Google Scholar]
- 20.Giannouli E, Webster K, Roberts D, Younes M. Response of ventilator-dependent patients to different levels of pressure support and proportional assist. Am J Respir Crit Care Med. 1999;159:1716–25. doi: 10.1164/ajrccm.159.6.9704025. [DOI] [PubMed] [Google Scholar]
- 21.Du H, Yamada Y. Expiratory asynchrony. Respir Care Clin. 2005;11:265–80. doi: 10.1016/j.rcc.2005.02.001. [DOI] [PubMed] [Google Scholar]


