Abstract
Background:
Anesthesia and surgery-induced neuroendocrine stress response can be modulated by appropriate premedication. The present study was designed to assess the clinical efficacy of dexmedetomidine versus fentanyl premedication for modulation of neuroendocrine stress response by analyzing the perioperative variation of blood glucose level during laparoscopic cholecystectomy under general anesthesia.
Subjects and Methods:
In a prospective randomized double-blind study, 60 adult consented patients of either sex with ASA I and II, scheduled for elective laparoscopic cholecystectomy under general anesthesia and meeting the inclusion criteria, were allocated into two groups. Group D patients (n = 30) were given intravenous dexmedetomidine 1μg/kg and Group F patients (n = 30) received fentanyl 2 μg/kg, given over a 10-min period, before induction of anesthesia. Perioperative blood glucose levels were analyzed preoperatively, at 30 min after beginning of surgery, and 2.5 h after surgery. Anesthetic and surgical techniques were standardized. All patients were also assessed for intraoperative hemodynamic changes of heart rate and mean arterial pressure at specific timings.
Results:
Blood glucose concentration has shown 20% increase after surgery. The differences between groups were not statistically significant as observed by analyzing the variation of serial perioperative blood glucose estimation. Both premedicants had attenuated the hemodynamic and neuroendocrine stress response of pneumoperitoneum and general anesthesia. The dexmedetomidine group showed more stabilization of intraoperative hemodynamics of mean arterial blood pressure and heart rate when compared to fentanyl group.
Conclusion:
During the laparoscopic cholecystectomy, dexmedetomidine and fentanyl, both premedicants have effectively modulated the neuroendocrine stress response of general anesthesia as assessed by analysis of perioperative blood glucose variation, but dexmedetomidine was better.
Keywords: Dexmedetomidine, fentanyl, laparoscopic cholecystectomy, neuroendocrine stress response, pneumoperitoneum
INTRODUCTION
Patients about to undergo anesthesia for surgery are almost inevitably anxious. There are several factors for these underlying fears which reflect the stress of the circumstances. Surgical procedures also induce complex stress responses, manifested by metabolic, neurohumoral, and immunological changes.[1,2] Hyperglycemia is a feature of the metabolic response to surgery and depends on patient age, anesthetic technique, severity of tissue trauma, type and magnitude of surgery, total operative time, amount of intraoperative blood loss, and postoperative pain.[3,4,5]
The laparoscopy cholecystectomy is considered as a low stress level surgery with fewer pulmonary complications and more rapid convalescence, but it predictably leads to increased hemodynamic stress responses.[6,7] Decreasing the stress response to surgery is of high relevance to the anesthesiologist. The choices of premedication and anesthetic techniques are able to influence the neurohormonal stress response by modulating the pathophysiological pathways.[8,9,10] Various pharmacological agents like nitroglycerine, beta blocker, and opioids were used to decrease surgical stress of laparoscopic procedures to improve outcome, with their own limitations.[11,12]
Dexmedetomidine, a α2-adrenergic agonist, has shown clinically useful drug profile due to its sympatholytic, hypnotic, sedative, anxiolytic, analgesic, and anesthetic sparing effects without respiratory depression. It has also shown attenuation of the hemodynamic responses associated with laryngoscopy by reducing norepinephrine release.[13,14]
The objective of the present study was to investigate the influence of dexmedetomidine and fentanyl premedication on modulation of neuroendocrine stress response during laparoscopic cholecystectomy under general anesthesia by analyzing the variation of perioperative serial blood glucose levels.
SUBJECTS AND METHODS
Selection of patients and randomization
The protocol of this double-blind prospective randomized study was approved by the Institutional Ethical Committee, and written informed consent was obtained from all patients. Sixty otherwise healthy adult patients of ASA physical status I and II of either sex, aged 32-65 years, scheduled for elective laparoscopic cholecystectomy under general anesthesia from August 2011 to February 2012 were enrolled. None of the patients were suffering from cardiac, pulmonary, hepatic, renal, or metabolic disorders, or were receiving medications which affect sympathetic response or hormonal secretions. Complicated surgeries of more than 2 h were also excluded. All patients were evaluated preoperatively with review of biochemical investigations.
