Abstract
Background:
In spite of being the preferred induction agent for LMA insertion, propofol has many undesirable side effects including dose-related cardiorespiratory depression and local pain at injection site. Ketofol as a novel induction agent has been introduced recently with comparable efficacy and improved hemodynamic control
Objective:
To investigate ketofol as a suitable induction agent alternative to propofol for insertion of LMA in children considering insertion conditions, hemodynamic stability, local pain at injection site, and recovery.
Materials and Methods:
In this randomized, double-blind study, 100 children were randomly assigned into two groups of 50 patients each in which induction was performed with either propofol or ketofol. Providers were given one 20 ml syringe [represent either 2 mg/kg of propofol (P group) or 0.75 mg/kg of ketamine and 1.5 mg/kg of propofol (KP group)] and one 10 ml syringe for rescue if needed [represent 1 mg/kg of propofol (P group) or 0.25 mg/kg of ketamine and 0.5 mg/kg of propofol (KP group)]. After monitoring with bispectral index (BIS), general anesthesia was induced by infusion with a syringe perfuser at a constant rate of 250 ml/h with either of the two agents till the BIS values decreased to 40. Mean arterial pressure (MAP), heart rate (HR) were measured every 30 seconds up to 5 minutes after LMA placement. The time till BIS values decreased to 40 was measured. All children were evaluated for incidence of apnea, pain on injection, jaw relaxation, conditions for LMA insertion, and complications such as muscle rigidity, hallucinations, and excessive secretions.
Results:
Induction time (time to reach BIS of 40) was faster in the KP group (150 ± 23.5 seconds) than in the P group (205 ± 37.4 seconds). The incidence of injection pain was significantly lower in the KP group (10%) than in the P group (80%). Excellent jaw relaxation and full mouth opening were higher in the KP group [45 patients (90%)] than in the P group [38 patients (76%)]. Excellent LMA insertion conditions were observed in 45 patients (90%) in the KP group and 38 patients (76%) in the P group. The KP group showed preserved hemodynamic stability (mean blood pressure, heart rate) with less incidence and duration of apnea compared to the P group.
Conclusion:
ketofol is a safe and effective alternative induction agent for LMA insertion in children with rapid onset of action and lower incidence of injection pain. It provided better LMA insertion conditions, improved hemodynamic stability with less prolonged apnea when compared with propofol.
Keywords: Bispectral index, hemodynamic, ketofol, LMA, propofol
INTRODUCTION
Propofol is the preferred induction agent for Laryngeal mask airway (LMA) insertion which is widely used for providing general anesthesia in children. Propofol is a nonopioid, nonbarbiturate, sedative-hypnotic agent with rapid induction and recovery times and antiemetic effects.[1] It allows easy insertion of LMA by depressing airway reflexes. However, adverse effects include dose-dependent cardiorespiratory depression, injection pain, and having no analgesic properties.[2,3] Ketamine causes little or no cardio-respiratory depression and unlike propofol, has pain relieving properties. Ketamine use as a single induction agent, however, is limited by emergence hallucinations, elevation of blood pressure and heart rate due to its sympathomimetic effects, and increased intracranial pressure.[4,5] Effectiveness of the two agents – propofol and ketamine – in combination (ketofol) has been recently demonstrated and may provide a novel induction agent with favorable hemodynamics and reduced side effects attributed to either drugs.[6] To date, ketofol has been used most extensively for procedural sedation in the Emergency Department with encouraging results,[3,7] but has not yet been standardized as an induction agent. Bispectral index (BIS) monitoring has emerged as a convenient and versatile tool to titrate hypnotic agents and to reduce drug consumption. BIS is a dimensionless number scaled from 100 to 0, with 100 representing an awake electroencephalogram and 0 representing electrical silence.[8] This study was designed to investigate ketofol as a suitable induction agent alternative to propofol for insertion of LMA in children considering insertion conditions, hemodynamic stability, local pain at injection site, and recovery score.
