Abstract
Many anti-emetics are used in clinical practice. Palonosetron hydrochloride is one of them. It is a novel, centrally acting antiemetic, and anti-nausea agent. This drug is an antagonist of serotonin receptor subtype 3 (5-HT3). This drug has longer duration of action which makes it useful in the prevention and treatment of acute and delayed onset of nausea and vomiting. This drug was initially used for chemotherapy induced nausea and vomiting. Federal drug agency (FDA) has approved it for prevention and treatment of post-operative nausea and vomiting. The literature search for this article was done using Google scholar and Pubmed using the terms “Palonosetron,” “longer duration of action,” “nausea,” “vomiting,” and “postoperative”.
Keywords: Antiemetics, anesthesia, nausea, palonosetron, post-operative, vomiting, 5-HT3
INTRODUCTION
Nausea is the sensation of being about to vomit. Vomiting or emesis is the expulsion of stomach contents through the mouth. Like pain it is also an unpleasant sensation one can have. Postoperative nausea and vomiting (PONV) are one of the most distressing side effects following anesthesia. It can result in morbidity, delayed hospital discharge and unexpected hospital admission, thereby increasing the total medical costs.[1] More importantly PONV has been a major cause of decreased patient satisfaction.[2] Patients and health care professionals report the avoidance of PONV is of equal or sometimes even greater concern than avoidance of postoperative pain.[3,4] Although the overall incidence of PONV in modern anesthetic practice has been reported as 30%, in high risk group it is reported to be as high as 70%.[5,6] The vomiting reflex and site of action of various antiemetics are shown in [Figure 1].
Figure 1.
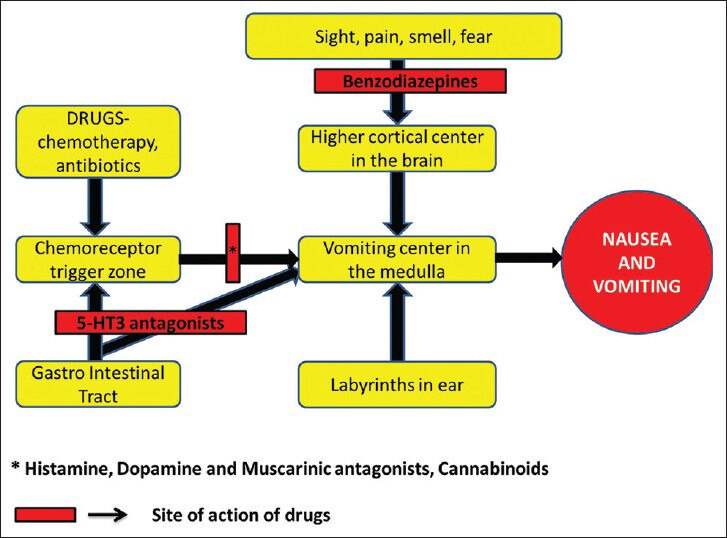
Vomiting reflex and site of action of various drugs
Different pharmacological and non-pharmacological methods are practiced to prevent or to treat nausea and vomiting.[7]
The classification of antiemetic drugs is described in [Table 1].
Table 1.
Classification of antiemetics
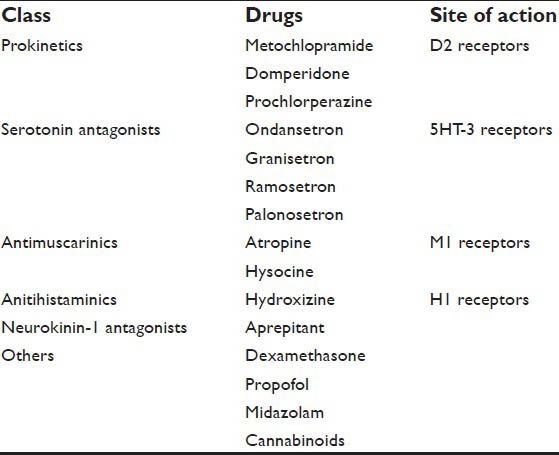
Currently, the drugs range from droperidol, metoclopramide, glucocorticoids to 5-HT3 antagonists.[8] These drugs are not free from side effects like, drowsiness (dose-dependent), dysphoria, restlessness, insomnia, extrapyramidal symptoms, and life threatening arrhythmias.[9] Considering these side effects, much safer drugs have been introduced. Single or multiple drug combinations have been used to prevent or to treat PONV. The combination of different class of drugs with lower concentration are found to be very effective.[10] 5 Hydroxytryptamine receptor antagonists (5 HT3RAs) have been widely and effectively used against PONV because of their efficacy and a favorable side-effect profile. Palonosetron is the only drug of its class approved for prophylaxis against both acute and delayed chemotherapy-induced nausea and vomiting (CINV). Following successful Phase III clinical trials, the FDA approved its use for prevention of PONV in March 2008.[11] Palanosetron is a new, potent second generation5-HT3 antagonist drug with improved profile which acts for longer duration.[12] A recent study where palonosetron was compared with ondansetron in high-risk patients receiving fentanyl-based PCA after thyroidectomy and other laparoscopic surgeries revealed palonosetron is much superior to ondansetron especially 2-24 h after surgery.[13,14,15] PONV is a major concern to patient, anesthesiologist and to the surgeon. A new safe, long acting and cost effective drug should be utilized in clinical practice. This review is aimed to discuss the distinct structural and pharmacological properties of Polonosetrone and its role in the prevention of PONV. The literature search for this article was done using Google scholar and Pubmed using the terms “Palonosetron,” “longer duration of action,” “nausea, vomiting” and “postoperative”.
