Abstract
Background:
Caudal analgesia is the most commonly used technique providing intra- and postoperative analgesia for various pediatric infraumbilical surgical procedures but with the disadvantage of short duration of action after single injection. Caudal dexamethasone and magnesium could offer significant analgesic benefits. We compared the analgesic effects and side-effects of dexamethasone or magnesium added to caudal ropivacaine in pediatric patients undergoing inguinal hernia repair.
Materials and Methods:
A total of 105 (1-6 years) were randomly assigned into three groups in a double-blinded manner. After a standardized sevoflurane in oxygen anesthesia, each patient received a single caudal dose of ropivacaine 0.15% 1.5 mL/kg combined with either magnesium 50 mg in normal saline 1 mL (group RM), dexamethasone 0.1 mg/kg in normal saline 1 mL (group RD), or corresponding volume of normal saline (group R) according to group assignment. Postoperative analgesia, use of analgesics, and side-effects were assessed during the first 24 h.
Results:
Addition of magnesium or dexamethasone to caudal ropivacaine significantly prolonged analgesia duration 8 (5-11) h and 12 (8-16) h, respectively compared with 4 (3-5) h with the use of ropivacaine alone. The incidence of postoperative rescue analgesia was significantly higher in group R compared with groups RM and RD. The time to 1st analgesic dose was significantly longer in groups RM and RD (500 ± 190 and 730 ± 260 min) respectively compared with group R (260 ± 65 min). Group R patients achieved significantly higher Children's Hospital of Eastern Ontario Pain Scale and Faces Legs Activity Cry Consolability scores (4th hourly) compared with groups RM and RD patients (8th and 12th hourly, respectively).
Conclusion:
The addition of dexamethasone or magnesium to caudal ropivacaine significantly prolonged the duration of postoperative analgesia in children undergoing inguinal hernia repair. Also the time to 1st analgesic dose was longer and the need for rescue postoperative analgesic was reduced and without increase in incidence of side effects.
Keywords: Caudal analgesia, dexamethasone, magnesium, pediatric, ropivacaine
INTRODUCTION
Postoperative pain in children is difficult to assess and associated with strong emotional component, however undertreated. Caudal epidural anesthesia is a common technique providing intra and postoperative analgesia in pediatric infraumbilical surgical procedures[1,2] but with the disadvantage of short duration of action after single injection.[3] Ropivacaine offers some advantages over bupivacaine, for example, less cardiac and neurological toxicity, less motor blockade and prolonged sensory analgesia.[4]
Prolongation of caudal analgesia using a “single-shot” technique has been achieved by the addition of various adjuvants such as opioids,[5,6] ketamine,[7,8] clonidine,[9,10] and dexmedetomidine.[10] However, their use has been limited by adverse effects in children. Opioids carry risk of postoperative respiratory depression and ketamine has the potential of neurotoxicity if inadvertently injected intrathecally.[11]
Dexamethasone is a well-known corticosteroid with strong anti-inflammatory effects, started to be investigated for its analgesic efficacy. Epidurally administered dexamethasone could reduce the incidence and severity of postoperative pain in adults.[12] However, up till now still there is some controversy concerning the route of administration whether regional or systemic[13] and its additive analgesic effects if administrated as adjuvant. Also, there is no study concerning the use of dexamethasone, as an adjuvant agent for the caudal epidural block in children.
Recently, there is increasing interest to study magnesium analgesic effects.[14] It has antinociceptive effects in human and these effects are primarily based on the regulation of calcium influx into the cell. Magnesium is a physiological calcium antagonist and blocks N-methyl-D-aspartate (NMDA) receptor and such NMDA antagonism prevents the central sensitization from nociceptive stimulation.[15] Many studies[16,17,18] suggested that epidurally administered magnesium as an adjuvant could reduce the postoperative pain in adults. But few studies[19] are available about the use of magnesium as an adjuvant in caudal block for postoperative analgesia in pediatrics.
We performed prospective randomized double-blind study to examine the analgesic effect of dexamethasone or magnesium added to ropivacaine and ropivacaine alone in caudal analgesia on postoperative pain control in pediatric patients undergoing inguinal hernia repair.