MATERIALS AND METHODS
After obtaining institutional ethics committee approval and informed parental consent, 100 children of ASA class I and II, of both sex, age ranging from 3 to 15 years and undergoing elective short surgical procedures under general anesthesia were included in the study. Children who were at risk of regurgitation, with known allergy to either agents, or with difficult airway were excluded. Children were randomly assigned to one of two groups 50 patients each; P group (propofol), KP group (ketofol). Randomization was by the envelope method and the investigator and observer were blinded. Upon arrival at the operating room, patients were preoxygenation with 100% oxygen for 3 minutes during which the standard monitors; electrocardiogram, noninvasive blood pressure and pulse oximetry were attached to the patient. BIS monitor electrodes (Aspect Medical System, Vista™, MA, USA) were applied on the skin of left side of the forehead after cleansing with alcohol. One minute before induction, baseline readings were recorded. Providers were given one 20 ml syringe and one 10 ml syringe for rescue if needed. The clinician and observer were blinded to the medication and doses being administered during induction. The 20 ml syringe in both groups looked identical (appeared to be propofol only) but depending on the group they were randomized to, it represented either 2 mg/kg of propofol (P group) or 0.75 mg/kg of ketamine and 1.5 mg/kg of propofol (KP group). Normal saline was added to the syringes up to 20 ml for blinding purposes. The 10 ml rescue syringe, if used, (due to any patient responding to stimulus after induction) represented 1 mg/kg of propofol (P group) or 0.25 mg/kg of ketamine and 0.5 mg/kg of propofol (KP group). In all patients general anesthesia was induced using a syringe perfuser at a constant rate 250 ml/h and the response was titrated until the target level of BIS 40 was obtained. As soon as BIS was decreased to index values of 40, an appropriate size LMA was inserted by an experienced anesthetist who was unaware of which group the child was in. A maximum of three attempts were allowed for the insertion of the LMA and Assessments were done only for the first attempt. Following insertion of the LMA, the cuff was inflated with air until effective ventilation was established or the maximum recommended inflation volume was used. Patients were kept on spontaneous respiration and anesthesia was maintained with sevoflurane 1-2%.
Assessments
The times needed for loss of verbal contact and for loss of eyelash reflex were recorded. Injection pain and the conditions for LMA insertion were assessed by the same anesthetist. Pain on injection was graded using a four-point scale by Cameron et al.[9]; 0: no pain, 1: mild pain (grimace), 2: moderate pain (grimace + cry), 3: severe pain (cry + withdrawal). The conditions for LMA insertion were scored according to mouth opening (1: full, 2: partial, 3:nil), gagging or coughing (1: nil, 2: slight, 3: gross), swallowing (1: nil, 2: slight, 3: gross), laryngospasm (1: nil, 2: partial, 3: complete), head or limb movements (1: nil, 2: slight, 3: gross), ease of LMA insertion (1: easy, 2: difficult, 3: impossible).[10] Jaw relaxation was assessed according to Young's criteria[11]; (excellent: absolutely relaxed with no muscle tone, satisfactory: moderately relaxed with some muscle tone, poor: poorly relaxed with full muscle tone).
The overall LMA insertion conditions were graded as excellent, satisfactory, and poor[12] as follows:
Excellent: No adverse response, i.e., no gagging or coughing, no patient movement or laryngospasm.
Satisfactory: Adverse response to airway manipulations, but not affecting the insertion of LMA.
-
Poor: a. Moderate to severe adverse responses requiring additional boluses of drugs,
b. More than two attempts were required for LMA insertion.
