Abstract
Introduction:
Carbon dioxide pneumoperitoneum (PP) for laparoscopic surgery increases arterial pressure, heart rate, and systemic vascular resistance. In this randomized, double blind, prospective clinical study; we investigated the efficacy of magnesium sulfate to prevent adverse hemodynamic response associated with PP in patients undergoing laparoscopic cholecystectomy.
Materials and Methods:
Sixty patients, of either sex (18-65 years of age), undergoing elective laparoscopic cholecystectomy were randomly allocated in one of the two groups containing 30 patients each. Group M received magnesium sulfate 30 mg/kg intravenously as a bolus before PP. Group C received same volume of 0.9% saline.
Results:
Mean arterial pressure and heart rate were significantly less throughout the period of pneumoperitoneum in patients of group M. Intravenous labetalol was required in 40% (12 out of 30) of the patients in group C to control intraoperative hypertension and it was clinically significant in comparison to group M.
Conclusion:
Magnesium sulfate administered before PP attenuates adverse hemodynamic response and provides hemodynamic stability during PP created for laparoscopic surgery.
Keywords: Hemodynamic, laparoscopic surgery, magnesium sulfate, pneumoperitoneum
INTRODUCTION
Laparoscopic cholecystectomy was first performed by Phillipe Mouret in 1987[1] Since then it became popular very quickly. For laparoscopic cholecystectomy, carbon dioxide is commonly used to create pneumoperitoneum (PP).[2,3] Both carbon dioxide and PP cause adverse cardiovascular effects.[4] Adverse cardiovascular changes are characterized by abrupt elevation of arterial pressure, systemic vascular resistance, and decreased cardiac output.[5] These vasopressor responses are mainly due to increased release of catecholamines, vasopressin, or both.[6,7] Laparoscopic cholecystectomy is performed in reverse Trendelenburg position.[8] Cardiac output is further decreased secondary to decreased venous return caused by the above said position.
Severe increases in arterial pressure and heart rate can be deleterious to the patients, especially for the patients with compromised cardiac function.[9] Attenuation of circulatory response to PP is usually done by opioids[10] vasodilators[11] beta blocking agents,[12] and alpha-2 adrenergic agonists.[13]
Magnesium has the ability to block the release of catecholamines from both the adrenal gland and the adrenergic nerve terminals.[14] Apart from that, magnesium can produce vasodilatation by acting directly on blood vessels[15] and it also capable of attenuating vasopressin-stimulated vasoconstriction.[16]
Intravenously administered magnesium sulfate is capable of attenuating the adverse hemodynamic response associated with endotracheal intubation.[17] So we have hypothesized that intravenously administered magnesium sulfate would be efficacious to attenuate adverse hemodynamic response in patients undergoing elective laparoscopic cholecystectomy with carbon dioxide PP.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Ethical Committee of Calcutta National Medical College, Kolkata and informed consent was taken from each of the patients. Sixty American Society of Anesthesiologists (ASA) grade I and II patients, aged 18-65 years, undergoing elective laparoscopic cholecystectomy were assigned (using a computer derived random number sequence) to one of the two groups (each containing 30 patients): Group M (magnesium sulfate group) and Group C (control group). Group size of 30 patients was determined by power analysis study. Patients with hypermagnesemia;- known allergy to magnesium sulfate, hypertension; morbid obesity; and severe hepatic, renal, endocrine, and cardiac dysfunction were excluded from the study.
All the patients received tab. diazepam 10 mg orally on the night before surgery. On arrival to operation theatre, routine monitoring (ECG, pulse oximetry, noninvasive blood pressure (NIBP) was started and baseline vital parameters like heart rate, mean arterial pressure (MAP), and arterial oxygen saturation (SpO2) were recorded. An intravenous line was started. Patients were induced with fentanyl 2 μg/kg and propofol 2 mg/kg. Endotracheal intubation was facilitated by muscle relaxant rocuronium 0.7 mg/kg. Group M patients received magnesium sulfate 30 mg/kg intravenously as a bolus and group C received same volume of 0.9% saline. The study medication was prepared in identical 20 ml syringe: 8 ml sterile water was added to 12 ml magnesium sulfate (6000 mg) for group M patients (1 ml = 300 mg). 0.9% saline was used for group C patients. Study medication was prepared by an anesthesiologist who was blinded to the computer generated randomization schedule. Patients received one of these above said solution as a bolus intravenously immediately before pneumoperitonium.
Anesthesia was maintained with 33% O2 in N2O, 0.6% isoflurane and rocuronium. CO2 was insufflated into the peritoneal cavity to create PP. Intra-abdominal pressure (IAP) was maintained to 12 mmHg throughout the laparoscopic procedure. All the patients were positioned in a head-up tilt for about 15°. The patients were mechanically ventilated to keep ETCO2 between 35-40 mmHg.