MATERIALS AND METHODS
After obtaining Zagazig University ethical committee approval and informed parental consent was obtained for each case. A total of 105 American Society of Anesthesiologists I and II children, aged 1-6 years undergoing inguinal hernia repair were included in this prospective, randomized, double-blinded study. Patients were excluded from the study if they had a contraindication for caudal block including a hypersensitivity to any local anesthetics, magnesium sulphate or dexamethasone, known or suspected coagulopathy, any signs of infection at the puncture sites, or preexisting neurological disease.
The children were randomly assigned in three groups to receive, via caudal route in a double blind manner either, Normal saline 1 mL added to ropivacaine 0.15% 1.5 mL/kg (Group R, n = 35) or magnesium 50 mg in 1 mL normal saline added to ropivacaine 0.15% 1.5 mL/kg (Group MR, n = 35) or dexamethasone 0.1 mg/kg in 1 mL normal saline added to ropivacaine 0.15% 1.5 mL/kg (Group DR, n = 35).
After inhalational induction of general anesthesia, by an anesthetist who was unaware of the group allocation, with sevoflurane 8% and N2O in 50% O2 in spontaneous ventilation, the airway was secured by placement of an appropriate-sized laryngeal mask. Anaesthesia was maintained by 2.5%-3% sevoflurane in spontaneous ventilation. Caudal block was performed by the same anesthetist as follows: For caudal puncture a 5 cm short beveled 22 G caudal needle was used in the lateral decubitus position. After identifying the space using the loss of resistance technique with saline, the study solutions were injected slowly with repetitive intermittent aspiration. Intraoperatively, no additional drugs were given.
Surgery was allowed to begin 10 min after performing the block. The same urologist performed all surgical procedures. Standard monitoring was used during anesthesia and surgery. Heart rate and arterial pressure were recorded before operation and every 5 min until the end of surgery.
End-tidal sevoflurane concentration was adjusted according to clinical signs (arterial pressure or heart rate within 20% of baseline). After emergence from anesthesia, patients were managed by an observer blinded to group allocation in the postanesthetia care unit (PACU). Behavior during emergence was rated on a four-point scale:[20] (1, calm; 2, not calm but could be easily calmed; 3, not easily calmed, moderately agitated or restless; and 4, combative, excited, or disoriented). Emergence time (the time from the end of surgery to opening the eyes on calling the patient's name), a delayed anesthetic emergence (considered if > 20 min elapsed from the end of surgery to exiting the operating theatre), or all were also noted.
Postoperative pain was assessed at the end of surgery, 30 min, 1, 2, and 3 h after surgery and at the time of discharge from the PACU, and then every 4 h for the first 18 h after operation using the Children's Hospital of Eastern Ontario Pain Scale (CHEOPS, 0-10)[21] and Faces Legs Activity Cry Consolability tool (FLACC, 0-10).[22] Pethidine 1 mg/kg i.m. was administered as rescue analgesia in the PACU if two coupled observations separated by a 5 min waiting period yielded both CHEOPS and FLACC ≥4. The duration of adequate caudal analgesia (from the time of caudal injection to the first time, the CHEOPS and FLACC pain scale score were noted to be ≥4) was also recorded. Motor block in the lower extremity was assessed with a modified Bromage scale (0 = no residual motor block, 1 = inability to raise extended legs, 2 = unable to flex knee, 3 = unable to flex ankle).
After transferring to the ward, rescue analgesic consumption, pain scores, and adverse effects such as postoperative nausea and vomiting (PONV), respiratory depression, urinary retention, hypotension, and bradycardia were monitored by a staff nurse for 24 h. Analgesia was provided with oral paracetamol (15 mg/kg). The time to first supplemental oral paracetamol demand from the end of surgery to the first registration of a visual analog score (0-10) ≥5 was recorded. The time to first micturition after caudal injection and the incidence of bladder catheterization. The initiation of clear liquid and time of discharge home were also recorded.
Statistical analysis
We calculated that 32 patients in each group would be needed to detect an intergroup difference in the average time to first rescue analgesic of at least 20% (α = 0.05, ß = 0.9). The sample size was increased to 35 patients in each group. Data were analyzed using SPSS for windows 12.0 (Chicago, IL, USA). Numerical variables were presented as mean ± standard deviation or median (95% confidence inerval) and categorical variables were presented as frequency (%). One-way analysis of variance was used for between-group comparisons of numerical variables, if its assumptions were fulfilled, otherwise for non-parametric; the Kruskal-Wallis test was used. Tukey's honestly significant difference test or the Mann–Whitney test was used, whenever appropriate iate, as post hoc tests. Chi-square test (x2) was used for between-group comparisons between categorical variables. A P value of 0.05 was considered statistically significant.