CHEMICAL FORMULATION
Chemical formula of Palonosetron is (3aS)-2-[(S)-1-Azabicyclo [2.2.2]oct-3-yl]-2,3,3a, 4,5,6-hexahydro- 1-oxo-1 Hbenz[de] isoquinoline hydrochloride. The empirical formula is C19 H24 N2O.HCl. Its molecular weight is 332.87.[16] The structure of palonosetron is structurally different from other 5-HT3 antagonists by a fused tricyclic ring system conjugated to a quinuclidine moiety as shown in [Figure 2].
Figure 2.
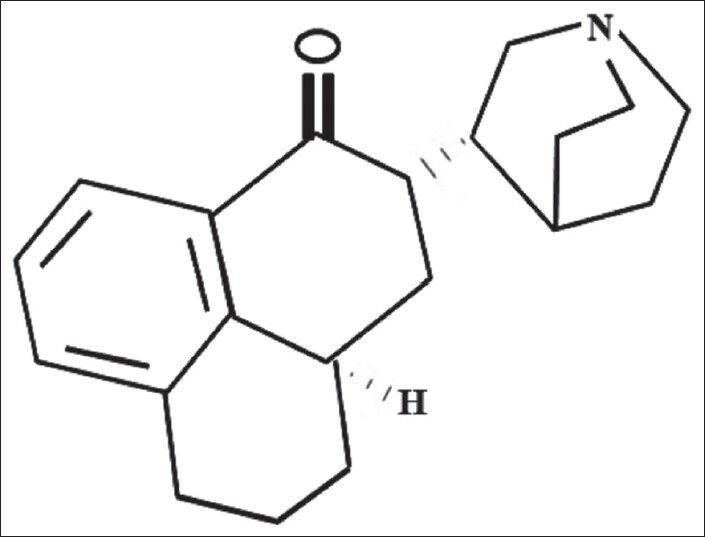
Structure of Palonosetron
PHARMACODYNAMICS
Palonosetron is the newest 5-HT3 (serotonin) receptor antagonist. Unlike other drugs in this class, it exhibits simple bimolecular binding and positive co-operatively in binding to its receptors. The molecular structure does not mimic that of serotonin and it therefore does not bind at the serotonin binding site of the 5-HT3 receptor.[17] Palonosetron has a three-member ring moiety bound to a quinuclidine ring and does not resemble serotonin. Hence, it does bind more tightly to the receptor and allow multiple palonosetron molecules to bind to a single receptor, and make it less likely to be displaced by serotonin molecules.[18] Prolonged duration of action of the drug is mainly due to palonosetron-triggered receptor internalization. This was confirmed in a study by visualizing by confocal fluorescence microscopy using cells transfected with 5-HT3 receptor fused to enhanced cyan fluorescent protein. When granisetron and ondansetron were studied, these drugs showed minimal to no effect on receptor internalization or prolonged inhibition of receptor function.[19]
PHARMACOKINETICS
Absorption
Following oral administration, it is well absorbed with its absolute bioavailability reaching 97%.
Distribution
The volume of distribution is approximately 8.3 ± 2.5 L/kg. Approximately 62% of the drug is bound to plasma proteins.
