Abstract
Objectives:
Pregabalin and clonidine have anti-nociceptive properties. This study assesses their efficacy in prolonging the analgesic effect of spinal anesthesia and post-operative analgesic requirement in patients undergoing vaginal hysterectomy.
Materials and Methods:
A total of 90 females in the age group of 30-60 years were randomly allocated in to three groups of 30 each, to receive either oral clonidine (150 μg) or oral pregabalin (150 mg) or oral multivitamin as placebo 1.5 h before spinal anesthesia with 3ml (15 mg) of 0.5% hyperbaric bupivacaine. Intensity of pain was measured on a visual analog scale (VAS) at the end of operation (0 h) then at 1,2,4,6,12 and 24 h thereafter. Diclofenac sodium intramuscularly 1 mg/kg was provided when the VASscore was >4 in the study period. Sedation was defined by Ramsay sedation scale at 0,6,12 and 24 h. Side-effects such as nausea and vomiting, respiratory depression and dryness of mouth were noted.
Results:
The VAS scores were significantly less in the pregabalin group compared with the clonidine group at 6,12 and 24 h post-operatively with a P < 0.0001. More sedation was seen in the clonidine group than in the pregabalin group (P < 0.05). Analgesic consumption and VAS scores were lower in clonidine and pregabalin group compared with the placebo group (P < 0.05).
Conclusion:
Oral pregabalin (150 mg) prolongs the post-operative pain relief after spinal anesthesia but produces less sedation compared with oral clonidine (150 μg).
Keywords: Clonidine, post-operative analgesia, pregabalin, Ramsay sedation scale, vaginal hysterectomy, visual analog scale score
INTRODUCTION
Pain is derived from the word poena meaning punishment. Pain is an unpleasant sensation that originates from ongoing and impending tissue damage. Acute pain accompanies almost all surgical procedures. Adequate pain relief provides a quick return to normal physiological function and prevents the development of chronic pain. Traditional analgesia in the post-operative period is based on opioids, non-steroidal anti-inflammatory drugs (NSAIDS) and regional techniques. Administration of high doses of opioids during the post-operative period can result in higher incidence of complications such as respiratory depression, sedation, vomiting, constipation, pruritus, immune dysfunction and urinary retention.[1] NSAIDS may lead to gastrointestinalbleeding, renal toxicity and thromboembolic complications. Regional analgesia techniques require additional intervention and have the potential risk of complications such as hypotension, bradycardia and toxicity of the administered drug. Hence, the search for an ideal drug continues. A drug, which has anxiolytic property without the adverse effects of traditional analgesics mentioned, may be an attractive choice for post-operative analgesia.
Pregabalin is a lipophilic gamma-amino-butyric-acid (GABA) analog with anti-convulsant, anxiolyitc and sleep modulating properties. Pregabalin was shown to be effective in neuropathic pain, incisional injury and inflammatory injury.[2,3,4,5] Clonidine is a selective partial alpha2 receptor agonist, which has sedative and anti-nociceptive properties. The aim of our study was to compare the duration of post-operative analgesia with oral pregabalin and clonidine after spinal anesthesia and the number of doses of diclofenac sodium injection required during the first 24 h after surgery among the study groups. We also assessed the side effects of the study drugs such as respiratory depression, nausea, vomiting and dryness of mouth.
MATERIALS AND METHODS
This is randomized, double-blind, controlled study carried out with the permission of the Institutional Ethics’Committee and after obtaining written informed consent of the patients. A total of 90 female patients in the age group 30 to 60 years of American Society of Anesthesiologists (ASA)-physical status I and II undergoing elective vaginal hysterectomy were selected for the study. The group size of 30 was determined by power analysis based on standard deviation (SD) data from previously published reports.[6,7] Patients having a body mass index > 30 kg/m2, suffering from central nervous system disorders, having chronic pain conditions or had taken analgesics in the last 48 h were excluded from the study. Those having contraindications of spinal anesthesia were exempted from the study.