RESULTS
Patients in the three groups were comparable regarding age, weight, sex distribution, and duration of surgery [Table 1]. Failure of caudal block was not reported in any patient. postoperative analgesia persisted for a longer duration in groups RM and RD, 8 (5-11) h and 12 (8-16) h, respectively compared with 4 (3-5) h in group R, with a (P < 0.001) [Table 2]. The emergence time of group R was 3.8 ± 1.3 min and that of groups RM and RD was 4.2 ± 1.6 and 4.0 ± 1.5 min, respectively (P = 0.35). The emergence behaviour score of group R was 3.5 ± 0.4 min and that of groups RM and RD was 1.6 ± 0.3 and 1.5 ± 0.3 min, respectively (P < 0.001) [Table 2]. Children in group R were restless and agitated compared with groups RM and RD children who were calm and cooperative.
Table 1.
Demographic data and duration of surgery. Data are mean±standard deviation. There was no statistically significant difference among the three groups
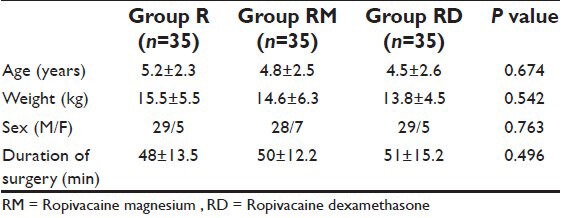
Table 2.
Analgesia duration, emergence time, and emergence behaviour score. Data are median (95% confidence interval) or mean±standard deviation. *P<0.001 compared with groups RM and RD. #P<0.001compared with group RD
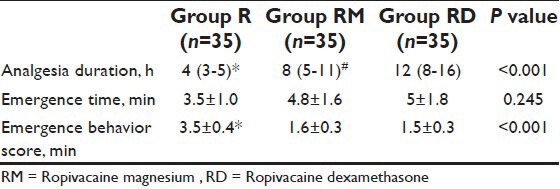
The incidence of rescue pethidine in the PACU and rescue oral paracetamol in ward was significantly higher in group R children compared with groups RM and RD children (P < 0.01) [Table 3]. Nine of the 35 child in group R, four of the 35 in group RM, and two of the 35 in group RD received both fentanyl rescue in PACU and oral paracetamol in ward (P < 0.01). The time to first oral paracetamol administration was significantly longer in groups RM and RD children (500 ± 190 and 730 ± 260 min), respectively compared with group R (260 ± 65 min) (P < 0.001) [Table 3].
Table 3.
Postoperative rescue analgesics. Data are proportion (%) or mean±standard deviation. *P<0.01 compared with groups RM and RD
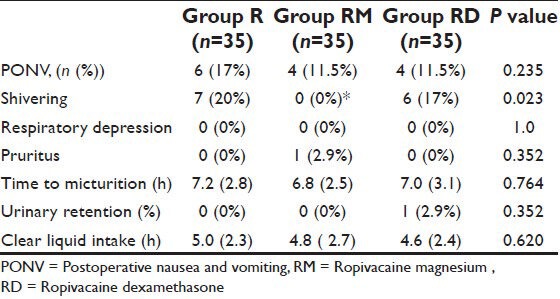
There was a significant difference between the groups in the CHEOPS and FLACC scores measured 4th hourly in the postoperative period where group R patients achieved significantly higher CHEOPS and FLACC scores compared with Groups RM and RD patients which diminished after receiving rescue analgesic. Group RM children achieved CHEOPS and FLACC scores of 4 at 8th hour, whereas Group RD children had CHEOPS and FLACC scores of 4 at 12th hour of postoperative period and diminished after receiving rescue analgesic [Figures 1 and 2]. Motor blockade assessed by modified Bromage scale was comparable between the three groups [Figure 3]. The incidence of PONV and pruritus was comparable between the three groups. Shivering occurred in 7 (20%) Group R and in 6 (17%) Group RD compared with no patient in group RM (P < 0.05). Postoperative respiratory depression was not observed in any group. Mean times to first micturition and clear liquid intake were comparable between the three groups. One child in Group RD required catheterization [Table 4]. The three groups did not vary in respect to sedation or other adverse effect.