Metabolism and Elimination
Palonosetron is eliminated by different routes and approximately half of it is metabolized to two primary metabolites: N-oxide-palonosetron and 6-Shydroxy- palonosetron. These have less than 1% of the 5-HT3 receptor antagonist activity of palonosetron. In vitro metabolism studies have suggested that CYP2D6 and to a lesser extent, CYP3A4 and CYP1A2 are involved in the metabolism of palonosetron.
Dosage and formulation
Palonosetron injection is available as a solution as 5 ml vial containing 0.25 mg (0.05 mg/ml). In the US, FDA has already approved for oral formulation of palonosetron for CINV.[20] An effective dose of palonosetron 0.50 mg oral has been favored for the prevention of CINV in patients receiving moderately emetogenic chemotherapy without a side effect disadvantage. Probably, this single dose in high risk patient before anesthesia may prevent PONV.[21]
Special population
There is no drug dosage adjustment required for renal, hepatic function impairment and also for elderly age group.
Pregnancy and lactation
No well controlled human study has been done. Eventhough the animal studies are favorable to use it, such study will not predict human response. It is designated category B agent and should be used cautiously whenever required.[22] There is no currently available data for the safe use in lactating women.[11]
Drug interaction
Most of the drugs are having effect on cytochrome P450 pathway. This drug does have minimal effect on this pathway. There is no report on induction or inhibition of this enzyme. There is a report of profound hypotension and altered consciousness due to the use of apomorphine.[23]
Indications
This is indicated for acute onset, moderate degree of nausea and vomiting which is induced by chemotherapy. Recently, it is used by anesthesia care providers to prevent postoperative nausea vomiting. Major disadvantages of PONV are delayed recovery leading to prolonged hospital stay, wound dehiscence, pulmonary aspiration, bleeding, dehydration, and electrolyte imbalance that can occur if vomiting is prolonged.[24] PONV is not only a burden to the patient but also for the care givers. FDA has approved the use of a single Inj Palonosetron 0.075 mg for post-op nausea and vomiting for preventing it up to 24 h after surgery.[25] Efficacy beyond 24 h has not been demonstrated. The dose required for PONV is also lesser than the dose used in chemotherapy induced nausea and vomiting.[13] This drug can be used in day care surgery. PONV is one of the worrisome factor in day care surgery. Studies have proven this drug is a better choice than ondansetron in such scenarios due to prolonged duration of action with good safety profile. Successfully, the drug has been used in day care surgeries and hence preventing post discharge nausea and vomiting (PDNV).[26]
Contraindications
It is contraindicated in patients with a known hypersensitivity reaction, patients with prolonged corrected QT interval (QTc) in electrocardiogram, high-dose anthracycline therapy, hypokalemia, and hypomagnesemia. Some studies showed palonosetron does not prolong the QTc in electrocardiogram,[27] but it should be used with caution.
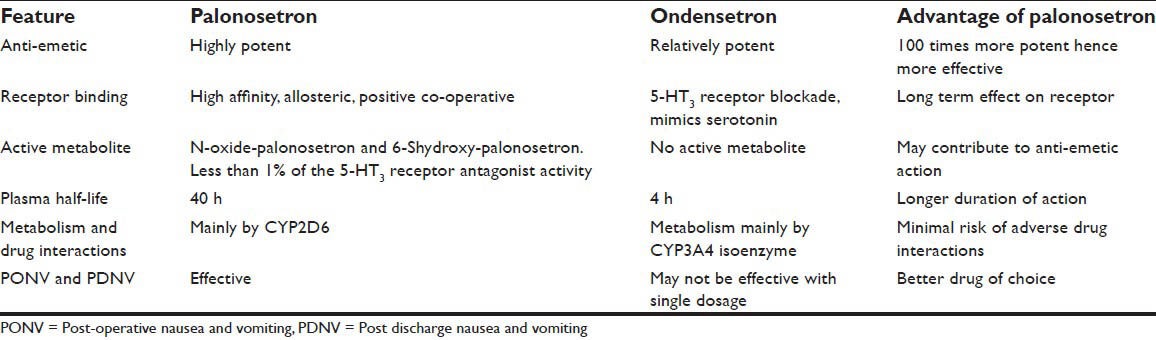
Comparison of palonosetron with the most commonly used antiemetic ondansetron is described in Table 2.
Table 2.