All patients were clinically evaluated pre-operatively and relevant laboratory investigations were obtained. The selected patients were randomly allocated in to three groups of 30 each based on a computer generated randomization table. The anesthesiologists who provided the drugs and those who recorded the data were all blinded to the group identities. Each group was given the study drug orally 1.5 h before the procedure in the following manner: Group A: Received150 μg of oral clonidine; Group B: received 150 mg of oral pregabalin; Group C: Received oral multivitamin [Figure 1].
Figure 1.
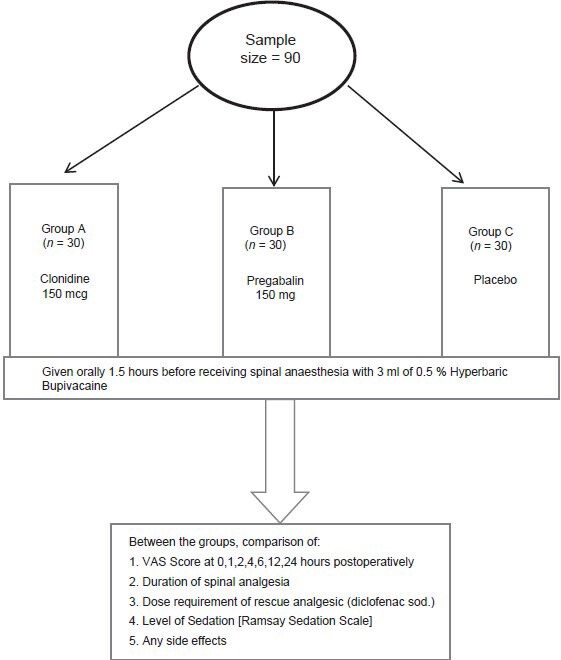
Method of the study
In the operation theater, baseline non-invasive blood pressure, electrocardiogram, pulse rate (PR) and oxygen saturation (SpO2%) were monitored. In the operating room, intravenous access was done with an 18 G cannula and Ringers’lactate infusion (500 ml) was infused at the rate of 10 ml/kg over 30 min and there after at the rate of 5 ml/kg/h through the rest of the intra-operative period. Spinal anesthesia was given in the sitting position through midline approach at L3-L4 intervertebral space using 26 G spinal needle and 3 ml of 0.5% hyperbaric bupivacaine was given. Patients were positioned in the supine position after intrathecal injection. Onset of sensory block was assessed bilaterally by loss of pinprick sensation with a short hypodermic needle. After achieving the sensory block up to T10 dermatome level and motor block of 3 on Bromage scale [Table 1] we allowed the surgery to begin.
Table 1.
Bromage scale

Intra-operative hemodynamic monitoring was done. When the mean arterial pressure (MAP) fell below 20% of the baseline value, phenylephrine 1 μg/kg was given intravenously and repeated after 5 min if required. If PR was <60 beats/min atropine 0.6 mg was given. No sedatives or analgesics were given during the intra-operative period. After operation patients were shifted to the post-anesthesia care unit. In the post-operative period intensity of pain was measured on a 10-point visual analog scale (VAS) at end of operation [0 hr] then 1, 2, 4, 6, 12 and 24 h after the operation.
Those patients who had a VAS score of >4 were given injection diclofenac sodium 1 mg/kg intramuscularly as a rescue analgesic. The number of doses of analgesic given in the first 24 h was documented. Sedation was scored on Ramsay sedation scale (RSS) at 0, 6, 12 and 24 h after operation. We also noted other side-effects such as respiratory depression, nausea and vomiting and dryness of mouth. Any incidence of respiratorydepression (respiratory rate <8 breaths/min or SpO2 <90% without supplementation) was to be corrected with supplemental oxygen through facemask.
RSS
Statistical analysis was performed by entering data in Microsoft Excel database and subsequently analyzed by standard statistical software like SPSS (StatisticalProductfortheSocialSciences version 19; manufacturer SPSS Inc. 223 South Wacker Drive, 11th floor, Chicago, IL 60606-6412, U.S.A.). Results are expressed in mean ± SD. Numerical variables were compared between the groups using unpaired t-test. The significance of change in VAS score from first to final assessment was evaluated by repeated measures ANOVA. All analysis was two-tailed and P < 0.05 was considered to be statistically significant and a P < 0.0001 as highly significant.