Figure 1.
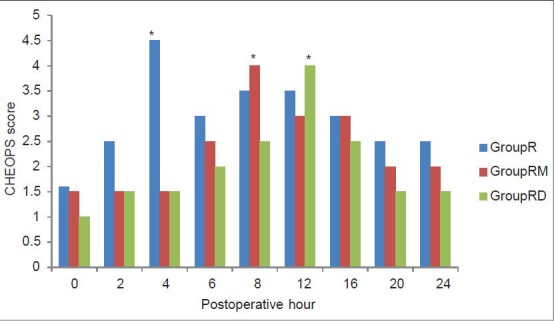
Postoperative pain score (Children's Hospital of Eastern Ontario Pain Scale) in the three groups. *Significantly higher than the other two groups
Figure 2.
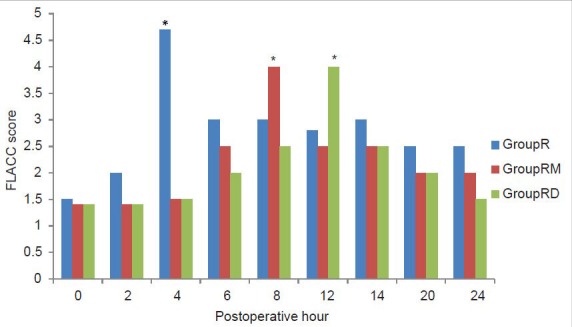
Postoperative pain score (Faces Legs Activity Cry Consolability) in the three groups. *Significantly higher than the other two groups
Figure 3.
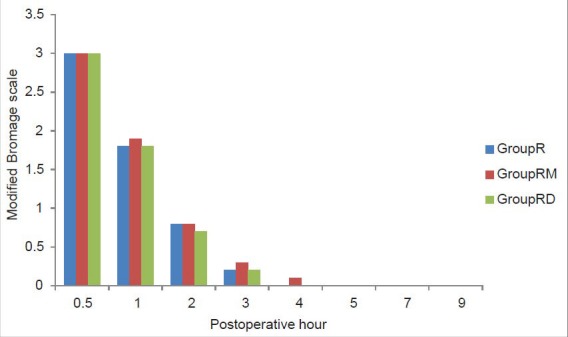
Postoperative residual motor block (assessed by modified Bromage scale) in the three groups
Table 4.
Postoperative adverse effects. Data expressed as frequency (%) or mean (standard deviation)
DISCUSSION
Many studies[16,17,18] suggested that epidurally administered magnesium as an adjuvant could reduce the postoperative pain in adults. But few studies[19] are available about the use of magnesium as an adjuvant in caudal block for postoperative analgesia in pediatrics. On the contrary, dexamethasone has been successfully administered epidurally for postoperative analgesia in adults.[12] Nevertheless, there are still some concerns regarding its route of administration whether regional or systemic[13] and its additive analgesic effects if administrated as adjuvant. In this study, we found that the duration of adequate caudal analgesia without the need for rescue pethidine is significantly higher in the groups receiving ropivacaine-magnesium [median (95% confidence interval (CI):8 (5-11) h] or ropivacaine–dexamethasone [median (95% CI): 12 (8-16) h] than the group receiving ropivacaine alone [median (95% CI): 4 (3-5) h] However, there was statistically significant difference between dexamethasone and magnesium as regards the analgesia duration. Also, rescue oral paracetamol was lower and the time to first oral paracetamol administration was significantly longer with the addition of dexamethasone or magnesium compared with caudal ropivacaine alone with improved emergence behavior score and no increased incidence of side effects.