Comparison of palonosetron and ondensetron
Safety and tolerability
5-HT3 blockers are commonly associated with side effects like dizziness, headache, somnolence, drowsiness and cardiac rhythm disturbances.[13] These adverse effects may be dose limiting. The recent study showed palonosetron was well tolerated without severe adverse events.[28] When drug was administered to healthy volunteers of both sex, i.e. a single dose intravenous palonosetron even up to nine times the approved intravenous dose, no dose-related electrocardiograph effects or any evidence of QTc interval prolongation were observed.[29] Studies have shown this drug was safely and successfully used for the prevention of CINV.[30,31]
CONCLUSION
Palonosetron, a newer second generation 5-HT3 blocker is already popular in treating acute onset, moderate nausea and vomiting in patients receiving chemotherapy. As droperidol and ondansetron have entered the black box of FDA due to safety issue, palonosetron is going to be popular in routine anesthesia practice for PONV. Since it is a potent anti-emetic, cost effective and no or minimal side effects make this drug superior. Due to its prolonged duration of action the drug can also be considered to prevent PDNV in day care surgeries. It is difficult to answer whether palonosetron replaces all other available anti-emetics, as there is limited number of published studies in anesthetized patient. The drug interactions, safety profile in anesthesia practice, side effect profile and pharmacoeconomic impact need to be confirmed in further studies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chatterjee S, Rudra A, Sengupta S. Current concepts in the management of postoperative nausea and vomiting. Anesthesiol Res Pract 2011. 2011:748031. doi: 10.1155/2011/748031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anesthesia and surgery: Results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84:6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 3.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? Anesth Analg. 1999;89:652–8. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Lee A, Gin T, Lau AS, Ng FF. A comparison of patients’ and health care professionals’ preferences for symptoms during immediate postoperative recovery and the management of postoperative nausea and vomiting. Anesth Analg. 2005;100:87–93. doi: 10.1213/01.ANE.0000140782.04973.D9. [DOI] [PubMed] [Google Scholar]
- 5.Gan TJ. Postoperative nausea and vomiting: Can it be eliminated? JAMA. 2002;287:1233–6. doi: 10.1001/jama.287.10.1233. [DOI] [PubMed] [Google Scholar]
- 6.Habib AS, Chen YT, Taguchi A, Hu XH, Gan TJ. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: A retrospective database analysis. Curr Med Res Opin. 2006;22:1093–9. doi: 10.1185/030079906X104830. [DOI] [PubMed] [Google Scholar]
- 7.Rowbotham DJ. Recent advances in the non-pharmacological management of postoperative nausea and vomiting. Br J Anaesth. 2005;95:77–81. doi: 10.1093/bja/aei125. [DOI] [PubMed] [Google Scholar]
- 8.Ku CM, Ong BC. Postoperative Nausea and Vomiting: A Review of Current Literature. Singapore Med J. 2003;44:366–74. [PubMed] [Google Scholar]
- 9.Cozanitis DA, Rosenberg PH. Intense inner agitation: An overlooked side effect of droperidol. Acta Anaesthesiol Scand. 2012;56:261–2. doi: 10.1111/j.1399-6576.2011.02582.x. [DOI] [PubMed] [Google Scholar]
- 10.Bugedo G, Gonzalez J, Asenjo C, De la Cuadra JC, Gajardo A, Castillo L, et al. Ondansetron and droperidol in the prevention of postoperative nausea and vomiting. Br J Anaesth. 1999;83:813–4. doi: 10.1093/bja/83.5.813. [DOI] [PubMed] [Google Scholar]
- 11.Muchatuta NA, Paech MJ. Management of postoperative nausea and vomiting: Focus on palonosetron. Ther Clin Risk Manag. 2009;5:21–34. [PMC free article] [PubMed] [Google Scholar]
- 12.Aapro MS. Palonosetron as an antiemetic and anti-nausea agent in oncology. Ther Clin Risk Manag. 2007;3:1009–20. [PMC free article] [PubMed] [Google Scholar]
