Abstract
Eclampsia is one of the most common emergencies encountered by anesthesiologists which involve a safe journey of two lives. The definition, etiology, pathophysiology, treatment guidelines along with a special reference to management of labour pain and caesarean section are discussed. Eclampsia is commonly faced challenging case in our day to day anaesthesia practice,but less is discussed in our anaesthesia text books. Lot of controversies with regard to fluid management and monitoring still remain unanswered
Keywords: Anesthesia, caesarean, eclampsia
INTRODUCTION AND DIAGNOSIS
Any pregnant woman presenting with a convulsion in an emergency setting should be taken as eclampsia unless proved. Greek meaning of eclampsia is fancied perception of flashes of light, as the entity is associated with visual disturbances. Eclampsia is defined as the occurrence of one or more generalized convulsions and/or coma in the setting of pre-eclampsia and in the absence of other neurologic conditions before, during, or after labor.[1]
The differential diagnosis includes epilepsy, cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, cerebral venous thrombosis, cerebral edema, malignant hypertension, benign and malignant cerebral tumors, cerebral abscess, viral, bacterial, parasitic infestations, hyponatremia, hypocalcemia, hypoglycemia, and hyperglycemia.[2,3]
Risk factors for eclampsia include nulliparity, multiple gestation, molar pregnancy, triploidy, pre-existing hypertension or renal disease, previous severe preeclampsia or eclampsia, nonimmune hydrops fetalis, and systemic lupus erythematosus.[4]
We have tried to discuss its pathophysiology and management with a special emphasis on quick and scientific anesthetic intervention to have a successful outcome in sick patients.
Etiology
Hypothesis of mechanism of endothelial damage leading to pre-eclampsia and eclampsia [Figure 1][5].
Figure 1.
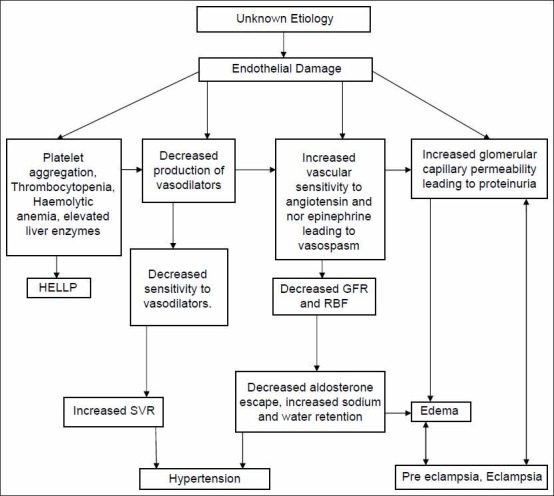
Hypothesis of mechanism of endothelial damage leading to preeclampsia-eclampsia
Pathogenesis of seizures
There is a loss of autoregulation of cerebral blood flow (CBF) (60-120 mmHg) causing increased CBF making some segments of vessels dilated, ischemic, and increasingly permeable. Cerebral vasospasm, ischemia, edema, hemorrhage, and hypertensive encephalopathy are probably associated in pathogenesis.[6]
Clinical features
The clinical features of pre-eclampsia are described earlier. When seizure adds on, it becomes eclampsia. The characteristics of seizure specific for eclampsia are described as follows.
It has an abrupt onset of facial congestion with eye protrusion, foam from mouth, and biting of tongue. It typically begins as facial twitching and followed by a tonic phase that persists for 15-20 s. Then it progresses to a generalized clonic phase characterized by apnea, which lasts for approximately 1 min. The breathing typically resumes with a long stertorous inspiration and the patient enters a postictal state, with a variable period of coma. Cardiorespiratory arrest and pulmonary aspiration of gastric contents may complicate a seizure.