Magnesium is known to be an NMDA receptor antagonist. NMDA receptors have been implicated in the development of central sensitivity after noxious peripheral stimulation.[23] Magnesium prevents this central sensitization and whatever the route of administration, the true site of action of magnesium is at the spinal cord NMDA receptors.[24,25] Tanmoy et al.,[26] evaluated the effect of adding MgSO4 as adjuvants to epidural bupivacaine in lower abdominal surgery and reported reduction in time of onset and establishment of epidural block. Whereas, Arcioni et al.,[27] proved that combined intrathecal and epidural MgSO4 supplementation reduce the postoperative analgesic requirements. Farouk[16] found that the continuous epidural magnesium started before anesthesia provided preemptive analgesia and analgesic sparing effect that improved postoperative analgesia. Also, Bilir et al.,[18] showed that the time to first analgesia requirement was slightly longer with significant reduction in fentanyl consumption after starting epidural MgSO4 infusion postoperatively. Asokumar et al.,[28] found that addition of MgSO4 prolonged the median duration of analgesia after intrathecal drug administration. El-Kerdawy[29] studied patients received CSE followed by epidural infusion of magnesium (100 mg/h) and concluded that time to first analgesic requirement was significantly prolonged in the magnesium group compared with the control placebo group. Kim et al.,[17] conducted a trial to evaluate the effects of epidural magnesium on cumulative dose of ropivacaine in patients with patient-controlled epidural analgesia after a thoracotomy. Magnesium group received 100 mg of magnesium in the initial dose and 4 mg in the demand dose. They observed that the analgesic requirement was lower at 12, 24, and 48 h compared with the control group (received ropivacaine only). Also, Buvanendran et al.,[28] used magnesium intrathecally and demonstrated that 50 mg intrathecal use of magnesium prolonged analgesia in adults. In contrast to our results, Birbicer et al.,[19] who compared ropivacaine 0.25% plus 50 mg magnesium to ropivacaine 0.25% alone for caudal anesthesia in children. They concluded that that addition of magnesium as an adjuvant agent to local anesthetics for caudal analgesia has no effect on postoperative pain and analgesic need. On the contrary, Ko et al.,[24] found that perioperative intravenous administration of magnesium sulfate 50 mg/kg does not reduce postoperative analgesic requirements which could be attributed to the finding that the perioperative intravenous administration of MgSO4 did not increase cerebrospinal fluid magnesium concentration due to inability to cross blood brain barrier.
The effects of systemic dexamethasone in reducing postoperative pain and morbidity have been studied in children. Results have been conflicting; some studies demonstrating benefit and others not[30,31,32,33] In addition, most published studies for children have been limited to the otolaryngology procedures with wide ranges of dexamethasone (0.4-1.0 mg kg 21 with maximum doses from 8 to 50 mg). Also, Hong et al.,[13] concluded that an intravenous dexamethasone in combination with a caudal block with ropivacaine reduces the intensity of postoperative pain and prolongs analgesic duration after paediatric orchiopexy. In line with our results, Thomas and Beevi[34] found preoperative epidural administration of dexamethasone 5 mg, with or without bupivacaine, reduces postoperative pain and morphine consumption following laparoscopic cholecystectomy. Also, Khafagy et al.,[12] found epidural bupivacaine-dexamethasone admixture had almost the same analgesic potency as bupivacaine-fentanyl with opioid-sparing and antiemetic effects. A recent study[35] concluded that caudal ropivacaine/dexamethasone provided safe profound labor analgesia, spared the need for perineal anesthesia for episiotomy repair, and minimized the need for subsequent analgesia.
Wang et al.,[36] showed that epidural administration of dexamethasone 5 mg reduces the incidence and severity of postepidural backache following hemorroidectomy with no adverse effects over a 3-day follow-up period, In contrast Maillefert et al.,[37] found that a much larger dose of epidural dexamethasone (15 mg) may induce transient adrenal suppression.
Our results are in line with the local application of corticosteroid at the surgical site following lumbar discectomy.[38] In contrast to the findings from our study, intrathecal administration of corticosteroids in a rat model has been shown to have a mild effect on nociception-driven spinal sensitization.[39] Blanloeil et al.,[40] reported that epidural steroids do not decrease pain after thoracotomy, although in their study the opioid consumption was less in patients who received epidural steroids. Possible explanations to account for differences between these studies include sampling size differences and different steroids with different potencies.
The exact mechanism of dexamethasone analgesic effect is not fully understood. Systemic administration of steroids has been found to suppress tissue levels of bradykinin[41] and the release of neuropeptides from nerve endings,[42] both of which can enhance nociception in inflamed tissue. Dexamethasone inhibits the synthesis of the cyclooxygenase isoform-2 in peripheral tissues and in the central nervous system resulting in reduction in prostaglandin production which might contribute to analgesia.[43] Another possible mechanism is abolishment or suppression of inflammatory cytokine release with its subsequent nociceptive effects, in line with this assumption, Wang et al.,[44] found epidural dexamethasone as adjuvant to epidural analgesia, prevented elevation of maternal temperature, and prevented increased serum levels of interleukin-6, one of the potent inflammatory cytokines.