The patients were randomized into two study groups of 30 patients each according to computer-generated code. The drug preparation was done by an anesthesiologist who was blinded to the study protocol and the observer was kept blinded about the randomization schedule of the study protocol. All patients were operated by standard technique of laparoscopic cholecystectomy during morning hours to minimize variability in the secretion of hormones.
Anesthetic technique
All patients were premedicated with oral alprazolam 0.25 mg the night before surgery. On the day of surgery, they received premedication of glycopyrrolate 0.2 mg intramuscularly 30 min prior to induction of anesthesia. On arrival to operation room, routine, hemodynamic monitoring was performed by automatic blood pressure measurements, five-lead ECG monitor, and finger pulse oximetry. An intravenous infusion of ringer lactate was started, followed by intravenous metoclopramide 10 mg and midazolam 2 mg. Group D patients (n = 30) were given intravenous dexmedetomidine 1μg/kg and Group F patients (n = 30) were given fentanyl 2 μg/kg, over a 10-min period before induction of general anesthesia.
After preoxygenation for 3 min, the anesthesia was induced with propofol (2 mg/kg) and tracheal intubation was facilitated by vecuronium 0.1 mg/kg. Anesthesia was maintained with isoflurane 1-1.5% and 60% nitrous oxide in oxygen with supplementary fentanyl (50-100 μg) to maintain the heart rate and mean arterial pressure within 20% of preinduction values and/or heart rate <85 beats/min during surgical stimulation. The patient's lungs were initially mechanically ventilated with a tidal volume of 8 ml/kg, a respiratory rate of 12 breaths/min, and an I:E ratio of 1:2 in volume-controlled mode. Five minutes after securing the airway and abdominal insufflation by carbon dioxide, the lung mechanics were adjusted to maintain normocapnia (an end-tidal carbon dioxide value of 35-40 mm Hg) and intra-abdominal pressure was maintained between 12 and 15 mm Hg. The degree of muscle relaxation was maintained using the train-of-four ratio of <25% with supplemental doses of vecuronium bromide (0.05 mg). All patients were covered to maintain normothermia.
All patients were assessed for changes in hemodynamic parameters of heart rate and mean arterial pressure prior to premedication, before induction, after intubation, and after pneumoperitoneum, followed by every 5 min for 30 min, thereafter every 15 min till the end of surgery and after extubation. Intraoperatively, any bradycardia or tachycardia, hypotension or hypertension was managed as required.
At the end of surgery, the surgeon administered 10 ml ropivacaine 0.75% in the peritoneum through the trocar and the anesthetics were discontinued. The residual neuromuscular block was reversed with neostigmine 0.05 mg/kg and atropine 0.02 mg/kg, and the trachea was extubated when respiration was adequate and patient was able to obey simple commands. All patients received ketorolac 30 mg intravenously at the end of procedure. The patients were transferred to post-anesthesia care unit and were observed for any hemodynamic abnormalities, respiratory depression (respiratory rate <8 breaths/min), or hypoxemia (SpO2 < 94%), shivering, nausea, and vomiting, and managed accordingly.
Blood samples were analyzed by glucometer (Abbott Optium Xceed) for blood glucose level preoperatively, at 30 min after beginning of surgery, and at 2.5 h postoperatively.
Statistical analysis
A sample size was decided to ensure power 0.80 for detecting reduction by 20% in hemodynamic parameters. Assuming a 5% dropout rate, the final sample size was set at 60 patients. Statistical analysis was done with SPSS software for windows, arithmetic mean and standard deviation values for different variables were calculated, and statistical analyses were performed for each group. Paired t-test was used to analyze each variable versus baseline level and Chi-square test was used to analyze non-continuous parameters. Differences in blood glucose levels in both groups were analyzed by Student's t test. P < 0.05 was considered statistically significant.