In case of acute and severe hemodynamic fluctuations, the following medical interventions were taken: For bradycardia (heart rate < 60 beats/min), i.v. bolus dose of 0.6 mg inj. atropine; for hypotension (MAP < 60 mmHg) increased rate of infusion of i.v. fluid and/or i.v. bolus dose of inj. phenylephrine, and for hypertension (MAP > 110 mmHg) i.v. bolus dose of inj. labetalol.
At the end of the operation, ondansetron 4 mg was administered for prophylaxis against nausea and vomiting. Residual neuromuscular block was reversed by appropriate dose of neostigmine and glycopyrolate and tracheal extubation was performed. Heart rate and MAP were recorded at the following points of time: (1) Baseline, (2) before PP, (3) 15 minutes after PP, (4) 30 minutes after, PP (5) after release of PP, and (6) after extubation. Patients were observed for any adverse events like bradycardia, hypotension, and hypertension during postoperative period in postanesthesia care unit.
Statistical analysis
The results obtained from the study are presented in the following section in a tabulated manner. The results are expressed in Mean ± SD. Comparison between groups were performed with the Kruskal-Wallis one way ANOVA by Ranks or Fisher's exact test for small samples with a 5% risk. Mann-Whitney-Wilcoxon tests were performed when tests of normal distribution failed. P < 0.05 was considered to be statistically significant (Graph Pad In Stat version 3.05, Graph Pad Software, San Diego, CA).
RESULTS
The groups were comparable with respect to age, sex, body-weight, and duration of surgery [Table 1]. There was no significant difference in the preoperative MAP values between two groups [Table 2]. Before PP, there is also no significant difference in MAP values between the groups. MAP values in group M were significantly lower (P < 0.05) throughout the PP, after the release of PP, and after extubation compared to group C [Table 2].
Table 1.
Patient's characteristics and duration of surgery (mean±SD)
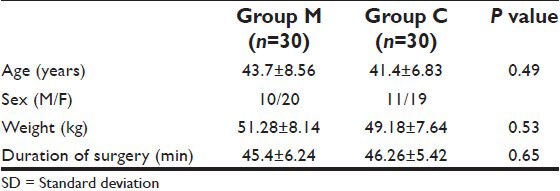
Table 2.
Changes in mean arterial pressure (mean±SD)
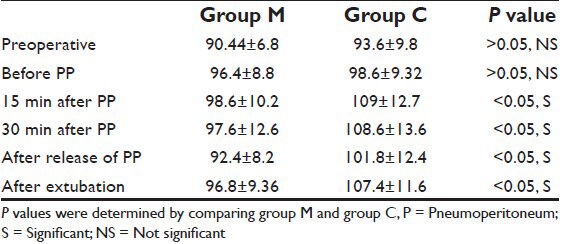
There was no significant difference in the preoperative heart rate between the groups [Table 3]. Before PP, there is also no significant difference in heart rate between the groups. Heart rate in group M were significantly lower throughout the PP (P < 0.05), after release of PP and after extubation in comparison to group C [Table 3].
Table 3.
Changes in heart rate (mean±SD)
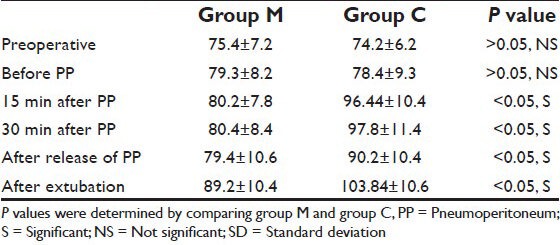
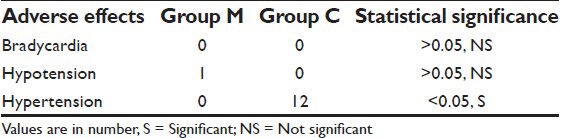
No patient suffered from bradycardia in our study. Only one patient in group M suffered from hypotension. Hypertension occurred in 12 patients of group C whereas no patient of group M suffered from hypertension. The difference was statistically significant (P < 0.05) [Table 4].
Table 4.
Distribution of patients according to adverse effects
DISCUSSION
In the study, we studied the effects of magnesium sulfate on hemodynamics in patients undergoing laparoscopic cholecystectomy.
During laparoscopic surgery, CO2 is routinely used to create PP.[1,2] Elevated IAP induced by PP and CO2 itself produce some adverse effects on the cardiovascular system [Figure 1].[3] Immediately after PP, plasma level of catecholamines and vasopressin is increased. Increased catecholamine level activates the renin-angiotensin aldosterone system (RAAS) leading to some characteristic hemodynamic alterations[4,5] which include decreased cardiac output, elevated arterial pressure, and increased systemic/pulmonary vascular resistance. Vasopressin also contributes to elevation of arterial pressure and systemic vascular resistance.[6] Laparoscopic cholecystectomy is performed in reverse Trendelenburg position.[7] This particular position causes diminished venous return which ultimately leads to further decrease in cardiac output.[8] As already mentioned in introduction, various pharmacological agents have been used to attenuate these adverse hemodynamic effects of PP.