The major complications of eclampsia include HELLP syndrome, intrauterine growth retardation, abruptio placentae, neurologic deficits, aspiration pneumonitis, DIC, pulmonary edema, renal failure and cardiac arrest.[7]
Role of imaging
Imaging is not necessary, as neurological abnormalities are transient in most cases. Moodley et al.[8] in their study on electroencephalogram and computerized cerebral tomography findings in eclampsia emphasized that imaging has limited clinical value and it can be performed on affected women with focal neurologic signs, atypical seizures, and/or delayed recovery.
Role of anesthesiologist
Role of anesthesiologist in eclampsia is to help obstetrician to control and prevent further convulsions, control blood pressure, establish a clear airway, prevent major complications, to provide labor analgesia and to provide anesthesia for cesarean section.
Control and prevention of convulsions
The elementary concepts of seizure control are to prevent maternal injury, ensure oxygenation, provide cardio respiratory support, and prevent aspiration. Magnesium sulfate (MgSO4) is the anticonvulsant drug of choice. In IV regimen (Zuspan) MgSO4 is given as 4 g IV bolus followed by 2 g/h as infusion. In IM regimen (Pritchard) 4 g of 20% MgSO4 IV and 10 g of 50% MgSO4 IM followed by 5 g IM every 4 h. Continuous infusion maintains steady-state plasma concentration than IM regimen. MgSO4 is continued for 24 h after last fit or delivery whichever is later. Side effects of MgSO4 therapy are potentiation of neuromuscular blockade, respiratory depression, hypotension, cardiac arrest, atonic PPH, and reduced beat to beat variability in the fetal heart rate. Hence, it is essential to monitor knee jerk, respiratory rate, and urine output during MgSO4 therapy. Serum Mg levels should be monitored in IV regimen as therapeutic window is very narrow. Therapeutic plasma level of Mg is 4-7 meq/l or 4.8-8.4 mg/dl (1 meq/l = 1.22 mg/dl). If seizure continues, or if seizures recur, give a second bolus (2 g) of magnesium sulfate. If seizures continue despite a further bolus of magnesium sulfate, treat with phenytoin (15 mg/kg) or diazepam (10 mg) or thiopentone (50 mg IV). The multinational Eclampsia Trial Collaborative Group study (1995) reveled that MgSO4 is superior to diazepam or phenytoin.[9] Resistant seizures should be managed by muscle relaxation and IPPV. The most common dilemma faced by critical care physician is whether MgSO4 can be given in cases of reduced GFR. The initial 4 g loading dose of magnesium sulfate can be safely administered regardless the renal function. This is because after distribution the loading dose achieves the desired therapeutic level and the infusion maintains the steady-state level. Thus, only the maintenance infusion rate should be altered with the diminished glomerular filtration rate. The renal function is estimated by measuring plasma creatinine. Whenever plasma creatinine levels are more than 1.0 mg/ml, serum magnesium levels are used to adjust the infusion rate.
Control of hypertension
The NICE Guidelines for hypertension management are:[10]
Antihypertensive treatment is started when systolic blood pressure is over 160 mmHg or a diastolic blood pressure is over 110 mmHg
Consider treatment at lower levels if other markers of potentially severe disease like heavy proteinuria or disordered liver or hematological test results are present
Labetalol, hydralazine, and nifedipine are the commonly used drugs
Atenolol, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor-blocking drugs (ARB), and diuretics should be avoided
Nifedipine is to be given orally. There is no role for sublingual nifedipine
Labetalol should be avoided in women with known bronchial asthma.
In an emergency setting, oral nifedipine 10-20 mg every 30 min to max of 50 mg or Inj. Labetalol 20, 40, 80, and 80 with a gap of 20 min intravenously based on the response to a maximum dose of 220 mg is given. Inj Hydralazine 5-10 mg every 20 min to a max dose 20 mg can also be given. If the result is not satisfactory, the last option is intravenous nitroglycerine.
Preanesthetic evaluation
Problems
The following is a brief list of possible problems when anaesthetizing a case of eclampsia.
