Abstract
Objectives:
The objective of the study is to compare the effect of dexmedetomidine versus lignocaine in attenuation of circulatory and airway responses during endotracheal extubation in craniotomies for intracerebral space occupying lesions (ICSOL).
Materials and Methods:
A total of 50 patients of American Society of Anesthesiologists Grade I and II of either sex, aged 18-50 years undergoing craniotomies for non-vascular ICSOL under general anesthesia were divided into two groups according to drug received. Group D (n = 25) received dexmedetomidine (0.5 mcg/kg) whereas group L (n = 25) received lignocaine (1.5 mg/kg). Both the drugs were given 5 min before the extubation over a period of 60 s. Values for heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), were recorded just before (A0) and 1, 3, 5 (A1, A3, A5) min after the study drug administration, at extubation (E) and 1, 3, 5, 10, 15 min after extubation (E1, E3, E5, E10 and E15). Respiratory rate, oxygen saturation and airway responses like coughing, breath-holding, laryngospasm/bronchospasm were recorded only at extubation (E) and 1, 3, 5, 10, 15 min after extubation (E1, E3, E5, E10, E15). Quality of extubation was recorded with four point scale. After extubation all these patients were also observed for sedation by Ramsey sedation score.
Results:
Both groups showed a statistically significant increase (D < L) in HR, SBP and DBP during (E) and immediately after extubation (E1) (P < 0.05). Dexmedetomidine (72%) produced a higher degree of sedation (Grade 3) as compare with lignocaine (0%) and with no incidence of coughing or breath holding (P < 0.05).
Conclusion:
Single dose of dexmedetomidine (0.5 mcg/kg) given 5 min before extubation produced significant attenuation of circulatory and airway responses produced during extubation as compared to Lignocaine (1.5 mg/kg) in ICSOL.
Keywords: Airway, circulatory reflexes, dexmedetomidine, extubation, lignocaine
INTRODUCTION
Endotracheal intubation is an integral part of the modern anesthesia techniques for major surgical procedures. Both intubation and extubation are associated with various cardiovascular and airway responses leading to tachycardia, hypertension, arrhythmias, myocardial ischemia, coughing, agitation, bronchospasm, increased bleeding, raised intracranial and intraocular pressure.[1] These transitory changes are of little consequences in American Society of Anesthesiologists (ASA) Grade I and II patients going for general surgical procedures, but could be of major concern for the anesthesiologist in patients especially with intracerebral space occupying lesions (ICSOL), where a sudden hypertension during or in immediate post-extubation phase could lead to raised cerebral blood flow (CBF), intracranial pressure (ICP) and decreased cerebral perfusion pressure (CPP) resulting into increased intracranial bleeding, high morbidity and mortality.[2,3] Up to 76-96% incidence of post-extubation bucking and coughing has been reported in the literature.[1,4,5] Much attention has been paid to attenuate these changes during intubation when compared with extubation. Intratracheal local anesthetic instillation,[6] intracuff lidocaine,[7] intravenous lignocaine,[8] short acting opioids such as fentanyl and remifentanil,[9,10] esmolol,[11] labetalol,[12] diltiazem,[13] prostaglandin-E1[14] and verapamil[15] have been used to attenuate these hemodynamic and respiratory responses during extubation in the past but with certain limitations.
Recently dexmedetomidine, a potent α2-adrenoreceptor agonist has been used to facilitate extubation in surgical intensive care unit,[16,17] but its role in the attenuation of hemodynamic and airway reflexes during extubation in general anesthesia is still scarce. A single dose of dexmedetomidine has been found effective in attenuation of the airway and circulatory reflexes during extubation.[18,19,20]
In this study, we compared the effects of intravenous dexmedetomidine and lignocaine on attenuation of circulatory and airway responses to endotracheal extubation after craniotomy for ICSOL under general anesthesia.
MATERIALS AND METHODS
After ethical committee approval and written informed consent, this double blind randomized, prospective clinical study was carried out on 50 patients of ASA Grade I and II of either sex, aged 18-50 years undergoing craniotomies for non-vascular ICSOL under general anesthesia. Patients with significant cardio-respiratory, hepatic, renal, metabolic disorder, chronic hypertension, bradycardia, severe hypovolemia and patient receiving antihypertensive, antiarrhythmic, adrenoceptor agonist or antagonist therapy were excluded from the study. Any patient who required post-operative ventilation was also excluded from the study.