RESULTS
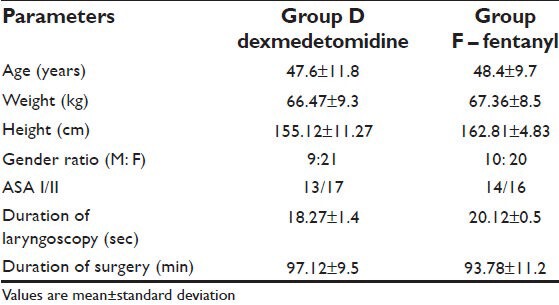
A total of 60 patients were randomly assigned to two groups of 30 patients each. Both groups were comparable in age, weight, height, and gender. There was no difference between groups for anesthetic technique and mean operative time. Duration of anesthesia did not differ among the study groups [Table 1].
Table 1.
Demographic profile of patient
During surgery, there were no significant changes in blood pressure and heart rate, confirming adequate depth of anesthesia. In spite of hemodynamic effects of pneumoperitoneum, all variations of blood pressure and heart rate never exceeded more than 15% of baseline value. Hemodynamic variables recorded at specified timings during the study are shown in Table 2. In dexmedetomidine group, the hemodynamic changes were more stabilized during perioperative period as compared to fentanyl group. Rapid intravenous infusion was needed in five patients of Group D to treat hypotension, but vasoactive drugs were not required as no patient had persistent or severe hypotension.
Table 2.
Heart rate and mean arterial blood pressure changes during laparoscopic cholecystectomy
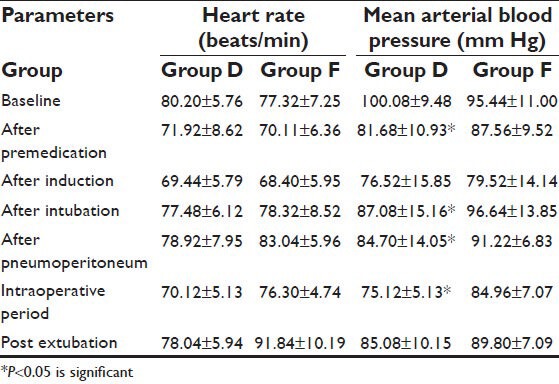
Skin body temperature was constantly maintained between 35.8°C and 36.5°C. Estimated blood loss did not exceed 342 ml (289 ± 57 ml) and did not necessitate blood transfusion. No significant anesthesiological or surgical complications occurred during the present study.
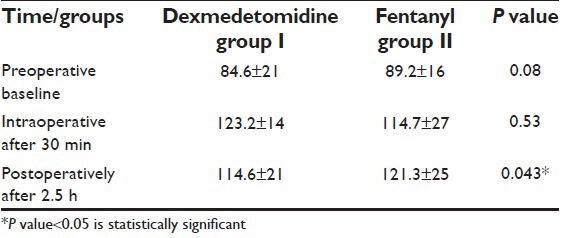
Blood glucose concentration significantly increased during the surgery and remained increased postoperatively in both groups. This postoperative increment was significantly more pronounced after the fentanyl premedication (121.3 ±25 vs. 114.6 ± 21 mg/dl; P < 0.05) [Table 3].
Table 3.