Poorly controlled hypertension
Albuminuria (decreased colloid osmotic pressure)
Thrombocytopenia
Central vascular depletion
Associated systemic illness like diabetes mellitus
Hypertensive response during intubation and extubation
-
Drug interaction with magnesium sulfate
- Airway edema
- Thromboembolism.
Assessment of target organ involved
Cardiovascular system: Hypertension control, LV function, and intravascular depletion (check osmolality)
Respiratory system: For signs of pulmonary edema
Renal: Degree of oliguria and creatinine level
Liver: Liver function test, clinical features of stretching of liver capsule
Coagulation profile: Platelet count, prothrombin time, activated partial thromboplastin Time
Airway examination: Degree of airway edema
ABG analysis: Acidemia.
Goals of anesthesia management
Seizure control
Blood pressure control: Appropriate treatment must be instituted if the diastolic pressure exceeds 110 mmHg
The possibility of increased ICP need not concern the anesthesiologist if the patient remains conscious, alert, and seizure free
Persistent coma and localizing signs may indicate major intracranial pathology, which would affect anesthetic management
Maintenance of fluid balance: intake should be restricted to 80 ml/h
Monitoring of maternal oxygenation by continuous pulse oximetry
Keep blood products available
Coagulation studies should be undertaken regardless of the platelet count
Judicious preloading if hypovolemia is suspected or post vasodilator therapy.
Management of labor pain
Epidural analgesia is possible in conscious eclamptic women with no evidence of increased ICP or coagulopathy and whose seizures have been well controlled. Most of us avoid epidural if neurologic deficit exists until the diagnosis becomes clear. The technique is as follows.
To start, hydration with 0.5-1 l of crystalloid is necessary. Maternal electrocardiogram, blood pressure, as well as fetal heart rate should be monitored continuously. Administration of oxygen with a facemask or nasal cannula is beneficial. Among the local anesthetics, a low concentration of bupivacaine, 0.125%, with 2 μg/ml of fentanyl as an initial bolus provides excellent analgesia with minimal motor block. Lesser the motor block, greater the benefits with regard to fetal head rotation. The mixture can also be given as epidural infusion at the rate of 10-12 ml/h. Other local anesthetics, such as ropivacaine and levobupivacaine may be used but the superiority of these agents compared to bupivacaine is not established till now. In the combined spinal epidural technique, the intrathecal dose, an opioid alone such as fentanyl or sufentanil may be used or a combination of 1.25-2.5 mg of bupivacaine with 25 μg fentanyl. Opioids, especially in large doses are cautiously administered for the possibility of exacerbation of increased ICP from respiratory depression. It is better to avoid overzealous preloading with intravenous fluids before establishing low-dose epidural analgesia and combined spinal epidural analgesia. Newsome et al.[11] in their study on hemodynamic effects of lumbar epidural anesthesia in severe preeclampsia have suggested that with judicious hydration and slow induction of block, hypotension can be minimized with little change in CVP, CI, and PCWP.
Ergometrine should be avoided in the third stage of labor as it may elevate the blood pressure further. Instead, oxytocin 20 IU in a liter of Ringer's lactate solution is to be given intravenously at 10 drops/min. The second stage is assisted by forceps in all eclamptic patients having a vaginal delivery, to minimize maternal efforts at bearing down and prevent further increase in blood pressure.