These patients were randomly (lottery method) divided into 2 groups according to study drugs as follows: Group D (n = 25) received dexmedetomidine 0.5 mcg/kg and group L (n = 25) received Lignocaine 1.5 mg/kg 5 min before extubation. All patients were premedicated with intramuscular glycopyrrolate 0.2 mg 30 min before induction of anesthesia. After securing an intravenous access, heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), respiratory rate (RR) and oxygen saturation (SpO2) were recorded with a multiparameter monitor (BeneView T5; Mindray, China). After preoxygenation for 3 min, general anesthesia was induced with thiopental sodium 5 mg/kg and pentazocine 0.5 mg/kg. Tracheal intubation was done using suxamethonium 2 mg/kg and anesthesia was maintained on O2:N2O (66:33), halothane 0.5% and vecuronium (loading dose 0.1 mg/kg and intermittent doses of 0.02 mg/kg) throughout the surgical procedure.
After closure and dressing of the surgical wound, halothane was discontinued and the study drugs were given, in 10 ml saline dilution, by slow intravenous injection over a period of 60 s by a resident doctor who was not involved in the management of the case to avoid bias. Residual muscle paralysis was reversed with neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg). Once patient became conscious and responded to verbal commands extubation was performed. All patients were given O2 by face mask during the recovery period. Values for HR, SBP, DBP, were recorded just before (A 0) and 1, 3, 5 (A1, A3, A5) min after the study drug administration and at extubation (E), 1, 3, 5, 10, 15 min after extubation (E1, E3, E5, E10, E15). RR, SpO2 and airway responses like coughing, breath holding, laryngospasm or bronchospasm were recorded at extubation (E) and 1, 3, 5, 10 and 15 min after extubation (E1, E3, E5, E10, E15).
At the end of extubation quality of extubation was recorded with four point scale (Grade 0: No Coughing; Grade 1: Minimal Coughing [once or twice]; Grade 2: Moderate coughing [3-4 times]; Grade 3: Severe coughing [5 or more times]).
After extubation, all these patients were also observed for sedation by Modified Ramsey sedation[21] score as: Grade 1: Anxious and agitated or restless or both; Grade 2: Co-operative, oriented and calm; Grade 3: Responsive to command only; Grade 4: Exhibiting brisk response to light/tap/auditory stimulus; Grade 5: Exhibiting sluggish response to light/tap/auditory stimulus; Grade 6: Unresponsive.
Any change in HR and blood pressure (BP) (±20% of pre drug administration value) if occurred were recorded and treated with appropriate drugs. Any other side-effect of study drugs if occurred was also recorded.
The recorded observations in both the study groups were subjected to statistical analysis using Student's t-test (Joe Gilman and Mark Myatt 1998, Brixton Books 2000 version l.02). Statistical significance was accepted as not significant and significant at P > 0.05 and P < 0.05 respectively. Statistical significance of sedation and extubation scores were obtained by applying Mann-Whitney U-test.
RESULTS
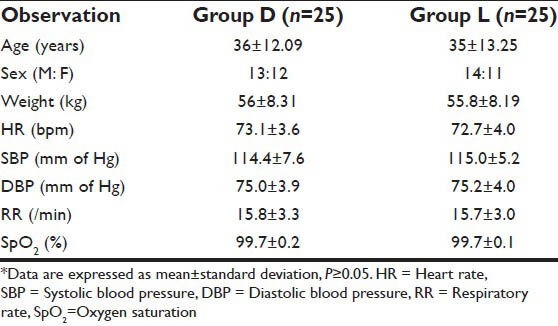
No statistical differences were found between the two study groups with respect to age, sex, weight, pre-induction HR, SBP, DBP, RR and SpO2 [Table 1].
Table 1.
Demographic data*
Circulatory parameters
In group D, a statistically significant (P < 0.05) decrease in HR, SBP and DBP was observed up to A5 stage whereas in group L, at this stage these changes were statistically insignificant (P > 0.05) [Table 2].
Table 2.