Perioperative blood glucose concentration (mg/dl) during laparoscopic cholecystectomy
DISCUSSION
The present study has compared the clinical efficacy of dexmedetomidine versus fentanyl premedication for modulation of neuroendocrine stress response by analyzing the variation of perioperative blood glucose level during laparoscopic cholecystectomy under general anesthesia. The near-stable intraoperative hemodynamic values were an indication of adequate analgesic and sedative effects of dexmedetomidine and fentanyl premedication. The hemodynamic results of our study are in agreement with the results of recent studies with dexmedetomidine and fentanyl.[14,15,16]
Blood glucose concentration increased during and after surgery in both groups, which is a common metabolic response to surgical trauma. This increment, however, was more pronounced in fentanyl group than in dexmedetomidine group. Bessey et al. have suggested that increased circulating concentrations of catecholamines, glucagons, and cortisol can evoke the changes in carbohydrate metabolism, occurring immediately after trauma.[17] The characteristic metabolic effect of cortisol is to decrease the rate at which insulin activates the glucose uptake system; hence, surgical stress led to greater activation of neuroendocrine response and hepatic gluconeogenesis under perioperative conditions, which corresponds to hemodynamic behavior and postoperative pain. Iwaska et al. have observed impaired glucose tolerance and diminished insulin secretion in response to intravenous glucose during prolonged radical neck surgery.[18]
Normotensive patients can develop sympathetic overactivity during Intraoperative period due to pain, light plane of anesthesia, and hypercapnia. This adrenergic response is caused by nociceptive pathways and humoral mediators originating from the surgical site, which are detrimental in elderly and hemodynamically compromised patients. Numerous surgical and anesthetic techniques have been used to reduce the incidence and severity of these hemodynamic responses. Clinical evidence showed that the choice of the anesthetic techniques influences the stress response by modulating the pathophysiological pathways that induce neurohormonal and immunological alterations.[10,18,19]
Dexmedetomidine, a α2-adrenergic agonist, has been in use as an anesthetic adjuvant during balanced anesthetic regimen and its preoperative use has attenuated the intraoperative hemodynamic response by reducing the nociceptive transmission and decreasing norepinephrine concentration in serum.[13,14] In our study, intravenous dexmedetomidine 1 μg/kg or fentanyl 2 μg/kg has effectively modulated the neuroendocrine response of laryngoscopy and laparoscopy.
Blood glucose levels have been shown to correlate with catecholamine levels in trauma patients. There is strong correlation between hyperglycemia and poor outcome. Thorell et al. have recently investigated that how intensive insulin therapy leads to normoglycemia in critically ill trauma patients.[20] Multiple theories have been proposed for better outcome of trauma patients, including a decreased infection rate, reduced endothelial dysfunction, and reduced hyperglycemic axonal damage.[21] Yendamuri et al. have shown significant correlation between glucose level at admission and urinary tract infection.[22]
In our study, attempts were done to standardize anesthetic and postoperative pain management to avoid influence on glucose metabolism in patients, so the alterations in postoperative glucose metabolism presumably were the consequences of surgical trauma.
CONCLUSION
Premedication with dexmedetomidine and fentanyl has effectively modulated the neuroendocrine stress response of laparoscopic cholecystectomy under general anesthesia, as assessed by analyzing the variation of blood glucose levels. The dexmedetomidine premedication was better when compared to fentanyl premedication. Modulating the stress response to surgery might enable laparoscopic cholecystectomy in obese, hypertensive, and cardiac compromised patients, and may be a key factor in improving outcome of these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Weissman C. The metabolic response to stress: An overview and update. Anesthesiology. 1990;73:308–27. doi: 10.1097/00000542-199008000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Marana E, Scambia G, Maussier ML, Parpaglioni R, Ferrandina G, Meo F, et al. Neuroendocrine stress response in patients undergoing benign ovarian cyst surgery by laparoscopy, minilaparotomy and laparotomy. J Am Assoc Gynecol Laparosc. 2003;10:159–65. doi: 10.1016/s1074-3804(05)60291-5. [DOI] [PubMed] [Google Scholar]