CONCLUSION
The addition of dexamethasone or magnesium to caudal 0.15% ropivacaine significantly prolonged the duration of postoperative analgesia in children undergoing inguinal hernia repair with significant advantage of dexamethasone over magnesium as regard analgesia duration. Also, the need for rescue postoperative analgesic was reduced with fewer incidences of emergence agitation and without prolongation of motor blockade or an increase in incidence of side effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Dalens B, Hasnoui A. Caudal anesthesia in pediatric surgery: Success rate and adverse effects in750 consecutive patients. Anesth Analg. 1989;68:83–9. [PubMed] [Google Scholar]
- 2.Giaufre’ E, Dalens B, Gombert A. Epidemiology and morbidity of regional anesthesia in children: A one-year prospective survey of the French-Language Society of Pediatric Anesthesiologists. Anesth Analg. 1996;83:904–12. doi: 10.1097/00000539-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Varghese ST, Hannallah RS. Postoperative pain management in children. Anesthesiol Clin North America. 2005;23:163–84. doi: 10.1016/j.atc.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Habre W, Bergesio R, Johnson C, Hackett P, Joyce D, Sims C. Pharmacokinetics of ropivacaine following caudal analgesia in children. Paediatr Anaesth. 2000;10:143–7. doi: 10.1046/j.1460-9592.2000.00454.x. [DOI] [PubMed] [Google Scholar]
- 5.Hannallah RS. Outpatient anesthesia. In: Cote’C, Todres ID, Ryan JF, Goudsouzian NG, editors. A Practice of Anesthesia for Infants, Children. 3rd ed. Philadelphia: W. B. Saunders; 2001. p. 61. [Google Scholar]
- 6.Constant I, Gall O, Gouyet L, Chauvin M, Murat I. Addition of clonidine or fentanyl to local anesthetics prolongs the duration of surgical analgesia after single shot caudal block in children. Br J Anesth. 1998;80:294–8. doi: 10.1093/bja/80.3.294. [DOI] [PubMed] [Google Scholar]
- 7.Naguib M, Sharif AM, Seraj M, el Gammal M, Dawlatly AA. Ketamine for caudal analgesia in children comparison with caudal bupivacaine. Br J Anaesth. 1991;67:559–64. doi: 10.1093/bja/67.5.559. [DOI] [PubMed] [Google Scholar]
- 8.Semple D, Findlow D, Aldrige LM, Doyle E. The optimal dose of ketamine for caudal epidural blockade in children. Anaesthesia. 1996;51:1170–2. doi: 10.1111/j.1365-2044.1996.tb15063.x. [DOI] [PubMed] [Google Scholar]
- 9.Cook B, Grubb DJ, Aldridge LA, Doyle E. Comparison of the effects of adrenaline, clonidine and ketamine on the duration of caudal analgesia produced by bupivacaine in children. Br J Anaesth. 1995;75:698–701. doi: 10.1093/bja/75.6.698. [DOI] [PubMed] [Google Scholar]
- 10.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 11.Malinovsky JM, Lepage JY, Cosian A, Mussini JM, Pinaudt M, Souron R. Is ketamine or its preservative responsible for neurotoxicity in rabbit? Anesthesiology. 1993;78:109–15. doi: 10.1097/00000542-199301000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Khafagy HF, Refaat AI, El-Sabae HH, Youssif MA. Efficacy of epidural dexamethasone versus fentanyl on postoperative analgesia. J Anesth. 2010;24:531–6. doi: 10.1007/s00540-010-0949-7. [DOI] [PubMed] [Google Scholar]
- 13.Hong JY, Han SW, Kim WO, Kim EJ, Kil HK. Effect of dexamethasone in combination with caudal analgesia on postoperative pain control in day case paediatric orchiopexy. Br J Anaesth. 2010;105:506–10. doi: 10.1093/bja/aeq187. [DOI] [PubMed] [Google Scholar]