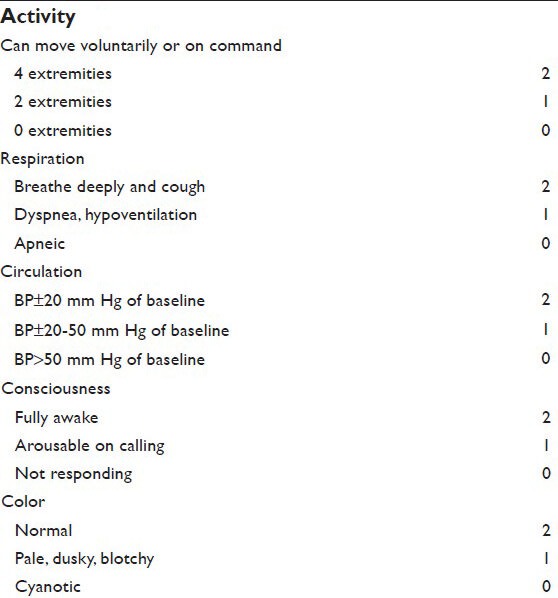
Mean arterial pressure (MAP), heart rate (HR), SpO2 were recorded before induction of anesthesia (t0), immediately following induction of anesthesia (t1), immediately after LMA placement (t2), 3 and 5 minutes after LMA placement (t3, t4). Complications such as bradycardia, muscle rigidity, and excessive secretions, were recorded. Incidence of apnea (absence of spontaneous respiration for > 20 seconds) was noted. At the end of surgery and after LMA removal, incidence of postoperative nausea and vomiting was recorded. Recovery was evaluated using the Aldrete score (1-10 range) (Appendix).[13]
Appendix.
Aldrete's postanesthesia recovery scoring system[13]
Statistical analysis
A sample size of 37 patients per group was needed to detect an intergroup difference of at least 20% (α =0.01, two-sided, power = 90%) with two-sample t-test. All values were expressed as mean ± SD, median (range) or number (percent) unless otherwise stated. All statistical analyzes were performed using SPSS for Windows 13.0. (SPSS Inc., Chicago, IL, USA). The categorical data were compared using a Chi-square test. Parametric data were compared using one-way analysis of variance (ANOVA) and within-group comparisons at different time intervals were assessed using a paired t-test. A value of P < 0.05 was considered statistically significant.
RESULTS
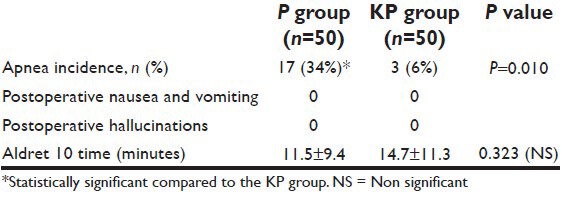
Demographic data were similar between the two groups regarding patients’ weight, age, gender, height, and duration of surgery [Table 1]. Verbal contact and eye lash reflex were lost earlier in patients in the KP group (33.5 ± 2.10 and 35.6 ± 1.9 seconds respectively) than patients in the P group (38.0 ± 2.8 and 41.6 ± 2.2 seconds respectively) (P < 0.05) [Figure 1]. The incidence of injection pain was significantly lower in the ketofol KP group (10%) than in the propofol P group (80%) (P < 0.001). The distribution of pain scores is shown in Table 2. Induction time (time to reach BIS of 40) was shorter in the ketofol group (150 ± 23.5 seconds) than in the propofol group (205 ± 37.4 seconds) (P < 0.005) [Figure 2]. The median summed score describing the overall LMA insertion conditions was significantly better in the KP group [6.4 (6-7)] compared with P group [7.5 (6-9)] (P = 0.023) [Table 3]. The incidence of excellent (absolute) jaw relaxation and full mouth opening was higher in the KP group 45 patients (90%) than in the P group 38 patients (76%) (P < 0.005). Excellent LMA insertion conditions were observed in 45 patients (90%) in the KP group and 38 patients (76%) in the P group. Poor insertion conditions were observed in one patient (2%) in the KP group and 6 patients (12%) in the P group [Table 3]. The mean blood pressure was significantly decreased in the P group compared to the KP group at all measurement points (P < 0.05) and was significantly lower than the basal level (t0) at t1, t2, t3, t4 in the P group (P < 0.005) [Table 4]. The heart rate was significantly decreased in the P group (P < 0.05), while it remained comparable to basal level in the KP group at all measurement points [Table 5]. The difference between the two groups was found to be statistically significant (P < 0.005). The incidence of apnea was higher in the P group than in the KP group, 17 patients (34%) vs. 3 patients (6%) respectively (P < 0.05). The mean duration of apnea was higher in the P group than in the KP group (47.65 ± 11.20 seconds vs. 30.50 ± 3.10 seconds respectively) (P < 0.05). There was no significant difference in the recovery score between the P group and KP group [Table 6].