RESULTS
A total of 90 patients who underwent vaginal hysterectomy under spinal anesthesia were included in the study and were randomly allocated to three groups of 30 each. The ASA physical status was similar in all the three groups. The demographic profile of all the three groups was comparable with respect to age, weight and height, duration of surgery [Table 2]. Baseline PR, MAP and SpO2 are comparable in all three groups. There were no statistically significant changes in these parameters in subsequent recordings [Figures 2 and 3].
Table 2.
The demographic profile and duration of surgery

Figure 2.
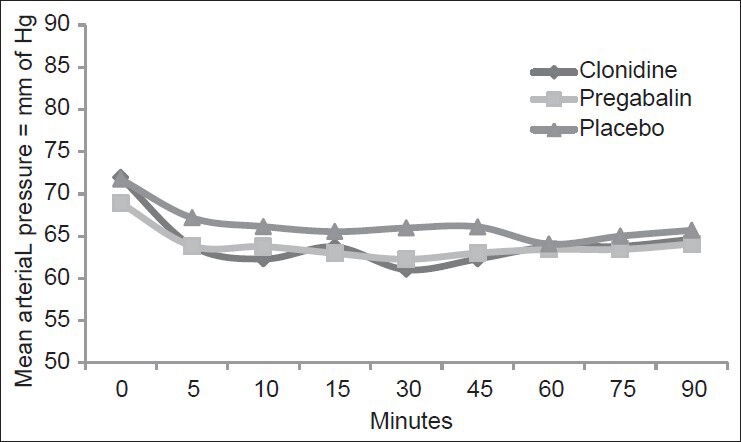
Comparison of mean arterial pressure
Figure 3.
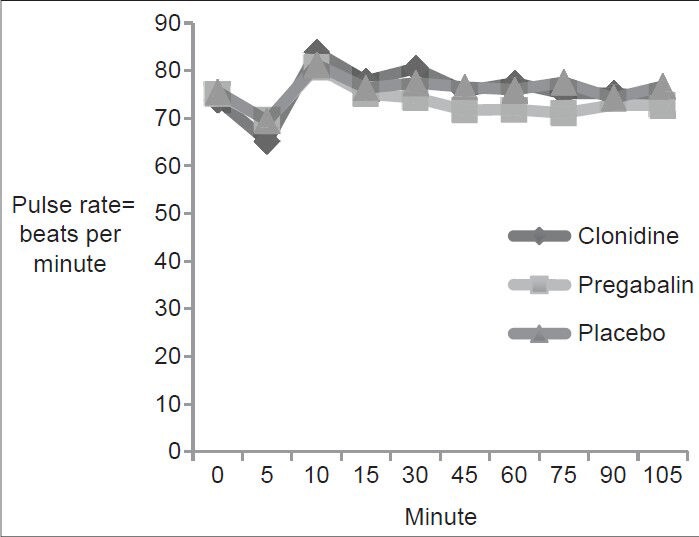
Comparison of the pulse rate
The difference in intensity of pain scores on a VAS scale between the study Group A (0.52 ± 0.51, 2.58 ± 0.56, 5.35 ± 0.95, 4.51 ± 0.99, 4.82 ± 0.51, 5.61 ± 0.80, 4.55 ± 1.03) or Group B (0.39 ± 0.56, 2.55 ± 0.85, 4.94 ± 1.34, 4.10 ± 0.87, 4.14 ± 0.85, 3.52 ± 0.89, 3.58 ± 0.96) compared to Group C (0.77 ± 0.72, 4.87 ± 1.09, 6.48 ± 0.63, 4.48 ± 0.81, 5.29 ± 0.78, 6.58 ± 1.12, 6.00 ± 1.18) at 0, 1, 2, 4, 6, 12 and 24 h of post-operative period respectively was highly significant statistically with a P < 0.0001. The duration of analgesia was significantly more in the Group B compared to Group A after 6 h of post-operative period P < 0.05 [Table 3, Figure 4].