Comparison of circulatory and respiratory changes at various time intervals in two study groups
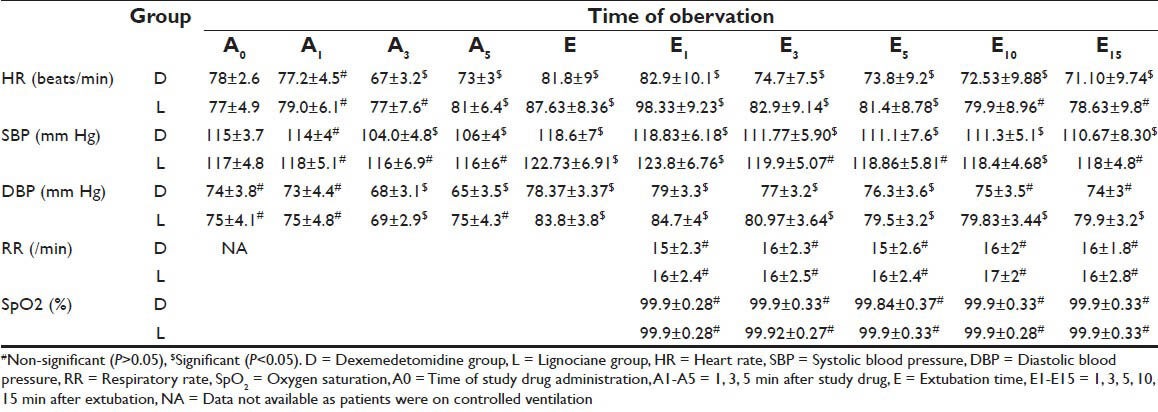
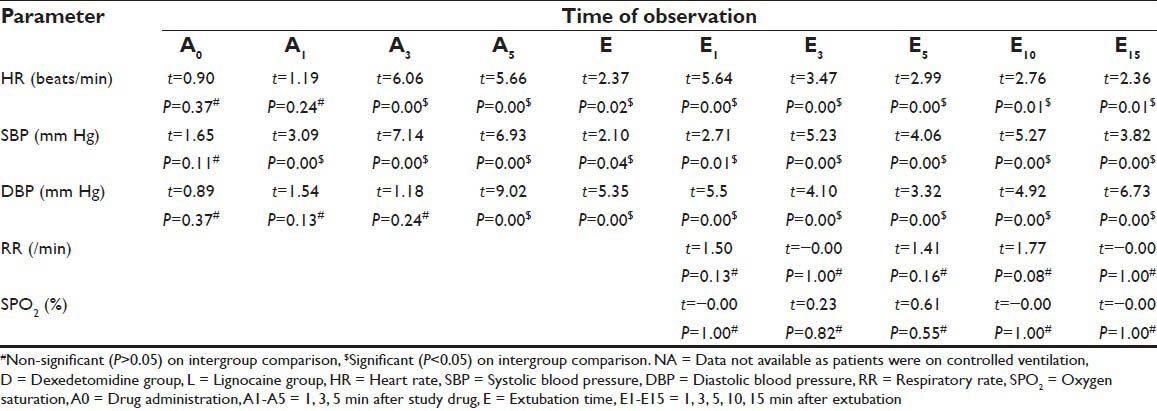
During (E) and immediately after extubation (E1) stages of study, patients in both the study groups showed a statistically significant increase (D < L) from A0 values in HR, SBP and DBP (P < 0.05) [Figure 1]. Thereafter in group D these values continued to decrease significantly (P < 0.05) and remained below the pre drug administration value (A0) at the end of the study (E15). Whereas in group L, these values although decreased from E and E1 values but remained above the A0 values at the end of study period (A15) (P > 0.05). In intergroup comparison, these changes were statistically significant (P < 0.05) from A5 stage until end (A15) of the study period [Table 3]. None of the patient required any medical intervention for fluctuation in circulatory parameters.
Figure 1.