Table 1.
Demographic data of patients. Values are mean± (SD) or number (proportion)
Figure 1.
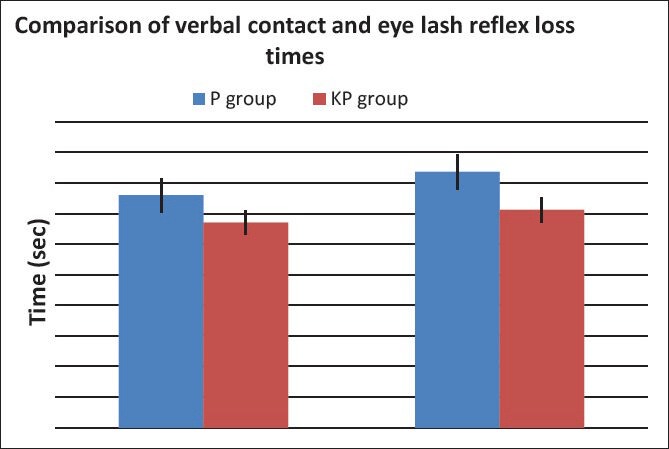
Verbal contact and eye lash reflex loss times, propofol vs. ketofol
Table 2.
Incidence of injection pain (values are number) -propofol vs. ketofol

Figure 2.
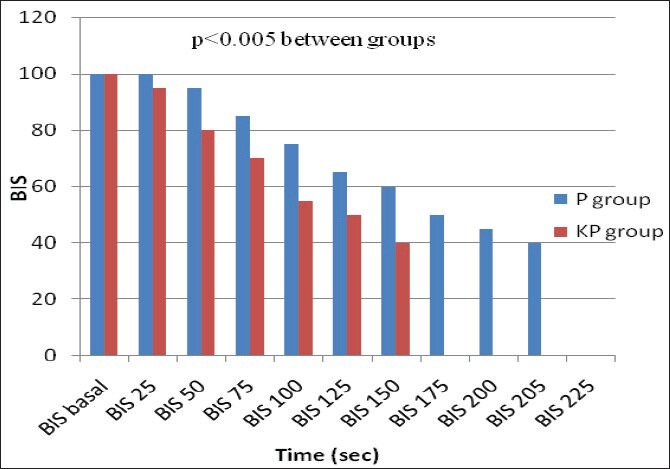
Time to reach BIS to 40 (induction time), propofol vs. ketofol
Table 3.
LMA insertion conditions (number) or median (range) -propofol vs. ketofol
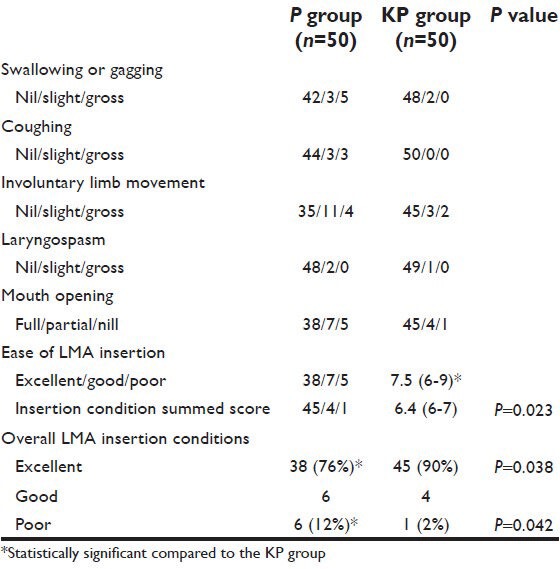
Table 4.