Management of anesthesia for caesarian section
Regional anesthesia
Spinal or epidural anesthesia can be given safely if the patient is conscious, seizure free with stable vital signs with no signs of raised ICP. Moodley et al.[12] found no difference in maternal and neonatal outcomes when comparing epidural versus general anesthesia for cesarean section in conscious women with eclampsia. Spinal anesthesia with low-dose bupivacaine with fentanyl is a good option. Safety of spinal anesthesia has been studied in eclamptics by Razzaque et al.[13] who concluded that spinal is safer than GA for LSCS in eclamptics. A prospective cohort comparison by Antonie[14] et al. in patients with severe pre-eclampsia concluded that pre-eclamptic patients experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients. Hyperbaric bupivacaine (7.5 mg) with 25 μg fentanyl provides adequate anesthesia for cesarean section. If a CSE technique is instituted, the presence of the epidural catheter provides the flexibility to extend the level and the duration of block. Contraindications to regional anesthesia include patient refusal, DIC, placental abruption. With regard to administering spinal anesthesia in patients on Aspirin, it has been recommended by American society of regional anesthesia[15] that a low-dose aspirin therapy is not a contraindication to regional technique. Regional anesthesia is considered safe when the platelet count is more than 75 000 per micro liter. Platelet count more than 50 000 per micro liter is generally considered a contraindication. Within the range 50-75 thousands per micro liter an individual assessment (considering patient risks and coagulation tests) is necessary. The anesthesiologist should also maintain vigilance toward the pulmonary function, urinary output, evidence of aortocaval compression and epidural-induced systemic hypotension that may lead to decreased uteroplacental blood flow. Small incremental intravenous doses (50 ug) of phenylephrine may be used to treat hypotension temporarily while additional intravenous fluid is infused judiciously.
General anesthesia
General anesthesia (GA) is the choice in unconscious, obtunded patients with evidence of increased ICP. Anesthesia is achieved with an opioid and relaxant technique and deliberate hyperventilation. The important considerations are
Airway edema
Possibility of difficult airway management
Although cholinesterase levels decrease, the duration of action of succinylcholine and ester local anesthetics is seldom affected
Exaggerated hypertensive responses to endotracheal intubation
Drug interaction between magnesium and muscle relaxants
A small dose of a volatile halogenated agent may prevent awareness
Extubation done in the left lateral position when patient is fully conscious or else patient is transferred to ICU for ventilatory support depending on the preoperative condition and intraoperative behavior.
Should we monitor fluid management?
Initial finding seen in most cases is low CVP and high left-sided filling pressures (PCWP). If urine output is adequate there is no necessity for any special monitoring. If urine output is not adequate, a fluid challenge is done with 250-500 ml of crystalloid infused over 20 min. If response is seen additional fluid boluses may be given cautiously. If there is no response to the initial fluid bolus, CVP or PCWP monitoring becomes necessary. Pulmonary artery catheter is indicated in severe pulmonary edema, oliguria unresponsive to fluid therapy and intractable hypertension.[16]
CVP monitoring concepts
Currently a volume expansion to CVP of at least 6-8 mmHg is considered to be safe and effective. Young et al.[17] in their study on hemodynamic, invasive, and echocardiographic monitoring in hypertensive parturients found that the CVP–PCWP gradients in severe pre-eclampsia may be as high as 8-10 mmHg. Therefore, a CVP of 8 mmHg might correspond to a PCWP as high as 18 mmHg. This results in volume overload and possibly pulmonary edema. Hence, the aim of a volume expansion to achieve a CVP of 4 mmHg or less may be better in eclamptics.
When is intra-arterial blood pressure indicated?[18]
Even though individual cases differ, invasive blood pressure monitoring is required in the following situations.
Sustained high BP
Potential rapid BP fluctuations
Inability to obtain BP by cuff
Repeated sampling
Use of peripheral vasodilators
Concepts about pulmonary edema.
Pulmonary edema is a dangerous complication that may be either cardiogenic or noncardiogenic.[19] Cardiogenic pulmonary edema is due to either impaired left ventricular systolic or diastolic function. The presence of low CO, high PCWP, high CVP, and high SVR characterizes systolic dysfunction, whereas diastolic dysfunction is associated with normal or high CO, high PCWP, and a normal SVR. Noncardiogenic pulmonary edema results from such factors as increased capillary permeability, iatrogenic fluid overload, an imbalance between colloid osmotic pressure (COP) and hydrostatic pressure, or a combination of these factors. The management varies according to the cause and the clinical dysfunction.