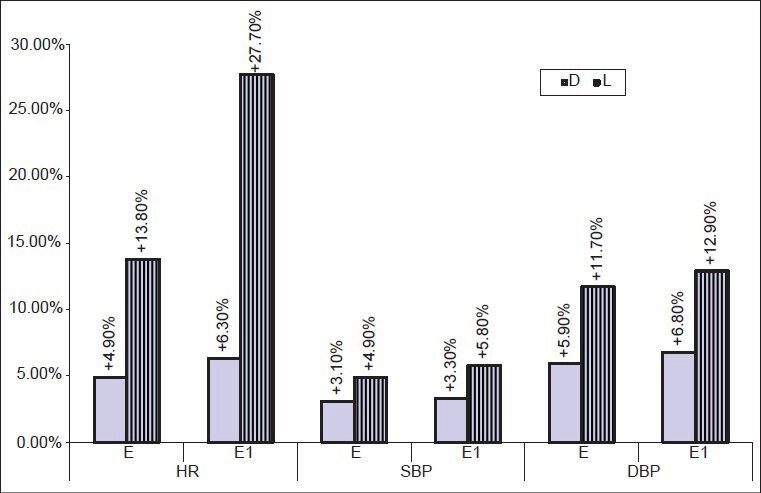
Percent change in HR, SBP, DBP (mean ± standard deviation) at extubation (E) and 1 min after extubation (E1) from the basal value (D0). (HR-heart rate; SBP-systolic blood pressure; DBP-diastolic blood pressure E-at extubation; E1-1 min after extubation; D-Dexmedetomidine group; L-lignocaine group)
Table 3.
Inter group comparison (D versus L) of circulatory and respiratory changes at various time intervals between group D and group L
Respiratory parameters
No statistically significant difference (P > 0.05) was found in RR and SpO2 after extubation (E) until end of the study in both groups.
Airway responses
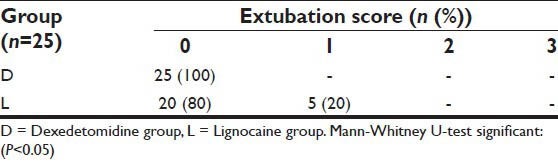
In group L, only 5 patients (20%) had Grade 1 cough during extubation as against none in group D. No patients had breath-holding/laryngospasm/bronchospasm during or after extubation in both the study groups. The difference between two groups was significant by applying Mann-Whitney U-test for extubation score (P < 0.05). The extubation scores are illustrated in Table 4.
Table 4.
Extubation score in two study groups
Sedation
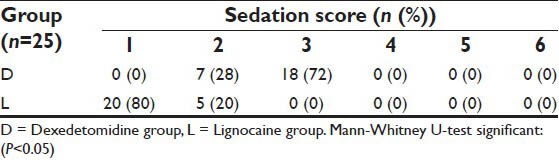
In group D, a higher degree of sedation (Grade 3 and 2) was obtained in 72% (n = 18) and 28% (n = 7) patients respectively after extubation. In contrast, only 20% (n = 5) patients had mild sedation (Grade 2) whereas 80% (n = 20) patients were anxious and agitated or restless or both (Grade 1) in group L [Table 5]. The difference between two groups was significant by applying Mann-Whitney U-test for extubation score (P < 0.05).
Table 5.
Sedation score after extubation in two study groups
DISCUSSION
The effect of the changes in circulatory and airway response during intubation has been discussed at length as compared to extubation in the literature. Many theories have been put forward to sudden increase in PR and BP during extubation such as a rise in catecholamine,[22] airway irritation due to suction, intense pain from surgical wounds and emergence.[13] This could be disastrous in patients operated for cardiac, neuro or ophthalmic lesions. Sudden increase in CBF due to disturbed auto-regulation can lead to increased ICP and decreased CPP may result in herniation of brain contents or cerebral ischemia.[2] Leech et al.[3] observed a rise of up to 55 mm of Hg in ICP during the removal of the endotracheal tube. We designed this study with the aim to compare the attenuating effects of single dose dexmedetomidine and lignocaine on hemodynamic and the airway responses during extubation in patients undergoing craniotomy for ICSOL. In this study, we could confirm the earlier finding that the dexmedetomidine causes reduction in both HR and BP.[23,24]
Patients of both groups showed a significant rise in HR and BP during (E) and after extubation (E1) possibly because of light planes of anesthesia due of discontinuation of halothane and N2O just before extubation. Muzzi et al.[12] also observed increased BP in 91% neurosurgical patients with the discontinuation of volatile agents till extubation. Lesser rise in HR during E and E1 (4.9%, 6.3% vs. 13.8%, 27.7%) and SBP (3.1%, 3.3% vs. 4.9%, 5.8%) in group D when compared to group L (P < 0.05) could be due to dexmedetomidine induced sedation, analgesia and decreased catecholamine levels, inhibition of central sympathetic outflow, stimulation of the presynaptic α-2 receptors.[18,25,26] The dose-dependent reduction of HR with dexmedetomidine is primarily mediated by the decrease in sympathetic tone, partly by baroreceptor reflex and enhanced vagal activity.[17,27,28] Higher degree of sedation obtained in this study is due to its action on α2 adrenoreceptors, reduced sympathetic activity and the level of arousal. Absence of airway responses such as cough, breath holding and desaturation during and after extubation observed with dexmedetomidine in this study are in accordance with other authors.[18]