- 3.Traynor C, Hall GM. Endocrine and metabolic changes during surgery: Anaesthetic implications. Br J Anaesth. 1981;53:153–60. doi: 10.1093/bja/53.2.153. [DOI] [PubMed] [Google Scholar]
- 4.Russell RC, Walker CJ, Bloom SR. Hyperglucagonemia in the surgical patient. Br Med J. 1975;1:10–2. doi: 10.1136/bmj.1.5948.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schricker T, Lattermann R, Schreiber M, Geisser W, Georieff M, Radermacher P. The hyperglycemic response to surgery: Pathophysiology, clinical implications and modulation by the anesthetic technique. Clin Intensive Care. 1998;9:118–28. [Google Scholar]
- 6.Cunningham AJ, Brull SJ. Laparoscopic cholecystectomy: Anesthetic implications. Anaesth Analg. 1993;76:1120–33. doi: 10.1213/00000539-199305000-00035. [DOI] [PubMed] [Google Scholar]
- 7.Jean IJ. Anaesthesia for Laparoscopic surgery. In: Miller RD, editor. Anesthesia. 7th ed. New York: Churchill Livingstone; 2010. pp. 2185–202. [Google Scholar]
- 8.Kehlet H. Manipulation of the metabolic response in clinical practice. World J Surg. 2000;24:690–5. doi: 10.1007/s002689910111. [DOI] [PubMed] [Google Scholar]
- 9.Galley HF, DiMatteo MA, Webster NR. Immunomodulation by anesthetic, sedative and analgesic agents: Does it matter? Intensive Care Med. 2000;26:267–74. doi: 10.1007/s001340051149. [DOI] [PubMed] [Google Scholar]
- 10.Marana E, Scambia G, Colicci S, Maviglia R, Maussier ML, Marana R, et al. Leptin and perioperative neuroendocrine stress response with two different anaesthetic techniques. Acta Anaesthesiol Scand. 2008;52:541–6. doi: 10.1111/j.1399-6576.2008.01589.x. [DOI] [PubMed] [Google Scholar]
- 11.Casati A, Fanelli G, Albertin A, Deni F, Daneili G, Grifoni F, et al. Small doses of remifentanil or sufentanil for blunting cardiovascular changes induced by tracheal intubation: A double blind comparison. Eur J Anaesthesiol. 2001;18:108–12. doi: 10.1046/j.1365-2346.2001.0790e.x. [DOI] [PubMed] [Google Scholar]
- 12.Tripathi DC, Shah SK, Dubey SR, Doshi SM, Rawal PV. Hemodynamic stress response during laparoscopic cholecystectomy: Effect of two different doses of intravenous clonidine premedication. J Anaesthesiol Clin Pharmacol. 2011;27:475–80. doi: 10.4103/0970-9185.86586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcangeli A, D’Alo C, Gaspari R. Dexmedetomidine use in general anesthesia. Curr Drug Targets. 2009;10:687–95. doi: 10.2174/138945009788982423. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharjee DP, Nayek SK, Dawn S, Bandopadhyay G, Gupta K. Effect of Dexmedetomidine on hemodynamics in patients undergoing Laparoscopic Cholecystectomy- A comparative study. J Anesth Clin Pharma. 2010;26:45–8. [Google Scholar]
- 15.Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth. 2006;18:24–8. doi: 10.1016/j.jclinane.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Joris JL, Chiche JD, Canivet JL, Jacquet NJ, Legros JJ, Lamy ML. Hemodynamic changes induced by laparoscopy and their endocrine correlates: Effect of clonidine. J Am Coll Cardiol. 1998;32:1389–96. doi: 10.1016/s0735-1097(98)00406-9. [DOI] [PubMed] [Google Scholar]
- 17.Bessey PQ, Watters JM, Aoki TT. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg. 1984;200:264–81. doi: 10.1097/00000658-198409000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaka H, Itoh K, Miyakawa H, Kitano T, Taniguchi K, Honda N. Glucose intolerance during prolonged sevoflurane anesthesia. Can J Anaesth. 1996;43:1059–61. doi: 10.1007/BF03011909. [DOI] [PubMed] [Google Scholar]
- 19.Marana E, Annetta MG, Meo F, Parpaglioni R, Galeone M, Maussier ML, et al. Sevoflurane improves the neuroendocrine stress response during laparoscopic pelvic surgery. Can J Anaesth. 2003;50:348–54. doi: 10.1007/BF03021031. [DOI] [PubMed] [Google Scholar]
- 20.Thorell A, Rooyackers O, Myrenforis P, Soop M, Nygren J, Ljungqvist OH. Intensive insulin treatment in critically ill trauma patients normalizes glucose by reducing endogenous glucose production. J Clin Endocrinol Metab. 2004;89:5382–6. doi: 10.1210/jc.2004-1118. [DOI] [PubMed] [Google Scholar]
- 21.Van den Berghe G. how does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114:1187–95. doi: 10.1172/JCI23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33–8. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]