Renal dysfunction and oliguria
In severe preeclampsia, vasospasm and endothelial dysfunction lead to reduction in GFR. Rising serum creatinine and oliguria signal rapid deterioration of the renal function. Clark et al.[20] in their study on managing hemodynamic subsets of oliguria in pre-eclampsia described three distinct types of hemodynamic findings. First group showed classic signs of hypovolemia as evidenced by low filling pressures, elevated SVR, and hyperdynamic cardiac function. These responded well to IV fluids. The second group showed normal or elevated filling pressures, elevated CO, and a high SVR. These patients were treated with vasodilators and fluid restriction. Third group showed elevated SVR and PCWP, and depressed cardiac function responded well to after-load reduction. So treatment for oliguria is situation based.
Thromboprophylaxis
Women with pre-eclampsia are at increased risk of thromboembolic disease. Before delivery, all patients should have antiembolic stockings or low molecular weight heparin while immobile. Following delivery, low molecular weight heparin (dose adjusted on early pregnancy weight) should be given daily until the patient is fully mobile (seven days if delivered by Caesarean section). Low molecular weight heparin should not be given until 4-6 h after spinal anesthesia. An epidural catheter should be left in place for at least 12 h after low molecular weight heparin administration. Following removal of an epidural catheter low molecular weight heparin should not be given for 4-6 h.[15]
Postpartum management
In postpartum, close monitoring is done of vital signs, fluid intake and output, and symptoms for at least 48 h. These women usually receive large amounts of intravenous fluids during labor, delivery, and postpartum. In addition, during the postpartum period there is mobilization of extracellular fluid leading to increased intravascular volume. As a result, women with eclampsia, particularly those with abnormal renal function, those with abruptio placentae, and those with pre-existing chronic hypertension, are at increased risk for pulmonary edema and exacerbation of severe hypertension. Hence, it is essential to continue the vigilance in the postpartum period. Regarding intravenous fluids, following delivery, the woman should be fluid restricted in order to wait for the natural diuresis that usually occurs sometime around 36-48 hours post delivery. The total amount of fluid (the total of intravenous and oral fluids) should be restricted to 80 ml/h. Fluid restriction will usually be continued for the duration of magnesium sulfate treatment; however, increased fluid intake may be allowed at an earlier time point in the presence of significant diuresis. Parenteral magnesium sulfate should be continued for at least 24 h after delivery and/or for at least 24 h after the last convulsion. Regarding antihypertensive therapy, methyldopa can be withheld in favor of calcium channel blockers, beta blockers, or alpha blockers.
CONCLUSION
The definitive treatment is delivery. However, it is inappropriate to deliver an unstable mother even if there is fetal distress. Once seizures are controlled, severe hypertension treated and hypoxia corrected, delivery can be expedited. Always consider prophylaxis against thromboembolism. The fluid management along with magnesium and antihypertensive therapy with strict hemodynamic vigilance is to be continued in the postpartum period.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. Pregnancy hypertension. 23rd ed. Chap. 34. New York: McGraw-Hill; 2010. Williams Obstetrics; pp. 706–56. [Google Scholar]