Other researchers also confirmed that a single dose of dexmedetomidine (0.5 mcg/kg) facilitated extubation with stable hemodynamics and easy recovery in patients after intracranial and intraocular interventions.[18,19,20] Recently many more researchers have found dexmedetomidine very effective in attenuation of hemodynamic responses in varying doses[29,30]
CONCLUSION
Dexmedetomidine in a single dose of 0.5 mcg/kg given 5 min before extubation provided significant attenuation of circulatory and airway responses during extubation as compared to lignocaine in craniotomies for ICSOL.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg. 2004;99:1253–7. doi: 10.1213/01.ANE.0000132779.27085.52. [DOI] [PubMed] [Google Scholar]
- 2.Hartley M, Vaughan RS. Problems associated with tracheal extubation. Br J Anaesth. 1993;71:561–8. doi: 10.1093/bja/71.4.561. [DOI] [PubMed] [Google Scholar]
- 3.Leech P, Barker J, Fitch W. Proceedings: Changes in intracranial pressure and systemic arterial pressure during the termination of anaesthesia. Br J Anaesth. 1974;46:315–6. doi: 10.1093/bja/46.4.315-a. [DOI] [PubMed] [Google Scholar]
- 4.Kim ES, Bishop MJ. Cough during emergence from isoflurane anesthesia. Anesth Analg. 1998;87:1170–4. doi: 10.1097/00000539-199811000-00036. [DOI] [PubMed] [Google Scholar]
- 5.Estebe JP, Dollo G, Le Corre P, Le Naoures A, Chevanne F, Le Verge R, et al. Alkalinization of intracuff lidocaine improves endotracheal tube-induced emergence phenomena. Anesth Analg. 2002;94:227–30. doi: 10.1097/00000539-200201000-00044. [DOI] [PubMed] [Google Scholar]
- 6.Jee D, Park SY. Lidocaine sprayed down the endotracheal tube attenuates the airway-circulatory reflexes by local anesthesia during emergence and extubation. Anesth Analg. 2003;96:293–7. doi: 10.1097/00000539-200301000-00058. [DOI] [PubMed] [Google Scholar]
- 7.Fagan C, Frizelle HP, Laffey J, Hannon V, Carey M. The effects of intracuff lidocaine on endotracheal-tube-induced emergence phenomena after general anesthesia. Anesth Analg. 2000;91:201–5. doi: 10.1097/00000539-200007000-00038. [DOI] [PubMed] [Google Scholar]
- 8.Gefke K, Andersen LW, Friesel E. Lidocaine given intravenously as a suppressant of cough and laryngospasm in connection with extubation after tonsillectomy. Acta Anaesthesiol Scand. 1983;27:111–2. doi: 10.1111/j.1399-6576.1983.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 9.Nishina K, Mikawa K, Maekawa N, Obara H. Fentanyl attenuates cardiovascular responses to tracheal extubation. Acta Anaesthesiol Scand. 1995;39:85–9. doi: 10.1111/j.1399-6576.1995.tb05597.x. [DOI] [PubMed] [Google Scholar]
- 10.Aouad MT, Al-Alami AA, Nasr VG, Souki FG, Zbeidy RA, Siddik-Sayyid SM. The effect of low-dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Anesth Analg. 2009;108:1157–60. doi: 10.1213/ane.0b013e31819b03d8. [DOI] [PubMed] [Google Scholar]
- 11.Lim SH, Chin NM, Tai HY, Wong M, Lin TK. Prophylactic esmolol infusion for the control of cardiovascular responses to extubation after intracranial surgery. Ann Acad Med Singapore. 2000;29:447–51. [PubMed] [Google Scholar]
- 12.Muzzi DA, Black S, Losasso TJ, Cucchiara RF. Labetalol and esmolol in the control of hypertension after intracranial surgery. Anesth Analg. 1990;70:68–71. [PubMed] [Google Scholar]