Changes in mean blood pressure (MAP) in mm Hg (mean±SD) -propofol vs. ketofol
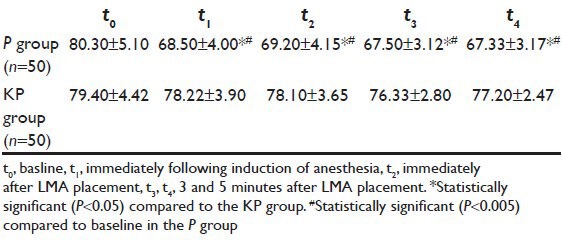
Table 5.
Changes in heart rate (HR) (beats/min) (mean±SD) -propofol vs. ketofol

Table 6.
Untoward effects and recovery (mean±SD) or n (%)- propofol vs. ketofol
DISCUSSION
Smooth and successful insertion of LMA necessitates the adequate depth of anesthesia to suppress pharyngeal and laryngeal reflexes and prevent airway complications. Currently, propofol is the most widely used induction agent for LMA insertion as it provides rapid induction and excellent jaw relaxation, but it has many disadvantages such as pain at injection site, involuntary limb movement, prolonged apnea, and hypotension. Dose of propofol required to produce adequate conditions for LMA insertion is suggested to be more than 3 mg/kg in children.[14] When a high dose of propofol is used, it causes cardiorespiratory depression.[15] In an attempt to reduce propofol adverse cardiorespiratory depressant effects, the combined use of propofol and ketamine (ketofol) has been proposed with great success but little is known in the literature about the use of ketofol as an induction agent compared to propofol.[16,17]
The main finding of this study was that ketofol as an induction agent has rapid onset of action, lower incidence of injection pain, and better overall conditions and ease of LMA insertion. It provided better hemodynamic stability and lower incidence of adverse effects when compared to propofol.
In this study, the onset of clinical hypnosis, proved by the times needed for loss of verbal contact and eye lash reflex, was earlier in the ketofol group compared to the propofol group (P < 0.05). Consistent with our results, Frey and colleagues[18] stated that ketofol was associated with shorter time until sedation compared to propofol. Induction time (time to reach BIS of 40) was faster in the ketofol group (150 ± 23.5 seconds) than in the propofol group (205 ± 37.4 seconds). In contrast to our results, Sakai et al.[19] found an additive effect of propofol and ketamine for achieving the hypnotic endpoints; however, the mixture did not depress the BIS values in proportion to its hypnotic effect and they attributed this to the small concentrations of propofol used and/or ineffectiveness of ketamine on BIS. This contrast between this study and that of Sakai can be explained by different methodologies, different concentrations of the ingredients within the admixture, and different infusion time and rate.
The high incidence of pain on injection is an annoying problem during the use of propofol. Adding lidocaine to propofol to reduce this problem is a common practice but despite this the incidence of pain remains unacceptably high (20-30%).[20] Pain with propofol injection is attributed to the free aqueous concentration of propofol in the emulsion[21] or to the activation of kininogens.[22] In our study, the use of ketofol was found to significantly reduce incidence of pain on injection (10%), compared with propofol (80%) and these results were comparable with previous studies where pretreatment with intravenous ketamine provided a simple and safe method of reducing the incidence of pain on injection of propofol.[23,24,25,26] Ketamine may activate NMDA receptors either in the vascular endothelium or in the central nervous system. It seems likely that ketamine reduces the propofol injection pain by virtue of its local anesthetic property.[23,27]
In the present study, the incidence of absolute jaw relaxation and excellent LMA insertion conditions was higher in the ketofol group (90%) than in the propofol group (76%). In agreement with our results, Bahk et al. had compared various doses of propofol and ketamine with lidocaine spray for LMA insertion.[28] They found that ketamine with lidocaine spray may be better than propofol for LMA insertion. Also Begec et al.[29] concluded that the combination of ketamine and propofol may be preferred for LMA insertion in children.