- 13.Moon YE, Joo J, Kim JE, Lee Y. Anti-emetic Effect of ondansetron and palonosetron in thyroidectomy: A prospective, randomized, double-blinded study. Br J Anaesth. 2012;108:417–22. doi: 10.1093/bja/aer423. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Hong JY, Kim WO, Kil HK, Karm MH, Hwang JH. Palonosetron has superior prophylactic antiemetic efficacy compared with ondansetron or ramosetron in high-risk patients undergoing laparoscopic surgery: A prospective, randomized, double-blinded study. Korean J Anesthesiol. 2013;64:517–23. doi: 10.4097/kjae.2013.64.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharjee DP, Dawn S, Nayak S, Roy PR, Acharya A, Dey R. A comparative study between palonosetron and granisetron to prevent postoperative nausea and vomiting after laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol. 2010;26:480–3. [PMC free article] [PubMed] [Google Scholar]
- 16.Navari RM. Palonosetron for the prevention of chemotherapy-induced nausea and vomiting: Approval and efficacy. Cancer Manag Res. 2009;1:167–76. doi: 10.2147/cmr.s6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008;107:469–78. doi: 10.1213/ane.0b013e318172fa74. [DOI] [PubMed] [Google Scholar]
- 18.Kloth DD. New pharmacologic findings for the treatment of PONV and PDNV. Am J Health Syst Pharm. 2009;66:11–8. doi: 10.2146/ashp080462. [DOI] [PubMed] [Google Scholar]
- 19.Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, et al. Palonosetron triggers 5-HT (3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010;626:193–9. doi: 10.1016/j.ejphar.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Yang LP, Scott LJ. Palonosetron: In the prevention of nausea and vomiting. Drugs. 2009;69:2257–78. doi: 10.2165/11200980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Boccia R, Grunberg S, Franco-Gonzales E, Rubenstein E, Voisin D. Efficacy of oral palonosetron compared to intravenous palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: A phase 3 trial. Support Care Cancer. 2013;21:1453–60. doi: 10.1007/s00520-012-1691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Leon A. Palonosetron (Aloxi): A second-generation 5-HT3receptor antagonist for chemotherapy-induced nausea and vomiting. Proc (Bayl Univ Med Cent) 2006;19:413–6. doi: 10.1080/08998280.2006.11928210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenwood Village, CO: Thomson Healthcare; 2006. Micromedex Drug Information Database. [Google Scholar]
- 24.Habib AS, Gan TJ. Pharmacotherapy of postoperative nausea and vomiting. Expert Opin Pharmacother. 2003;4:457–3. doi: 10.1517/14656566.4.4.457. [DOI] [PubMed] [Google Scholar]
- 25.Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008;107:439–44. doi: 10.1213/ane.0b013e31817abcd3. [DOI] [PubMed] [Google Scholar]
- 26.Bajwa SS, Bajwa SK, Kaur J, Sharma V, Singh A, Singh A, et al. Palonosetron: A novel approach to control postoperative nausea and vomiting in day care surgery. Saudi J Anaesth. 2011;5:19–24. doi: 10.4103/1658-354X.76484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apfel CC, Jukar-Rao S. Is palonosetron also effective for opioid-induced and post-discharge nausea and vomiting? Br J Anaesth. 2012;108:371–3. doi: 10.1093/bja/aer516. [DOI] [PubMed] [Google Scholar]
- 28.Di Renzo N, Montanini A, Mannina D, Dondi A, Muci S, Mancuso S, et al. Single-dose palonosetron for prevention of chemotherapy-induced nausea and vomiting in patients with aggressive non-Hodgkin's lymphoma receiving moderately emetogenic chemotherapy containing steroids: Results of a phase II study from the GruppoItaliano per lo Studio dei Linfomi (GISL) Support Care Cancer. 2011;19:1505–10. doi: 10.1007/s00520-010-0974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morganroth J, Parisi S, Spinelli T, Moresino C, Thorn M, Cullen MT. High-dose palonosetron does not alter ECG parameters including QTc interval in healthy subjects: Results of a dose-response, double-blind, randomized, parallel E14 study of palonosetron vsmoxifloxacin or placebo. Presentation at the 14thEuropean Conference of Clinical Oncology (ECCO) ECCO. 2007:23–7. [Google Scholar]
- 30.Nadaraja S, Mamoudou AD, Thomassen H, Wehner PS, Rosthoej S, Schroeder H. Palonosetron for the prevention of nausea and vomiting in children with acute lymphoblastic leukemia treated with high dose methotrexate. Pediatr Blood Cancer. 2012;59:870–3. doi: 10.1002/pbc.24068. [DOI] [PubMed] [Google Scholar]
- 31.Sepulveda-Vildosola AC, Betanzos-Cabrera Y, Lastiri GG, Rivera-Márquez H, Villasis-Keever MA, Del Angel VW, et al. Palonosetron hydrochloride is an effective and safe option to prevent chemotherapy-induced nausea and vomiting in children. Arch Med Res. 2008;39:601–6. doi: 10.1016/j.arcmed.2008.04.007. [DOI] [PubMed] [Google Scholar]


