Abstract
Background:
This study was performed to test the effectiveness of topical tranexamic acid (TXA) in reducing blood loss in pelvic hemiarthoplasty surgeries compared with intravenous TXA, regarding the incidence of thromboembolic complications (deep vein thrombosis [DVT], pulmonary embolism (PE) and cerebrovascular stroke [CVS]).
Patients and Methods:
After obtaining institutional ethical approval 60 patients divided into three groups. Group A: Received intravenous TXA Group B: Received topical TXA Group C: Control group (placebo saline). All patients were received general anesthesia and post-operative bleeding, immediate and 24 h post-operatively, hemoglobin concentration, hematocrit, platelets and coagulation profile (prothrombin time, activated partial thromboplastin time and international normalized ratio) baseline, immediate and 24 h post-operatively. Thromboelastography was recorded baseline, immediate and 24 h post-operatively. Incidence of DVT, PE and CVS was recorded.
Results:
There was statistical significant elevation hemoglobin concentration and hematocrit in both Groups A and B, significant increase in blood loss in Group C, significant increase in number of patients receiving blood in Group C, there was a significant decrease in “r” and “k” times and a significant increase in maximum amplitude and α-angle in Group A, statistically significant increase in the incidence of thromboembolic events in the form of DVT, PE and CVS in Group A.
Conclusion:
Topical TXA is effective in decreasing post-operative blood loss with possible side-effects of this route of administration.
Keywords: Hip arthroplasty, intravenous, topical, tranexamic acid
INTRODUCTION
Orthopedic surgery may be associated with substantial blood loss requiring transfusion of erythrocytes. Surgery for hip fractures frequently requires blood transfusion, despite recent advances in techniques of orthopedic surgery and mechanical improvements of implants. Blood may be transfused before, during or following surgery.[1]
Surgery enhances coagulability and local fibrinolytic activity. Increases the level of coagulation factors and reduction of coagulation inhibitors together with enhanced platelets activity with the release of catecholamines contribute to hemostatis. The fibrionolytic system is activated by the surgical trauma causing the release of tissue plasminogen activators (t-PA). Furthermore, the release of t-PA triggered by thrombin activates fibrinolytic system. t-PA is the major enzyme in controlling the conversion of plasminogen to plasmin.[2]
The use of allogenic blood products increases the rate of transmission of infectious diseases, modulates the immune response and increases the rate of post-operative infection. Numerous methods of controlling bleeding such as thromboplastic agents; topical freezing saline; deliberate hypotension and administration of fibrinolytic inhibitors as aprotinin and tranexamic acid (TXA) have been used.[3,4,5]
Meanwhile, the nature of orthopedic illness and diseases, trauma and surgical repair or replacement of hip predisposes patients to the occurrence of venous thromboembolism. These complications are predictable and are the result of alteration of the natural equilibrium mechanisms in various disease states.[6] Untreated general surgical patients have a post-operative risk of deep venous thrombosis (DVT) of 19-25%. In comparison, DVT rate among high-risk orthopedic patients is substantially greater. Untreated patients following total hip replacement have a DVT rate of 50-60%. In these same series, the risk of fatal pulmonary embolism (PE) ranges 0.4-12.9%.[7,8,9]
TXA is a synthetic hydrophilic inhibitor of fibrinolysis. It blocks lysine binding sites of plasminogen by competitive mechanism. TXA complex are displaced from the surface of fibrin and like plasmin is prevented from binding to fibrinogen or fibrin monomers delaying lysis. With higher concentration, TXA acts as a non-competitive inhibitor of plasmin.[2] However, it is contraindicated in patients with history of thromboembolic diseases.[10,11] Topical use of this agent is reported in coronary artery bypass.[12]
Aim of the study
The primary objective of this study was to test the effectiveness of topical TXA in reducing blood loss in pelvic hemiarthroplasty surgeries as compared with intravenous TXA.
The secondary objective was to compare both techniques as regards the incidence of thromboembolic complications (DVT, PE and cerebrovascular stroke [CVS]).