Figure 1.
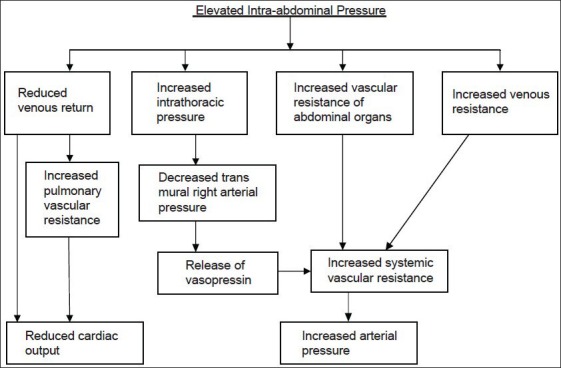
Schematic representation of hemodynamic changes due to elevated intra-abdominal pressure
As stated earlier, magnesium is effective in blocking the release of catecholamines from both adrenergic nerve terminals and the adrenal gland.[14] Besides, magnesium produces vasodilatation by acting directly on blood vessels.[15] In addition to catecholamines, vasopressin is a major contributor to the hemodynamic changes induced by PP. Magnesium attenuates vasopressin stimulated vasoconstriction.[16] In a study, James et al.[17] observed that i.v. magnesium sulfate was able to attenuate the adverse hemodynamic response of endotracheal intubation. Because of the ability of magnesium sulfate to attenuate adverse hemodynamic response, we have administered 30 mg/kg magnesium sulfate as a bolus before PP and observed its effect on hemodynamic response to PP Telci and etal.[18] used i.v. Magnesium sulfate in a dose of 30 mg/kg bolus before induction and 10 mg/kg/h continuous i.v. infusion in a study to observe the efficacy of magnesium sulfate to decrease anesthetic requirement.[18] We did not administer any infusion of magnesium intraoperatively as to observe the efficacy of magnesium sulfate to decrease anesthetic requirement was not our goal. Still we observed that i.v. magnesium sulfate in a bolus dose of 30 mg/kg before PP was able to attenuate the adverse hemodynamic response.
Diamant et al.[19] reported 35% decrease in cardiac output in dog with a raised IAP of 40 mmHg. Ishizaki et al.,[20] tried to evaluate the safe IAP during laparoscopic surgery. They observed significant fall in cardiac output at 16 mmHg of IAP and hemodynamic alterations were much less at 12 mm Hg of IAP. So in our study, we kept IAP 12 mm Hg. In spite of maintaining normocapnia and keeping IAP 12 mm Hg, there was significant rise of MAP and heart rate in patients of group C. However in group M, hemodynamic responses to PP were effectively blunted and both MAP and heart rate remained at a significantly lower level compared to group C. Thus in the present study, pre-pneumoperitoneum i.v. magnesium sulfate attenuated adverse hemodynamic response.
Regarding the incidence of adverse effects, no patient of either group suffered from bradycardia in our study and only single patient in group M suffered from hypotension. Hypertension occurred in 12 patients of group C for which they had to be treated with inj. labetalol whereas no patient of group M suffered from hypertension. To conclude, magnesium sulfate attenuates the elevation of MAP and heart rate during PP and thereby provides perioperative hemodynamic stability during laparoscopic surgery. Therefore, magnesium sulfate may be recommended for attenuation of adverse hemodynamic response during laparoscopic surgery.