Table 3.
Comparison of post-operative visual analog score
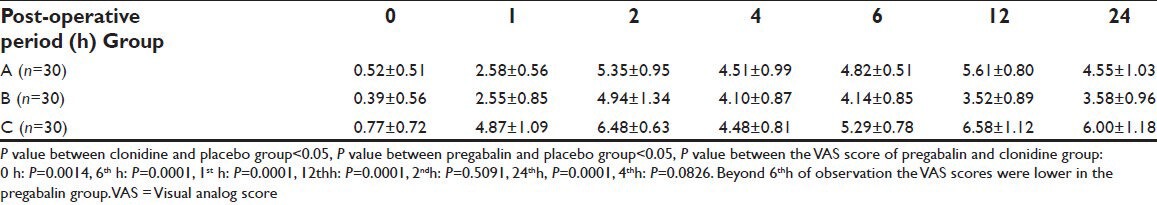
Figure 4.
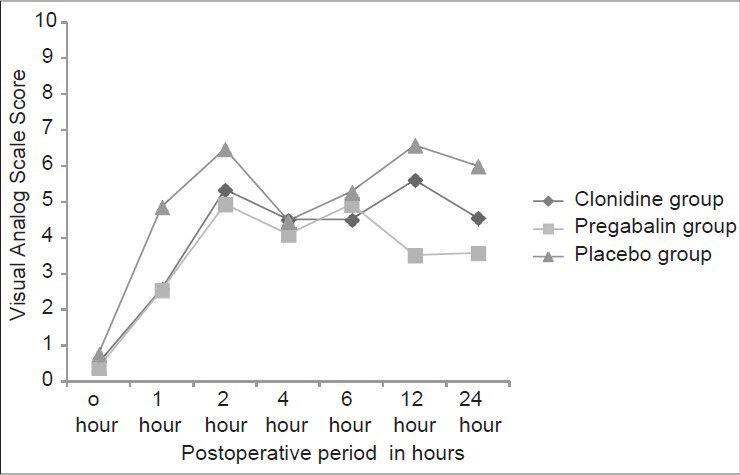
Comparison of the visual analog score scores of the clonidine, pregabalin and the placebo group
Incidence of sedation was higher in the Group A just at the end of operation with six patients (20%) having a RSS score of 4 compared to only one patient (3%) in the Group B (P < 0.05). During the rest of the observation period the patients in both the groups had a RSS of less than 3. In the placebo group, all the patient were observed to have a RSS score of 2 or 1 throughout the period of observation [Figures 5 and 6].
Figure 5.
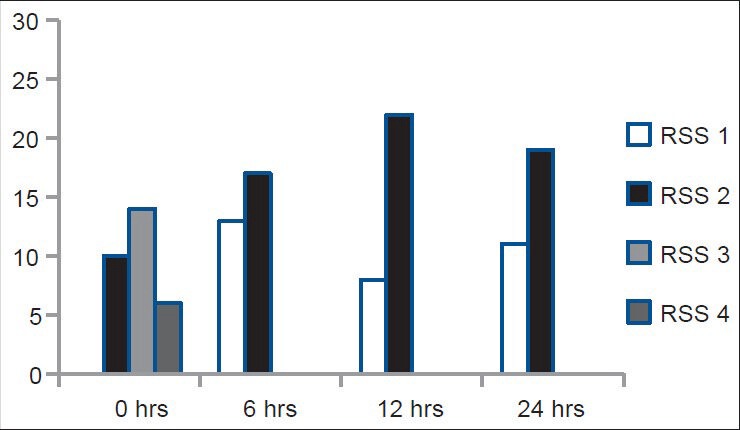
Ramsay sedation scale score of the clonidine group
Figure 6.