PATIENTS AND METHODS
The present study was carried out in a tertiary referral teaching hospital in a double-blind randomized placebo-controlled prospective study on 60 patients (age ranged from 50 to 60 years) undergoing hemiarthroplasty surgeries for fractured hip joint within 48 h of trauma. After obtaining approval from the ethical committee, informed consent was obtained from all patients. Patients were randomly allocated into one of the three study groups:
Group A: Received Intravenous TXA
Group B: Received topical TXA
Group C: Control group (placebo saline).
Patients in Group A received intravenous TXA 10 mg/kg in 20 ml normal saline as a bolus dose prior to skin incision, then by infusion of 5 mg/kg/h in the form of 500 mg TXA in 250 ml with rate of 80 ml/h until the end of surgery. Prior to wound closure 100 ml of normal saline was purred into the surgical field and left for 5 min before suction.
Patients in Group B received intravenous 20 ml of normal saline before skin incision the by infusion of normal saline with a rate of 80 ml/h until the end of surgery. Prior to wound closure, 100 ml normal saline with 1.5 grams of TXA was purred into the surgical field and left for 5 min before suction.
Patients in Group C received 20 ml of normal saline before skin incision then by infusion of 80 ml/h of normal saline until the end of surgery. Prior to wound closure, 100 ml of normal saline is purred in the surgical field and left for 5 min before suction.
All patients of the three studied groups received prophylactic anticoagulants in the form of clexane (low molecular weight heparin) 80 international units subcutaneously within 8 h pre-operatively and were continued post-operatively according to the standard practice.
All patients of the study three groups were admitted post-operatively to post-anesthesia care unit where they remained for 24 h under observation.
Exclusion criteria
Allergy to TXA
Acquired disturbances of color vision
Pre-operative anemia (hemoglobin <11 gm% in females and hemoglobin <12 gm% in males)
Preoperative use of anticoagulant therapy i.e., oral anticoagulants, heparin within 5 days of surgery, fibrinolytic disorders requiring intraoperative antifibrinolytic treatment
Coagulopathy i.e., pre-operative platelets count <150,000 mm3, international normalized ratio (INR) >1.4 and prolonged prothrombin time (PT) >1.4 s
A previous history of thromboembolic disease i.e.; DVT, CVS and PE
Significant co-morbidities
Severe ischemic heart disease, New York Heart Association Class III and IV
Previous myocardial infarction
Severe pulmonary disease
Plasma creatinine greater than 115 mmol/L in males and more than 100 μmol/L in females
Hepatic failure
Occurrence Intraoperative surgical/medical/anesthetic complications i.e.; myocardial infarction or neurovascular injury
Patients who need massive blood transfusion
Postoperative bleeding of surgical causes.
Anesthetic technique
Patients enrolled in the study had the same anesthetic technique: General anesthesia using propofol 1-2 mg/kg, fentanyl 1-2 microgram/kg and recuronium 0.1 mg/kg to facilitates endotracheal intubation and there after infusion using maintenance dose until the end of surgery. Sevoflurane 1-2 minimum alveolar concentration was used to maintain the anesthesia with 50% nitrous oxide in oxygen. Mechanical ventilation aiming to keep end tidal carbon dioxide (EtCo2) 30-35 mm Hg. Standard monitoring of Electrocardiography, pulse oximetry for SaO2, none invasive blood pressure, capnography for end tidal carbon dioxide, temperature and urine output. Post-operative pain was controlled using intravenous morphine titrated to the patients response. No non-steroidal analgesic anti-inflammatory was used.
Intraoperative replacement therapy has been planned in this study to be on the negative balance side as over hydration may result in dilutional anemia and we also avoided the use of more than 500 cc. colloid in the replacement therapy as in a dose more than this, colloids will affect the coagulation process.
Outcomes measured
Post-operative bleeding (surgical drains) immediate and 24 h post-operatively
Hemoglobin concentration, hematocrite, platelets and coagulation profile (PT, activated partial thromboplastin time [APTT] and INR) baseline, immediate and 24 h post-operatively
Number of patients requiring blood transfusion in the first 24 h post-operatively
Thromboelastogram (TEG) was recorded baseline, immediate and 24 h post-operatively. TEG 5000 hemostasis analyzer [Haemoscope Inc. USA] was used connected to computer with the TEG analytical software, version 4.1.54. Maintenance and quality control were performed in strict accordance with manufacturers recommendations. Blood samples were collected in 1.8 ml vacutainer tubes [bection Dickinson, USA] with 3.2% sodium citrate [0.109M trisodium citrate]
Incidence of DVT confirmed by symptoms and phlebography by ultrasound. PE confirmed by spiral computed tomography and CVS confirmed by computed tomographic scan or magnetic resonance imaging (MRI).