- 2.Kaplan PW, Repke JT. Eclampsia. Neurol Clin. 1994;12:565–82. [PubMed] [Google Scholar]
- 3.Sibai BM. Eclampsia. In: Goldstein PJ, Stern BJ, editors. Neurologic Disorders of Pregnancy. 2nd ed. Mount Kisco, New York: Futura Publishing Company; 1992. pp. 1–24. [Google Scholar]
- 4.Sibai BM. Eclampsia. VI. Maternal-perinatal outcome in 254 consecutive cases. Am J Obstet Gynecol. 1990;163:1049–55. doi: 10.1016/0002-9378(90)91123-t. [DOI] [PubMed] [Google Scholar]
- 5.Boxer LM, Malinow AM. Preeclampsia and eclampsia. Curr Opin Anesth. 1997;10:188–98. [Google Scholar]
- 6.Barton JR, Sibai BM. Cerebral pathology in eclampsia. Clin Perinatol. 1991;18:891–910. [PubMed] [Google Scholar]
- 7.Mattar F, Sibai BM. Eclampsia VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182:307–12. doi: 10.1016/s0002-9378(00)70216-x. [DOI] [PubMed] [Google Scholar]
- 8.Moodley J, Bobat SM, Hoffman M, Bill PL. Electroencephalogram and computerised cerebral tomography findings in eclampsia. Br J Obstet Gynaecol. 1993;100:984–8. doi: 10.1111/j.1471-0528.1993.tb15138.x. [DOI] [PubMed] [Google Scholar]
- 9.Eclampsia Trial Collaborative Group. Which anticonvulsant for women with eclampsia? Evidence from the collaborative eclampsia trial. Lancet. 1995;345:1455–63. [PubMed] [Google Scholar]
- 10.National Institute of Health and Clinical Excellence. Hypertension in Pregnancy, The management of hypertensive disorders during pregnancy. Clinical guidelines CG107 Issued. 2010. Aug, [Last accessed on 2013 Feb 1]. Available from: http://guidance.nice.org.uk/CG107 .
- 11.Newsome LR, Bramwell RS, Curling PE. Severe preeclampsia: Hemodynamic effects of lumbar epidural anesthesia. Anesth Analg. 1986;65:31–6. [PubMed] [Google Scholar]
- 12.Moodley J, Jjuuko G, Rout C. Epidural compared with general anaesthesia for caesarean delivery in conscious women with eclampsia. Br J Obstet Gynaecol. 2001;108:378–82. doi: 10.1111/j.1471-0528.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- 13.Razzaque M, Rahman K, Sashidharan R. Spinal is safer than GA for LSCS in eclamptics (abstract) Anesthesiology. 2001;94:A34. [Google Scholar]
- 14.Aya AG, Mangin R, Vialles N, Ferrer JM, Robert C, Ripart J, et al. Patients with Severe Preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: A Prospective cohort comparison. Anesth Analg. 2003;97:867–72. doi: 10.1213/01.ANE.0000073610.23885.F2. [DOI] [PubMed] [Google Scholar]
- 15.Horlocker TT, Wedel DJ, Rowlingson JC, Enneking FK, Kopp SL, Benzon HT, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines. Reg Anesth Pain Med. (3rd ed) 2010;35:64–101. doi: 10.1097/aap.0b013e3181c15c70. [DOI] [PubMed] [Google Scholar]
- 16.Ramanathan J, Bennett K. Preeclampsia: Fluids, drugs and anesthetic management. Anesthesiol Clin North Am. 2003;21:145–63. doi: 10.1016/s0889-8537(02)00054-8. [DOI] [PubMed] [Google Scholar]
- 17.Young P, Johanson R. Haemodynamic, invasive, and echocardiographic monitoring in the hypertensive parturient. Best Pract Res Clin Obstet Gynecol. 2001;15:605–22. doi: 10.1053/beog.2001.0203. [DOI] [PubMed] [Google Scholar]
- 18.Polley LS. Hypertensive disorders. Chestnut's Obstetric anaesthesia: Principles and practice. In: Chestnut DH, Polley LS, Tsen LC, Wong CA, editors. 4th ed. Philadelphia: Mosby Elsevier; 2009. pp. 975–1000. [Google Scholar]
- 19.Mabie WC, Ratts TE, Ramanthan KB. Circulatory congestion in obese hypertensive parturients: A subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553–8. [PubMed] [Google Scholar]
- 20.Clark SL, Greenspoon JS, Aldahl D. Severe pre-eclampsia with oliguria: Management of hemodynamic subsets. Am J Obstet Gynecol. 1986;154:490–4. doi: 10.1016/0002-9378(86)90588-0. [DOI] [PubMed] [Google Scholar]