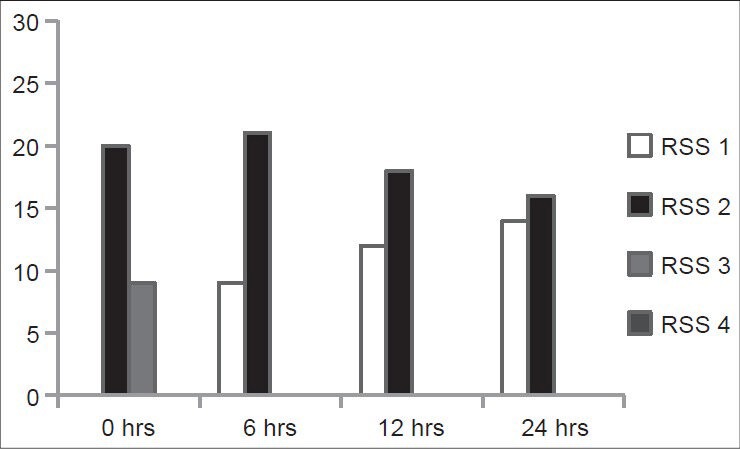
Ramsay sedation scale score of the pregablin group
Time to first demand of a rescue analgesic was more with clonidine (238.41 ± 7.32 min) and pregabalin (252.14 ± 5.02 min) than with placebo (178.24 ± 7.43 min) [Table 4].
Table 4.
Mean duration of spinal analgesia

In the Group C 80% patients required three doses each of 1 mg/kg of intramuscular diclofenac sodium as rescue analgesic whereas majority in the Group B (70%) and the Group A (57%) sufficed with only a single dose [Table 5, Figure 7].
Table 5.
Comparison of doses of intramuscular diclofenac sodium (1 mg/kg)
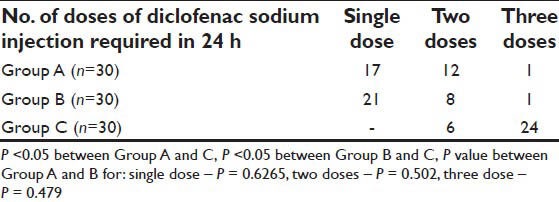
Figure 7.
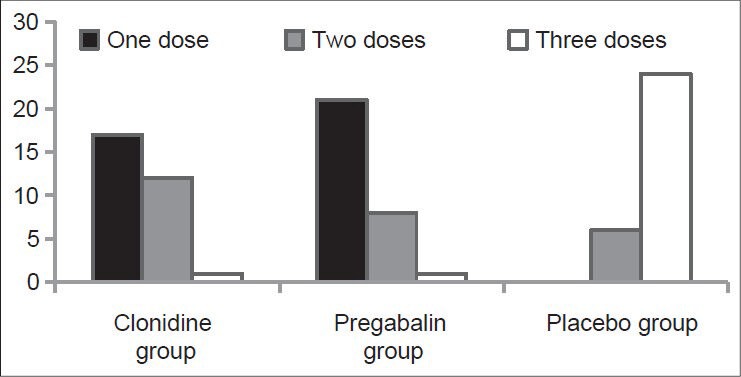
Diclofenac sodium requirement
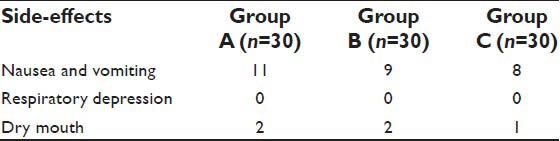
There was no incidence of respiratory depression or dry mouth in the study groups A and B The episodes of post-operative nausea and vomiting were comparable in all the three groups and not statistically significant [Table 6].
Table 6.
Side-effects of study drugs
DISCUSSION
Pain is the most common complaint in the post-operative period. Pain signals start a cascade of alterations in the somatosensory systems, which increases the response to subsequent stimuli and thus amplify pain.[8]
Clonidine is an α2 adrenergic agonist that produces dose dependent analgesia at spinal and supraspinal sites. Oral clonidine is almost completely absorbed and peak plasma concentration is reached after 1-3 h of administration. It is highly lipid soluble, crosses the blood brain barrier easily. Clonidine inhibits neurotransmission in both A-delta and C fibers and potentiates inhibitory effect of the local anesthetic on the C-fiber activity. Pregabalin is a GABA analog that binds to the α2-d subunit of the presynaptic voltage-dependent calcium channels that are widely distributed throughout the central and peripheral nervous systems.[9,10,11] It reduces the release of several neurotransmitters such as glutamate, norepinephrine, serotonin, dopamine and substance P.[12,13,14,15,16,17,18] Pregabalin is inactive at GABAA and GABAB receptors.[19] Its elimination half-life is 5.5-6.7 h independent of dose and repeated administration. Side-effects of pregabalin include dizziness, somnolence, dry mouth etc.