Statistical analysis
All measurements were expressed as mean ± the standard deviation of the mean. Statistical analyses were performed with SPSS for Windows version 18.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons of results between groups were carried out by the two-sample t-test for each normally distributed variable. For nonparametric readings Fisher Exact test was used to analyze nonparametric data. P < 0.05 was considered to indicate a significant difference.
RESULTS
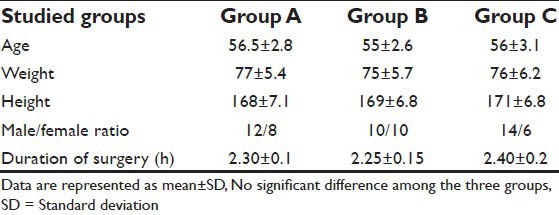
Regarding demographic data (age, weight, height, male/female ratio and surgery duration), there were no significant differences among all studied groups (P > 0.05) [Table 1].
Table 1.
Demographic data and surgery duration for all studied groups
Concerning hematological data, there were no significant differences in the baseline values in all studied groups (P > 0.05) [Table 2].
Table 2.
Hematological data during the 1st 24 h in all studied groups
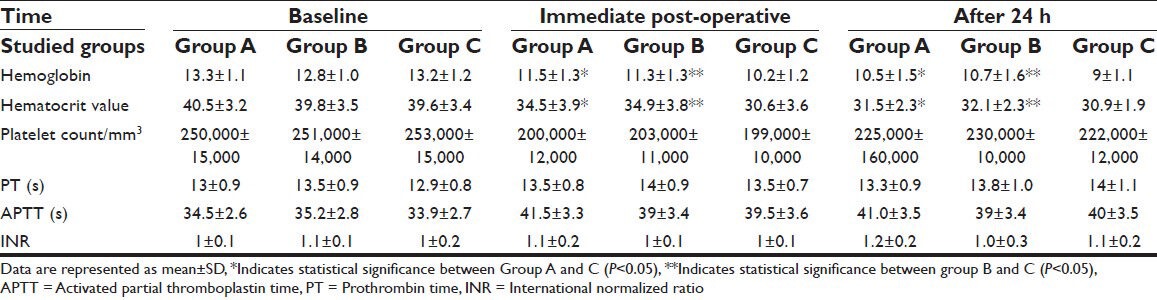
Values of hemoglobin and hematocrit immediate post-operatively and after 24 h showed no significant differences between Groups A and B while there was statistical significant elevation in both Groups A and B compared with Group C (P < 0.05).
Regarding other hematological data (platelet count, PT, APTT and INR) there were no significant differences among the groups immediate and after 24 h post-operatively (P > 0.05) [Table 2].
Considering the mean total blood loss during the first 24 h in all groups there was no significant difference between Groups A and B (P > 0.05) while there was a significant increase in mean blood loss in Group C compared to Groups A and B (P < 0.05) [Table 3].
Table 3.
Blood loss (surgical drains) in the 1st 24 h

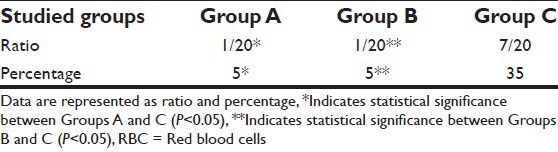
Regarding the primary outcome patients receiving packed red blood cells (RBCs) during the first 24 h post-operatively we found a significant increase in number of patients receiving packed RBCs in Group C compared to Groups A and B (P < 0.05) [Table 4].
Table 4.
Primary outcome patients receiving packed RBCs in the 1st 24 h
As a monitor to the biomarkers of coagulopathy and maximum clot strength for detection of the effect of TXA on the coagulation and the hypercoagulable state we used the TEG. With the analysis of “r” time, “k” time, maximum amplitude (MA), α-angle and LY 30 of the thromboelastography. Baseline values showed no significant differences among all groups (P > 0.05) [Table 5].