In our study, ketofol showed more hemodynamic stability compared to propofol for LMA insertion in children. When administered with propofol for induction of anesthesia, even in subanesthetic doses, ketamine may produce hemodynamic stability.[30,31,32] Ketamine 0.5 mg/kg administered before propofol 1.5 mg/kg for LMA insertion preserved hemodynamic stability compared with propofol alone in children[33] and in adult patients.[34] Akin et al.[35[ found hemodynamic stability of ketofol in children undergoing cardiac catheterization. Arora et al.[36] proved hemodynamic stability of ketofol in a 1:1 ratio for procedural sedation in adults. Ketofol was associated with improved hemodynamic stability during the first 10 minutes after induction compared with propofol and the authors concluded that ketofol has the potential to be used as an alternative agent for emergency induction during which stable hemodynamics are desirable.[37] The incidence and mean duration of apnea in the present study were higher in the propofol group than in the ketofol group. Consistent with our results, previous studies[16,18,33] concluded that the addition of ketamine to propofol is associated with less prolonged apnea and less risk of respiratory depression compared with propofol alone. Also low-dose ketamine added to propofol sedation attenuated propofol-induced hypoventilation and preserved response to carbon dioxide.[38] Complications as nausea and vomiting, increases in secretions, and emergence hallucinations were not seen in this study. Propofol was effective in eliminating side effects of a subanesthetic dose of ketamine.[39]
CONCLUSION
Ketofol is a safe and effective alternative induction agent for LMA insertion in children with rapid onset of action and lower incidence of injection pain. It provided better LMA insertion conditions, improved hemodynamic stability with less prolonged apnea when compared to propofol.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.White PF. Clinical pharmacology of intravenous induction drugs. Int Anesthesiol Clin. 1988;26:98–104. doi: 10.1097/00004311-198802620-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bassett KE, Anderson JL, Pribble CG, Guenther E. Propofol for procedural sedation in children in the emergency department. Ann Emerg Med. 2003;42:773–82. doi: 10.1016/s0196-0644(03)00619-x. [DOI] [PubMed] [Google Scholar]
- 3.Arora S. Combining ketamine and propofol (ketofol) for emergency department procedural sedation and analgesia: A review. West J Emerg Med. 2008;9:20–3. [PMC free article] [PubMed] [Google Scholar]
- 4.Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26:985–1028. doi: 10.1016/j.ajem.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Morgan GE, Mikhail MS, Murray MJ. Nonvolatile anesthetic agents. Clin Anesthesiology. 2002;8:151–77. [Google Scholar]
- 6.Nathan JS, Michael LB, Thomas MD, Matthew DK. Ketofol as a Sole Induction Agent is Associated with Increased Hemodynamic Indices in Low-Risk Patients. ASA abstracts. 2011;16:A485. [Google Scholar]
- 7.Morse Z, Sano K, Kanri T. Effects of a propofol–ketamine admixture in human volunteers. Pac Health Dialog. 2003;10:51–4. [PubMed] [Google Scholar]
- 8.Bonhomme V, Deflandre E, Hans P. Correlation and agreement between bispectral index and state entropy of the electroencephalogram during propofol anaesthesia. Br J Anaesth. 2006;3:340–6. doi: 10.1093/bja/ael171. [DOI] [PubMed] [Google Scholar]
- 9.Cameron E, Johnston G, Crofts S, Morton NS. The minimum effective dose of lignocaine to prevent injection pain due to propofol in children. Anaeshesia. 1992;47:604–6. doi: 10.1111/j.1365-2044.1992.tb02335.x. [DOI] [PubMed] [Google Scholar]