We selected the dose of oral clonidine to be 150 mcg as the incidence of bradycardia and hypotension is more with higher doses during spinal anesthesia.[20,21] A test dose of 150 mg of pregabalin was based on the studies where such a dose produced no acute hemodynamic alterations as well as sedation.[7,22] In our study, 150 mcg oral clonidine and pregablin 150 mg was given 1.5 h before spinal anesthesia, which prolonged the duration of analgesia and maintained the VAS scores at 0, 1, 2, 4, 6, 12 and 24 h in the lower range in comparison with the placebo group (P < 0.05). Rescue analgesic doses required was less in the Group A and Group B than in the Group C (P < 0.05). RSS score was more in Group A and B than Group C. There were no significant hemodynamic changes in all the groups [Figures 1 and 2].
Montazeri and Ghobadian found out that with oral clonidine in spinal anesthesia, the mean duration of sensory and motor blockade was increased.[23] Liu et al., Ota et al. and Singh et al. observed that oral clonidine 150-200 mcg given 1-1.5 h before spinal anesthesia caused significant prolongation of sensory analgesia.[20,21,24] Partahusniutojo showed that the mean duration of sensory analgesia was significantly prolonged with 150 mcg oral clonidine in spinal anesthesia.[25] Joleka et al. found that there was a decreased post-operative requirement of analgesics in pregabalin (300 mg) group compared with those in the diazepam (10 mg) group with increased incidence of dizziness and blurred vision in the pregabalin group.[26] Tiippana et al. did a meta-analysis of the Medline and Cochrane Central Register of Controlled Trials (CENTRAL) databases regarding perioperative administration of gabapentinoids and concluded that they effectively reduced post-operative pain, opioid consumption and their adverse effects.[27]
In our study, mean duration of sensory analgesia in GroupA (238.41 ± 7.32 min) and in Group B was (252.14 ± 5.02 min), which was statistically significant (P < 0.05). The VAS score at rest was significantly different between Group A and Group B during the observations made at 0, 1 h and thereafter most of the patients demanded a rescue analgesic. After giving the rescue analgesic the VAS score was significantly lower in Group B (P < 0.001) than Group A beyond 6 h of observation in the post-operative period. It was seen that 57% and 70% of patients in the Group A and Group B respectively required only a single dose of diclofenac sodium whereas 80% patients in the Group C had to be administered three doses of diclofenac in the 24 h study period. The difference was statistically significant and supported the role of pain prevention in surgery with clonidine and pregabalin. Ghafari et al., did a comparative study of oral clonidine (100 mcg) and gabapentin (300 mg) given pre-operatively in 99 women undergoing abdominal hysterectomy. They found out that both gabapentin and clonidine group had a post-operative VAS score and total morphine consumption less than that of the placebo group (P < 0.05). Gabapentin group had a better pain score than clonidine group.[6] Kohli et al. conducted a randomized trial on patients undergoing hysterectomy under spinal anesthesia and concluded that oral pregabalin provides immediate post-operative analgesia by prolonging the neuraxial block and reduces the frequency of administration of parenteral analgesics.[22] Mathiesen et al. showed that there was decreased 24 h analgesic consumption in patients undergoing total hip arthroplasty who had received pregabalin with paracetamol compared to those who had received only paracetamol.[28] Ittichaikulthol et al. found out that there was decreased verbal numerical rating scale pain score and decreased 24 h morphine consumption in pregabalin (300 mg) group in comparison with lorazepam (0.5 mg) given 1 h before abdominal hysterectomy.[29] All these studies suggest that both clonidine and pregabalin prolonged the duration of sensory analgesia after spinal blockade and reduce the analgesic requirement on the post-operative period. Their finding corroborates with our study.