Table 5.
Thromboelastography readings during the 1st 24 h for all studied groups
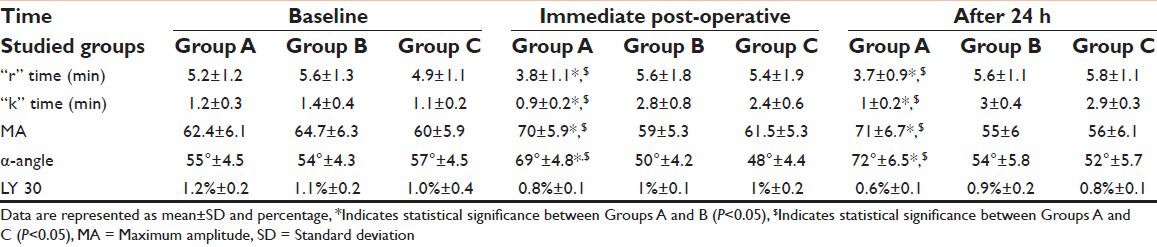
Immediate post-operatively and after 24 h there was a significant decrease in “r” time and “k” time and a significant increase in MA and α-angle in group A compared with Groups B and C (P < 0.05) [Table 5]. However, there was no significant differences in LY 30 among the three groups either immediate or after first 24 h post-operatively (P > 0.05) [Table 5].
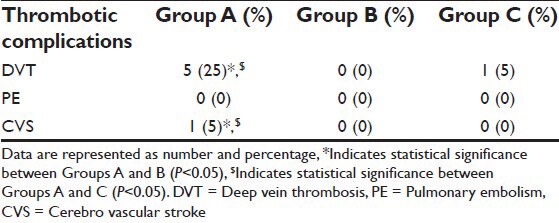
The main safety outcome was the incidence of thromboembolic events within 4 weeks post-operatively. These thromboembolic events were defined as the composite of DVT confirmed by symptoms and phlebography by ultrasound, PE confirmed by Spiral computed tomography and CVS confirmed by computed tomographic scan or MRI.
At the end of 4 weeks interval, thromboembolic events occurred in six patients in Group A, (five patients with DVT and one patient with CVS) and in Group C, one patient with DVT. There were no thromboembolic events in any of Group B patients. There was statistically significant increase in the incidence of thromboembolic events in the form of DVT in Group A patients compared to patients in Groups B and C (P < 0.05) [Table 6].
Table 6.
Incidence of thromboembolic events (DVT, PE and CVS) within 4 weeks post-operatively
DISCUSSION
Previous studies evaluated the use of TXA intravenously to reduce perioperative bleeding and the need for blood transfusion also some researchers tested the effect of topical use of TXA on the site of surgery in favor to get its benefit of reducing blood loss and enhancing hemostasis and to avoid the hypercoagulation effect of TXA when it is used systemically. In this study, we compared the intravenous TXA to topical TXA together with the placebo group.
Concerning hemoglobin and hematocrit levels, they were significantly higher in intravenous and topical TXA groups compared to placebo group. This is in agreement with studies showed that patients in TXA group also had a higher level of post-operative hemoglobin than the placebo group.[5,13]
In the present study regarding the coagulation profile (PT, APTT and INR) and platelet count, there were no significant changes among pre-operative levels, immediate and 24 h post-operatively. These findings are in agreement with other studies that used TXA for blood conservation in knee arthroplasty. Their data confirmed that TXA did not induce platelet activity or platelet count and at the same time there were no significant changes on coagulation profile.[9,13,14]
Results of our study showed significant reduction of blood loss in both intravenous and topical TXA groups in comparison to the placebo group within the first post-operative 24 h. These findings go with Wong et al.,[5] who proved that administration of TXA significantly reduced the total perioperative blood loss in adult patients having thoracic/lumbar instrumental spinal fusion surgery.
Following success of systemic use of Aprotinin and TXA in controlling post-coronary artery bypass graft (CABG) bleeding trials for topical use were started by Tatar et al.,[9] in 1993, who reported reduction of post-operative blood loss and the need for transfusion after topical aprotinin use in CABG. Similar results were reported by Khalil et al.,[10] and De Bonis et al.,[11] to start topical use of TXA.