- 14.James MF. Clinical use of magnesium infusions in anaesthesia. Anesth Analg. 1992;74:129–36. doi: 10.1213/00000539-199201000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-Daspartic acid receptor activation implications for the treatment of post-injury pain and hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 16.Farouk S. Pre-incisional epidural magnesium provides pre-emptive and preventive analgesia in patients undergoing abdominal hysterectomy. Br J Anaesth. 2008;101:694–9. doi: 10.1093/bja/aen274. [DOI] [PubMed] [Google Scholar]
- 17.Kim SM, Cho SH, Kim SH, Lee DG, Chae WS, Jin HC. The effects of epidural magnesium on postoperative pain management in patients with patient-controlled epidural analgesia after a thoracotomy. Korean J Anesthesiol. 2009;57:466–71. doi: 10.4097/kjae.2009.57.4.466. [DOI] [PubMed] [Google Scholar]
- 18.Bilir A, Gulec S, Erkan A, Ozcelik A. Epidural magnesium reduces postoperative analgesic requirement. Br J Anaesth. 2007;98:519–23. doi: 10.1093/bja/aem029. [DOI] [PubMed] [Google Scholar]
- 19.Birbicer H, Doruk N, Cinel I, Atici S, Avlan D, Bilgin E, et al. Could adding magnesium as adjuvant to ropivacaine in caudal anaesthesia improve postoperative pain control? Pediatr Surg Int. 2007;23:195–8. doi: 10.1007/s00383-006-1779-4. [DOI] [PubMed] [Google Scholar]
- 20.Ibacache ME, Muñoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia. Anesth Analg. 2004;98:60–3. doi: 10.1213/01.ANE.0000094947.20838.8E. [DOI] [PubMed] [Google Scholar]
- 21.Crellin D, Sullivan TP, Babl FE, O’Sullivan R, Hutchinson A. Analysis of the validation of existing behavioral pain and distress scales for use in the procedural setting. Paediatr Anaesth. 2007;17:720–33. doi: 10.1111/j.1460-9592.2007.02218.x. [DOI] [PubMed] [Google Scholar]
- 22.Willis MH, Merkel SI, Voepel-Lewis T, Malviya S. FLACC Behavioral Pain Assessment Scale: A comparison with the child's self-report. Pediatr Nurs. 2003;29:195–8. [PubMed] [Google Scholar]
- 23.O’Flaherty JE, Lin CX. Does ketamine or magnesium affect posttonsillectomy pain in children? Pediatr Anaesth. 2003;13:413–21. doi: 10.1046/j.1460-9592.2003.01049.x. [DOI] [PubMed] [Google Scholar]
- 24.Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS. Magnesium sulfate does not reduce postoperative analgesic requirements. Anesthesiol. 2001;95:640–6. doi: 10.1097/00000542-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Xiao WH, Bennet GJ. Magnesium suppresses neuro-pathic pain response in rats via spinal site of action. Brain Res. 1994;666:168–72. doi: 10.1016/0006-8993(94)90768-4. [DOI] [PubMed] [Google Scholar]
- 26.Ghatak T, Chandra G, Malik A, Singh D, Bhatia VK. Evaluation of the effect of magnesium sulphate vs. Midazolam as adjunct to epidural bupivacaine. Indian J Anesth. 2010;54:308–13. doi: 10.4103/0019-5049.68373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arcioni R, Palmisani S, Tigano S, Santorsola C, Sauli V, Romano S, et al. Combined intrathecal and epidural magnesium sulfate supplementation of spinal anesthesia to reduce postoperative analgesic requirements. Acta Anaesthesiol Scand. 2007;51:482–9. doi: 10.1111/j.1399-6576.2007.01263.x. [DOI] [PubMed] [Google Scholar]
- 28.Buvanendran A, Mccarthy RJ, Kroin JS, Leong W, Perry P, Tuman KJ. Intrathecal magnesium prolongs fentanyl analgesia: A prospective, randomized controlled trial. Anesth Analg. 2002;95:661–6. doi: 10.1097/00000539-200209000-00031. [DOI] [PubMed] [Google Scholar]
- 29.El-Kerdawy H. Analgesic requirements for patients undergoing lower extremity orthopedic surgery – the effect of combined spinal and epidural magnesium. Middle East J Anesthesiol. 2008;19:1013–25. [PubMed] [Google Scholar]