Both clonidine and pregabalin have sedative properties. 6 (20%) patients of Group A had a RSS score of 4 at 0 h, but were arousable by gentle tactile stimulation. In the Group B only 1 (3%) of the patients had a RSS score of 4 at 0 h of observation, but they remained awake, calm and cooperative. This suggested that clonidine produces more sedation than pregabalin after oral administration. Other studies also suggest sedation with oral clonidine 150 mcg and pregabalin 150 mg.[30,31] Incidence of nausea and vomiting, dry mouth was not significantly different among the groups.
We conclude that oral pregabalin in the dose of 150 mg prolonged the duration of spinal analgesia and provided better post-operative analgesia, less analgesic requirement and less sedation than oral clonidine in the dose of 150 μg in patients receiving spinal anesthesia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Dolin SJ, Cashman JN. Tolerability of acute postoperative pain management: Nausea, vomiting, sedation, pruritus, and urinary retention. Evidence from published data. Br J Anaesth. 2005;95:584–91. doi: 10.1093/bja/aei227. [DOI] [PubMed] [Google Scholar]
- 2.Nozaki-Taguchi N, Chaplan SR, Higuera ES, Ajakwe RC, Yaksh TL. Vincristine-induced allodynia in the rat. Pain. 2001;93:69–76. doi: 10.1016/S0304-3959(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 3.Field MJ, Holloman EF, McCleary S, Hughes J, Singh L. Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. J PharmacolExpTher. 1997;282:1242–6. [PubMed] [Google Scholar]
- 4.Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997;121:1513–22. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houghton AK, Lu Y, Westlund KN. S-(+)-3-isobutylgaba and its stereoisomer reduces the amount of inflammation and hyperalgesia in an acute arthritis model in the rat. J PharmacolExpTher. 1998;285:533–8. [PubMed] [Google Scholar]
- 6.Ghafari MH, Akrami M, Nouralishahi B, Sadegh A. Preoperative gabapentin or clonidine decreases postoperative pain and morphine consumption after abdominal hysterectomy. Res J BiolSci. 2009;4:458–63. [Google Scholar]
- 7.Rastogi B, Gupta K, Gupta PK, Agarwal S, Jain M, Chauhan H. Oral pregabalin premedication for attenuation of haemodynamicpressor response of airway instrumentation during general anaesthesia: A dose response study. Indian J Anaesth. 2012;56:49–54. doi: 10.4103/0019-5049.93344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 9.Arikkath J, Campbell KP. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. CurrOpinNeurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 10.Bian F, Li Z, Offord J, Davis MD, McCormick J, Taylor CP, et al. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: An ex vivoautoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res. 2006;1075:68–80. doi: 10.1016/j.brainres.2005.12.084. [DOI] [PubMed] [Google Scholar]
- 11.Belliotti TR, Capiris T, Ekhato IV, Kinsora JJ, Field MJ, Heffner TG, et al. Structure-activity relationships of pregabalin and analogues that target the alpha (2)-delta protein. J Med Chem. 2005;48:2294–307. doi: 10.1021/jm049762l. [DOI] [PubMed] [Google Scholar]
- 12.Gazulla J, Tintoré MA. The P/Q-type voltage-dependent calcium channel as pharmacological target in spinocerebellar ataxia type 6: Gabapentin and pregabalin may be of therapeutic benefit. Med Hypotheses. 2007;68:131–6. doi: 10.1016/j.mehy.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Dooley DJ, Donovan CM, Pugsley TA. Stimulus-dependent modulation of [(3) H] norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J PharmacolExpTher. 2000;295:1086–93. [PubMed] [Google Scholar]
- 14.Dooley DJ, Mieske CA, Borosky SA. Inhibition of K(+)-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. NeurosciLett. 2000;280:107–10. doi: 10.1016/s0304-3940(00)00769-2. [DOI] [PubMed] [Google Scholar]