Our results showed that the need for packed RBCs transfusion is reduced in both intravenous and topical TXA groups in comparison to placebo group built as statistically significant. Similar findings were found that the need for blood transfusion after topical aprotinin use in CABG were reduced.[8,12]
Other studies reported that the administration of TXA did not reduce the incidence of allogenic blood transfusion; However, there was tendency toward reduced amount of RBC transfusion in TXA group.[15,16]
At the same time other studies had evaluated the use of tranexamic acid given during total knee arthroplasty decreased blood loss by 48% and significantly reduced transfusion requirements. The blood sparing effect of tranexamic acid was most evident during the first 24 hours after operation where blood loss was significantly reduced compared to control groups.[2,17,18,19] These findings are in agreement of our results regarding blood loss and need for blood transfusion.
Hobson et al.,[20] used thromboelastogram as a monitor for coagulation in patients who received TXA during surgery. However, our study used thromboelastogram as a monitor for coagulation which showed that all parameters (significant decrease in r, k and significant increase in MA, α-Angle). These findings favor relative hypercoagulable state in intravenous TXA group in comparison to topical TXA and placebo groups and this in agreement with Mannuci et al.[21] who reported that the use of TXA may promote hypercoagulable state.
Other researchers reported that the use of TXA can decrease perioperative bleeding by attenuating the enhance fibronolytic activity that may enhance the coagulable state with the risk of thrombo embolic events[5,22] this confirms our results by thromboelostogram, which shows hypercoagulable state in intravenous TXA group.
Previous study, reported that clot stability by thrombelastography may have demonstrated evidence of increased fibrinolysis after use of tourniquet in the control group and its absence in the TXA group and this study reported incidence of hypercoagulale state in TXA group[2] which agrees with our findings in this study.
As there remains a concern that TXA may promote a hypercoagulale state, our study evaluated its safety on venous and arterial outcomes. We reported 30% increased the risk of thromboembolic event in intravenous group compared to topical group which was 0% and placebo group was 5%. These findings were statistically significant.
In a previous study, results showed a three-fold increased risk of vascular events with the use of intravenous TXA group compared to placebo.[23]
In contrast, previous study of elective hip or knee arthroplasty, reported that use of tranexamic cid did not increase the risk of venous or arterial thrombosis.[24] Furthermore, in previous studies on the use of tranexamic and thrombosis failed to show any thrombogenic effect. Again these previous studies that use antifibriotylic during total knee replacement, reported no clinically relevant thromboembolic episode identified.[2,25]
CONCLUSION
The use of TXA either intravenous or topical during hip hemiarthroplasty reduced the post-operative blood loss and the transfusion needs during the first 24 h post-operative.
However, topical use of tranexamic aicd has the adventage of more broad safety margin in thromboembolic events with lower incidence of developing thrombotic complication. So, in summary our study advised the routine use topical TXA in hip surgery with wide safety margin of complication and less risk of blood transfusion.
Large comparative trials are needed for further assessment of the efficacy and safety of topical use during surgery.