- 10.Cheam EW, Chui PT. Randomised double-blind comparison of fentanyl, mivacurium or placebo to facilitate laryngeal mask airway insertion. Anaesthesia. 2000;55:323–6. doi: 10.1046/j.1365-2044.2000.01214.x. [DOI] [PubMed] [Google Scholar]
- 11.Young HA, Clark RS, Dunder JW. Intubating condition with Ah8165 and suxamethonium. Anaesthesia. 1975;30:30–3. doi: 10.1111/j.1365-2044.1975.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 12.Yeo KS, Kua SW, Teoh GS, Onsiong MK. The use of thiopentone/propofol admixture for Laryngeal Mask Airway insertion. Anaesth Intensive Care. 2001;29:38–42. doi: 10.1177/0310057X0102900107. [DOI] [PubMed] [Google Scholar]
- 13.Aldrete JA, Krulik D. Postoperative recovery score. Anesth Analg. 1970;49:924–34. [PubMed] [Google Scholar]
- 14.Martlew RA, Meakin G, Wadsworth R, Sharples A, Baker RD. Dose of propofol for laryngeal mask airway insertion in children: Effect of premedication with midazolam. Br J Anesth. 1996;76:308–9. doi: 10.1093/bja/76.2.308. [DOI] [PubMed] [Google Scholar]
- 15.Chari P, Ghai B. Comparison of butorphanol-thiopentone versus fentanyl-thiopentone for LMA insertion. J Clin Anaesth. 2006;18:8–11. doi: 10.1016/j.jclinane.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Akin A, Esmaoglu A, Tosun Z, Gulcu N, Aydogan H, Boyaci A. Comparison of propofol with propofol–ketamine combination in pediatric patients undergoing auditory brainstem response testing. Int J Pediatr Otorhinolaryngol. 2005;69:1541–5. doi: 10.1016/j.ijporl.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49:23–30. doi: 10.1016/j.annemergmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Frey K, Sukhani R, Pawlowski J, Lucia A, Mikat M, Slogoff S. Propofol versus propofol ketamine sedation for retrobulbar nerve block: Comparison of sedation quality, intraocular pressure changes, and recovery profiles. Anesth Analg. 1999;89:317–21. doi: 10.1097/00000539-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Sakai T, Singh H, Mi WD, Kudo T, Matsuki A. The effect of ketamine on clinical endpoints of hypnosis and EEG variables during propofol infusion. Acta Anaesthesiol Scand. 1999;43:212–6. doi: 10.1034/j.1399-6576.1999.430216.x. [DOI] [PubMed] [Google Scholar]
- 20.Nyman Y, Van Hofman K, Georgiadi A, Ekasharg S, Lonnqqrist PA. Propofol injection pain in children: A prospective randomized double- blind trial of a new propofol formulation versus propofol with added lidocaine. Br J Anesth. 2005;95:222–5. doi: 10.1093/bja/aei156. [DOI] [PubMed] [Google Scholar]
- 21.Klement W, Arndt JO. Pain on injection of propofol: Effects of concentration and diluent. Br J Anesth. 1991;67:281–4. doi: 10.1093/bja/67.3.281. [DOI] [PubMed] [Google Scholar]
- 22.Scott RP, Saunders DA, Norman J. Propofol: Clinical strategies for preventing the pain of injection. Anaesthesia. 1988;43:492–4. doi: 10.1111/j.1365-2044.1988.tb06641.x. [DOI] [PubMed] [Google Scholar]
- 23.Tan CH, Onsiong MK, Kua SW. The effect of ketamine pretreatment on propofol injection pain in 100 women. Anaesthesia. 1998;53:302–5. doi: 10.1046/j.1365-2044.1998.00287.x. [DOI] [PubMed] [Google Scholar]
- 24.Barbi E, Marchetti F, Gerarduzzi T, Neri E, Gagliardo A, Sarti A, et al. Pretreatment with intravenous ketamine reduces propofol injection pain. Paediatr Anaesth. 2003;13:764–8. doi: 10.1046/j.1460-9592.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 25.Koo SW, Cho SJ, Kim YK, Ham KD, Hwang JH. Small-Dose ketamine reduces the pain of propofol injection. Anesth Analg. 2006;103:1444–7. doi: 10.1213/01.ane.0000243334.83816.53. [DOI] [PubMed] [Google Scholar]