ACKNOWLEDGEMENT
I want to thank all the members who were directly or indirectly involved in this project, and for their constant support to carry out my research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vecchio R, Macfayden BV, Palazzo F. History of laparoscopic surgery. Panminerva Med. 2000;42:87–90. [PubMed] [Google Scholar]
- 2.Hodgson G, Mc Clelland RM, Newton JR. Some effects of the peritoneal insufflation of carbon dioxide at laproscopy. Anaesthesia. 1970;25:382–90. doi: 10.1111/j.1365-2044.1970.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 3.Blobner M, Felber AR, Gögler S, Weigl EM, Jelen ES. Carbon dioxide uptake from the pneumoperitoneum during laparoscopic cholecystectomy. Anesthesiology. 1992;77:A37–40. [Google Scholar]
- 4.Richardson JD, Trinkl JK. Haemodynamic and respiratory alterations with increased intra abdominal pressure. J Surg Res. 1976;20:401–4. doi: 10.1016/0022-4804(76)90112-8. [DOI] [PubMed] [Google Scholar]
- 5.Lenz RJ, Thomas TA, Wilkins DG. Cardiovascular changes during laparoscopy. Studies of stroke volume and cardiac output using impedance cardiography. Anaesthesia. 1976;31:4–12. doi: 10.1111/j.1365-2044.1976.tb11738.x. [DOI] [PubMed] [Google Scholar]
- 6.Myre K, Rostrup M, Buanes T, Stokland O. Plasma catecholamines and haemodynamic changes during pneumoperitoneum. Acta Anaesthesiol Scand. 1998;42:343–7. doi: 10.1111/j.1399-6576.1998.tb04927.x. [DOI] [PubMed] [Google Scholar]
- 7.Walder AD, Aitkenhead AR. Role of vasopressin in the haemodynamic response to laparoscopic cholecystectomy. Br J Anaesth. 1997;78:264–6. doi: 10.1093/bja/78.3.264. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox S, Vandar LD. Trendelenburg and his position! A critique of its uses nad effectiveness. Anesth Analg. 1988;67:574–8. [PubMed] [Google Scholar]
- 9.McLaughlin JG, Scheeres D, Dean R, Bonnell BW. The adverse haemodynamic effects of laparoscopic cholecystectomy. Surg Endosc. 1995;9:121–4. doi: 10.1007/BF00191950. [DOI] [PubMed] [Google Scholar]
- 10.Lentschener C, Axler O, Fernandez H, Megarbane B, Billard V, Fouqueray B, et al. Haemodynamic changes and vasopressin release are not consistently associated with carbon dioxide pneumoperitoneum in humans. Acta Anaesthesiol Scand. 2001;45:527–35. doi: 10.1034/j.1399-6576.2001.045005527.x. [DOI] [PubMed] [Google Scholar]
- 11.Joris JL, Hamoir EE, Hartstein GM, Meurisse MR, Hubert BM, Charlier CJ, et al. Haemodynamic changes and catecholamine release during laparoscopic adrenalectomy for pheochromocytoma. Anesth Analg. 1999;88:16–21. doi: 10.1097/00000539-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Koivusalo AM, Scheinin M, Tikkanen I, Yli-Suomu T, Ristkari S, Laakso J, et al. Effects of esmolol on haemodynamic response to CO pneumoperitoneum for laparoscopic surgery. Acta Anaesthesiol Scand. 1998;42:510–7. doi: 10.1111/j.1399-6576.1998.tb05159.x. [DOI] [PubMed] [Google Scholar]
- 13.Joris JL, Chiche JD, Canivet JL, Jacquet NJ, Legros JJ, Lamy ML. Hemodynamic changes induced by laparoscopy and their endocrine correlates: Effects of clonidine. J Am Coll Cardiol. 1998;32:1389–96. doi: 10.1016/s0735-1097(98)00406-9. [DOI] [PubMed] [Google Scholar]
- 14.Lishajko F. Releasing effect of calcium and phosphate on catecholamines, ATP, and protein from chromaffin cell granules. Acta Physiol Scand. 1970;79:575–84. doi: 10.1111/j.1748-1716.1970.tb04760.x. [DOI] [PubMed] [Google Scholar]
- 15.Altura BM, Altura BT. Magnesium and vascular tone and reactivity. Blood Vessels. 1978;15:5–16. doi: 10.1159/000158148. [DOI] [PubMed] [Google Scholar]
- 16.Laurant P, Touyz RM, Schiffrin EL. Effect of magnesium on vascular tone and reactivity in pressurized mesenteric resistance arteries from spontaneously hypertensive rats. Can J Physiol Pharmacol. 1997;75:293–300. [PubMed] [Google Scholar]
- 17.James MF, Beer RE, Esser JD. Intravenous magnesium sulfate inhibits catecholamine release associated with tracheal intubation. Anesth Analg. 1989;68:772–6. [PubMed] [Google Scholar]
- 18.Telci L, Esen F, Ackora D, Erden T, Canbolat T, Akpir K. Evaluation of effects of magnesium sulfate in reducing intraoperative anesthetic requirements. Br J Anaesth. 2002;89:594–8. doi: 10.1093/bja/aef238. [DOI] [PubMed] [Google Scholar]
- 19.Diamant M, Benumof JL, Saidman LJ. Haemodynamics of increased intra-abdominal pressure: Interaction with hypovolemia and halothane anesthesia. Anaesthesiology. 1978;48:23–7. doi: 10.1097/00000542-197801000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Ishizaki Y, Bandai Y, Shimomura K, Abe H, Ohtomo Y, Idezuki Y. Safe intraabdominal pressure of carbon dioxide pneumoperitoneum during laparoscopic surgery. Surgery. 1993;114:549–54. [PubMed] [Google Scholar]