- 30.Mohamed SK, Ibraheem AS, Abdelraheem MG. Preoperative intravenous dexamethasone combined with glossopharyngeal nerve block: Role in paediatric postoperative analgesia following tonsillectomy. Eur Arch Otorhinolaryngol. 2009;266:1815–9. doi: 10.1007/s00405-009-0937-4. [DOI] [PubMed] [Google Scholar]
- 31.Hanasono MM, Lalakea ML, Mikulec AA, Shepard KG, Wellis V, Messner AH. Perioperative steroids in tonsillectomy using electrocautery and sharp dissection techniques. Arch Otolaryngol Head Neck Surg. 2004;130:917–21. doi: 10.1001/archotol.130.8.917. [DOI] [PubMed] [Google Scholar]
- 32.Vosdoganis F, Baines DB. The effect of single dose intravenous dexamethasone in tonsillectomy in children. Anaesth Intensive Care. 1999;27:489–92. doi: 10.1177/0310057X9902700509. [DOI] [PubMed] [Google Scholar]
- 33.Giannoni C, White S, Enneking FK. Does dexamethasone with preemptive analgesia improve paediatric tonsillectomy pain? Otolaryngol Head Neck Surg. 2002;126:307–15. doi: 10.1067/mhn.2002.122700. [DOI] [PubMed] [Google Scholar]
- 34.Thomas S, Beevi S. Epidural dexamethasone reduces postoperative pain and analgesic requirements. Can J Anaesth. 2006;53:899–905. doi: 10.1007/BF03022833. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed AA, Ibrahim WA, Safan TF. Dexamethasone as adjuvant to caudal ropivacaine as analgesic for labour. Ain Shams J Anesthesiol. 2012;5-1:33–41. [Google Scholar]
- 36.Wang YL, Tan PP, Yang CH, Tsai SC, Chung HS. Epidural dexamethasone reduces the incidence of backache after lumbar epidural anesthesia. AnesthAnalg. 1997;84:376–8. doi: 10.1097/00000539-199702000-00025. [DOI] [PubMed] [Google Scholar]
- 37.Maillefert JF, Aho S, Huguenin MC, Chatard C, Peere T, Marquignon MF, et al. Systemic effects of epidural dexamethasone injections. Rev Rheum Engl Ed. 1995;62:429–32. [PubMed] [Google Scholar]
- 38.Mirzai H, Tekin I, Alincak H. Perioperative use of corticosteroid and bupivacaine comb ination in lumbar disc surgery. A randomized controlled trial. Spine. 2002;27:343–6. doi: 10.1097/00007632-200202150-00003. [DOI] [PubMed] [Google Scholar]
- 39.Abram SE, Marsala M, Yaksh TL. Analgesic and neurotoxic effects of intrathecal corticosteroids in rats. Anesthesiology. 1994;81:1198–205. doi: 10.1097/00000542-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Blanloeil Y, Bizouarn P, Le Teurnier Y, Le Roux C, Rigal JC, Sellier E, et al. Postoperative analgesia by epidural methylprednisolone after posterolateral thoracotomy. Br J Anaesth. 2001;87:635–8. doi: 10.1093/bja/87.4.635. [DOI] [PubMed] [Google Scholar]
- 41.Hargreaves KM, Costello A. Glucocorticoids suppress levels of immune reactive bradykinin in inflamed tissue as evaluated by microdialysis probes. Clin Pharmacol Ther. 1990;48:168–78. doi: 10.1038/clpt.1990.132. [DOI] [PubMed] [Google Scholar]
- 42.Hong D, Byers MR, Oswald RJ. Dexamethasone treatment reduces sensory neuropeptides and nerve sprouting reactions in injured teeth. Pain. 1993;55:171–81. doi: 10.1016/0304-3959(93)90146-G. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira SH, Cunha FQ, Lorenzetti BB, Michelin MA, Perretti M, Flower RJ, et al. Role of lipocortin-1 in the anti-hyperalgesic actions of dexamethasone. Br J Pharmacol. 1997;121:883–8. doi: 10.1038/sj.bjp.0701211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang LZ, Hu XX, Liu X, Qian P, Ge JM, Tang BL. Influence of epidural dexamethasone on maternal temperature and serum cytokine concentration after labor epidural analgesia. Int J Gynaecol Obstet. 2011;113:40–3. doi: 10.1016/j.ijgo.2010.10.026. [DOI] [PubMed] [Google Scholar]