- 26.Kad N, Malik P, Dureja J, Thakur A. Ketamine pretreatment to alleviate the pain of propofol injection: A randomized, double blind study. Internet J Anesthesiol. 2009:20. [Google Scholar]
- 27.Durrani Z, Winnie AP, Zsigmoud EK, Burnett ML. Ketamine for intravenous regional anaesthesia. Anesth Analg. 1989;68:328–32. [PubMed] [Google Scholar]
- 28.Bahk JH, Sung J, Jang IJ. A comparison of ketamine and lidocaine spray with propofol for insertion of LMA in children. Anesth Analg. 2002;95:1586–9. doi: 10.1097/00000539-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Begec Z, Demirbilek S, Onal D, Erdil F, Ilksen Toprak H, Ozcan Ersoy M. Ketamine or alfentanil administration prior to propofol anaesthesia: The effects on ProSeal™ laryngeal mask airway insertion conditions and haemodynamic changes in children. Anaesthesia. 2009;64:282–6. doi: 10.1111/j.1365-2044.2008.05782.x. [DOI] [PubMed] [Google Scholar]
- 30.Furuya A, Matsukawa T, Ozaki M, Nishiyama T, Kume M, Kumazawa T. Intravenous ketamine attenuates arterial pressure changes during the induction of anaesthesia with propofol. Eur J Anaesthesiol. 2001;18:88–92. doi: 10.1046/j.0265-0215.2000.00784.x. [DOI] [PubMed] [Google Scholar]
- 31.Hui TW, Short TG, Hong W, Suen T, Gin T, Plummer J. Additive interactions between propofol and ketamine when used for anesthesia induction in female patients. Anesthesiology. 1995;82:641–8. doi: 10.1097/00000542-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Gupta A, Kaur S, Attri JP, Saini N. Comparative evaluation of ketamine-propofolt fentanyl-propofol and butorphanol- propofol on haemodynamics and laryngeal mask airway insertion conditions. J Anaesthesiol Clin Pharmacol. 2011;27:74–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Tomatir E, Atalay H, Gurses E, Erbay H, Bozkurt P. Effects of low dose ketamine before induction on propofol anesthesia for pediatric magnetic resonance imaging. Pediatr Anesth. 2004;14:845–50. doi: 10.1111/j.1460-9592.2004.01303.x. [DOI] [PubMed] [Google Scholar]
- 34.Goh PK, Chiu CL, Wang CY, Chan YK, Loo PL. Randomized double-blind comparison of ketamine-propofol, fentanyl-propofol and propofol-saline on haemodynamics and laryngeal mask airway insertion conditions. Anaesth Intensive Care. 2005;33:223–8. doi: 10.1177/0310057X0503300211. [DOI] [PubMed] [Google Scholar]
- 35.Akin A, Esmaoglu A, Guler G, Demircioglu R, Narin N, Boyaci A. Propofol and propofol ketamine in pediatric patients undergoing cardiac catheterization. Pediatr Cardiol. 2005;26:553–7. doi: 10.1007/s00246-004-0707-4. [DOI] [PubMed] [Google Scholar]
- 36.Arora S, Martin CL, Fernandez MM, Wagner JG, Hertbert M. Combining ketamine and propofol (ketofol) for procedural sedation in emergency department. Ann Emerg Med. 2007;50:S121. [Google Scholar]
- 37.Smischney NJ, Beach ML, Loftus RW, Dodds TM, Koff MD. Ketamine/propofol admixture (ketofol) is associated with improved hemodynamics as an induction agent: A randomized, controlled trial. J Trauma Acute Care Surg. 2012;73:94–101. doi: 10.1097/TA.0b013e318250cdb8. [DOI] [PubMed] [Google Scholar]
- 38.Mortero RF, Clark LD, Tolan MM, Metz RJ, Tsueda K, Sheppard RA. The effects of small dose ketamine on propofol sedation: Respiration, post operative mood, perception, cognition, and pain. Anesth Analg. 2001;92:1465–9. doi: 10.1097/00000539-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Guit JB, Koning HM, Coster ML, Niemeijer RP, Mackie DP. Ketamine as analgesic for total intravenous anaesthesia with propofol. Anaesthesia. 1991;46:24–7. doi: 10.1111/j.1365-2044.1991.tb09308.x. [DOI] [PubMed] [Google Scholar]