- 13.Nishina K, Mikawa K, Maekawa N, Obara H. Attenuation of cardiovascular responses to tracheal extubation with diltiazem. Anesth Analg. 1995;80:1217–22. doi: 10.1097/00000539-199506000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Nishina K, Mikawa K, Shiga M, Maekawa N, Obara H. Prostaglandin E1 attenuates the hypertensive response to tracheal extubation. Can J Anaesth. 1996;43:678–83. doi: 10.1007/BF03017950. [DOI] [PubMed] [Google Scholar]
- 15.Mikawa K, Nishina K, Maekawa N, Obara H. Attenuation of cardiovascular responses to tracheal extubation: Verapamil versus diltiazem. Anesth Analg. 1996;82:1205–10. doi: 10.1097/00000539-199606000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Siobal MS, Kallet RH, Kivett VA, Tang JF. Use of dexmedetomidine to facilitate extubation in surgical intensive-care-unit patients who failed previous weaning attempts following prolonged mechanical ventilation: A pilot study. Respir Care. 2006;51:492–6. [PubMed] [Google Scholar]
- 17.Arpino PA, Kalafatas K, Thompson BT. Feasibility of dexmedetomidine in facilitating extubation in the intensive care unit. J Clin Pharm Ther. 2008;33:25–30. doi: 10.1111/j.1365-2710.2008.00883.x. [DOI] [PubMed] [Google Scholar]
- 18.Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005;49:1088–91. doi: 10.1111/j.1399-6576.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 19.Turan C, Turan G, Ozgulltekin A, Dýncer E, Yuksel G. The effects of two different doses of dexmedetomidine on extubation: 9AP 3-5. Eur J Anaesthesiol. 2007;24:114. [Google Scholar]
- 20.Turan G, Ozgultekin A, Turan C, Dincer E, Yuksel G. Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816–20. doi: 10.1017/S0265021508004201. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowrie A, Johnston PL, Fell D, Robinson SL. Cardiovascular and plasma catecholamine responses at tracheal extubation. Br J Anaesth. 1992;68:261–3. doi: 10.1093/bja/68.3.261. [DOI] [PubMed] [Google Scholar]
- 23.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 24.Tanskanen PE, Kyttä JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97:658–65. doi: 10.1093/bja/ael220. [DOI] [PubMed] [Google Scholar]
- 25.Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–9. doi: 10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence CJ, De Lange S. Effects of a single pre-operative dexmedetomidine dose on isoflurane requirements and peri-operative haemodynamic stability. Anaesthesia. 1997;52:736–44. doi: 10.1111/j.1365-2044.1997.169-az0303.x. [DOI] [PubMed] [Google Scholar]
- 27.Afonso J, Reis F. Dexmedetomidine: Current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62:118–33. doi: 10.1016/S0034-7094(12)70110-1. [DOI] [PubMed] [Google Scholar]
- 28.Penttilä J, Helminen A, Anttila M, Hinkka S, Scheinin H. Cardiovascular and parasympathetic effects of dexmedetomidine in healthy subjects. Can J Physiol Pharmacol. 2004;82:359–62. doi: 10.1139/y04-028. [DOI] [PubMed] [Google Scholar]
- 29.Jain D, Khan R, Maroof M. Effect of dexmedetomidine on stress response to extubation. Internet J Anesthesiol. 2009;21 2009:21:1. [Google Scholar]
- 30.Aksu R, Akın A, Biçer C, Esmaoglu A, Tosun Z, Boyaci A. Comparison of the effects of dexmedetomidine versus fentanyl on airway reflexes and hemodynamic responses to tracheal extubation during rhinoplasty: A double-blind, randomized, controlled study. Curr Ther Res. 2009;70:209–20. doi: 10.1016/j.curtheres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