- 15.Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, et al. Inhibition of neuronal Ca (2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–36. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 16.Errante LD, Petroff OA. Acute effects of gabapentin and pregabalin on rat forebrain cellular GABA, glutamate, and glutamine concentrations. Seizure. 2003;12:300–6. doi: 10.1016/s1059-1311(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham MO, Woodhall GL, Thompson SE, Dooley DJ, Jones RS. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur J Neurosci. 2004;20:1566–76. doi: 10.1111/j.1460-9568.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- 18.Micheva KD, Taylor CP, Smith SJ. Pregabalin reduces the release of synaptic vesicles from cultured hippocampal neurons. MolPharmacol. 2006;70:467–76. doi: 10.1124/mol.106.023309. [DOI] [PubMed] [Google Scholar]
- 19.Lanneau C, Green A, Hirst WD, Wise A, Brown JT, Donnier E, et al. Gabapentin is not a GABAB receptor agonist. Neuropharmacology. 2001;41:965–75. doi: 10.1016/s0028-3908(01)00140-x. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Chiu AA, Neal JM, Carpenter RL, Bainton BG, Gerancher JC. Oral clonidine prolongs lidocaine spinal anesthesia in human volunteers. Anesthesiology. 1995;82:1353–9. doi: 10.1097/00000542-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ota K, Namiki A, Iwasaki H, Takahashi I. Dosing interval for prolongation of tetracaine spinal anesthesia by oral clonidine in humans. AnesthAnalg. 1994;79:1117–20. [PubMed] [Google Scholar]
- 22.Kohli M, Murali T, Gupta R, Khan P, Bogra J. Optimization of subarachanoid block by oral pregabalin for hysterectomy. J AnaesthesiolClinPharmacol. 2011;27:101–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Montazeri K, Ghobadian A. Oral clonidine as a premedication in spinal anesthesia: Effects on the duration of block and hemodynamic status a randomised double blind clinical trial. Journal of Research in Medical Sciences. 2002;7 No 4. [Google Scholar]
- 24.Singh H, Liu J, Gaines GY, White PF. Effect of oral clonidine and intrathecal fentanyl on tetracaine spinal block. AnesthAnalg. 1994;79:1113–6. doi: 10.1213/00000539-199412000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Partahusniutojo P. The effect of oral clonidine premedication on the duration of hyperbaric tetracaine spinal anesthesia. Philipp J Anesthesiol. 1999;11:32–9. [Google Scholar]
- 26.Jokela R, Ahonen J, Tallgren M, Haanpää M, Korttila K. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008;134:106–12. doi: 10.1016/j.pain.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. AnesthAnalg. 2007;104:1545–56. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]
- 28.Mathiesen O, Jacobsen LS, Holm HE, Randall S, Adamiec-Malmstroem L, Graungaard BK, et al. Pregabalin and dexamethasone for postoperative pain control: A randomized controlled study in hip arthroplasty. Br J Anaesth. 2008;101:535–41. doi: 10.1093/bja/aen215. [DOI] [PubMed] [Google Scholar]
- 29.Ittichaikulthol W, Virankabutra T, Kunopart M, Khamhom W, Putarawuthichai P, Rungphet S. Effects of pregabalin on post operative morphine consumption and pain after abdominal hysterectomy with/without salphingo-oophorectomy: A randomized, double-blind trial. J Med Assoc Thai. 2009;92:1318–23. [PubMed] [Google Scholar]
- 30.Filos KS, Patroni O, Goudas LC, Bosas O, Kassaras A, Gartaganis S. A dose-response study of orally administered clonidine as premedication in the elderly: Evaluating hemodynamic safety. AnesthAnalg. 1993;77:1185–92. doi: 10.1213/00000539-199312000-00018. [DOI] [PubMed] [Google Scholar]
- 31.White PF, Tufanogullari B, Taylor J, Klein K. The effect of pregabalin on preoperative anxiety and sedation levels: A dose-ranging study. AnesthAnalg. 2009;108:1140–5. doi: 10.1213/ane.0b013e31818d40ce. [DOI] [PubMed] [Google Scholar]