Significance of the study
The study investigated the effectiveness of Topical TXA in decreasing post-operative blood loss. It also investigated the possible side-effects of this route of administration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Zufferey P, Merquiol F, Laporte S, Decousus H, Mismetti P, Auboyer C, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105:1034–46. doi: 10.1097/00000542-200611000-00026. [DOI] [PubMed] [Google Scholar]
- 2.Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83:596–601. doi: 10.1093/bja/83.4.596. [DOI] [PubMed] [Google Scholar]
- 3.Bannister GC, Young SK, Baker AS, Mackinnon JG, Magnusson PA. Control of bleeding in cemented arthroplasty. J Bone Joint Surg Br. 1990;72:444–6. doi: 10.1302/0301-620X.72B3.2341445. [DOI] [PubMed] [Google Scholar]
- 4.Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs. 1985;29:236–61. doi: 10.2165/00003495-198529030-00003. [DOI] [PubMed] [Google Scholar]
- 5.Wong J, El Beheiry H, Rampersaud YR, Lewis S, Ahn H, De Silva Y, et al. Tranexamic Acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. 2008;107:1479–86. doi: 10.1213/ane.0b013e3181831e44. [DOI] [PubMed] [Google Scholar]
- 6.Beauty JH. Rosemont, IL: AAOS; 1999. Orthopedic Knowledge; pp. 63–72. [Google Scholar]
- 7.Ido K, Neo M, Asada Y, Kondo K, Morita T, Sakamoto T, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120:518–20. doi: 10.1007/s004029900132. [DOI] [PubMed] [Google Scholar]
- 8.Abul-Azm A, Abdullah KM. Effect of topical tranexamic acid in open heart surgery. Eur J Anaesthesiol. 2006;23:380–4. doi: 10.1017/S0265021505001894. [DOI] [PubMed] [Google Scholar]
- 9.Tatar H, Ciçek S, Demirkiliç U, Ozal E, Süer H, Oztürk O, et al. Topical use of aprotinin in open heart operations. Ann Thorac Surg. 1993;55:659–61. doi: 10.1016/0003-4975(93)90271-i. [DOI] [PubMed] [Google Scholar]
- 10.Khalil PN, Ismail M, Kalmar P, von Knobelsdorff G, Marx G. Activation of fibrinolysis in the pericardial cavity after cardiopulmonary bypass. Thromb Haemost. 2004;92:568–74. doi: 10.1160/TH03-07-0455. [DOI] [PubMed] [Google Scholar]
- 11.De Bonis M, Cavaliere F, Alessandrini F, Lapenna E, Santarelli F, Moscato U, et al. Topical use of tranexamic acid in coronary artery bypass operations: A double-blind, prospective, randomized, placebo-controlled study. J Thorac Cardiovasc Surg. 2000;119:575–80. doi: 10.1016/s0022-5223(00)70139-5. [DOI] [PubMed] [Google Scholar]
- 12.O’Regan DJ, Giannopoulos N, Mediratta N, Kendall SW, Forni A, Pillai R, et al. Topical aprotinin in cardiac operations. Ann Thorac Surg. 1994;58:778–81. doi: 10.1016/0003-4975(94)90748-x. [DOI] [PubMed] [Google Scholar]
- 13.Camarasa MA, Ollé G, Serra-Prat M, Martín A, Sánchez M, Ricós P, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: A randomized clinical trial. Br J Anaesth. 2006;96:576–82. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 14.Brown JR, Birkmeyer NJ, O’Connor GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation. 2007;115:2801–13. doi: 10.1161/CIRCULATIONAHA.106.671222. [DOI] [PubMed] [Google Scholar]
- 15.Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005;102:727–32. doi: 10.1097/00000542-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Abrishami A, Chung F, Wong J. Topical application of antifibrinolytic drugs for on-pump cardiac surgery: A systematic review and meta-analysis. Can J Anaesth. 2009;56:202–12. doi: 10.1007/s12630-008-9038-x. [DOI] [PubMed] [Google Scholar]
- 18.Yasim A, Aşik R, Atahan E. Effects of topical applications of aprotinin and tranexamic acid on blood loss after open heart surgery. Anadolu Kardiyol Derg. 2005;5:36–40. [PubMed] [Google Scholar]
- 19.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90:596–99. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 20.Hobson AR, Agarwala RA, Swallow RA, Dawkins KD, Curzen NP. Thrombelastography: Current clinical applications and its potential role in interventional cardiology. Platelets. 2006;17:509–18. doi: 10.1080/09537100600935259. [DOI] [PubMed] [Google Scholar]
- 21.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–11. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 22.Sperzel M, Huetter J. Evaluation of aprotinin and tranexamic acid in different in vitroand in vivomodels of fibrinolysis, coagulation and thrombus formation. J Thromb Haemost. 2007;5:2113–8. doi: 10.1111/j.1538-7836.2007.02717.x. [DOI] [PubMed] [Google Scholar]
- 23.Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, et al. Tranexamic acid in hip fracture surgery: A randomized controlled trial. Br J Anaesth. 2010;104:23–30. doi: 10.1093/bja/aep314. [DOI] [PubMed] [Google Scholar]
- 24.Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: A meta-analysis. Anaesth Intensive Care. 2003;31:529–37. doi: 10.1177/0310057X0303100507. [DOI] [PubMed] [Google Scholar]
- 25.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: A prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78:434–40. [PubMed] [Google Scholar]


