Abstract
Aim:
A double-blinded randomized controlled study to compare discharge time and patient satisfaction between two groups of patients submitted to open surgeries for abdominal malignancies using segmental thoracic spinal or general anesthesia.
Background:
Open surgeries for abdominal malignancy are usually done under general anesthesia, but many patients with major medical problems sometimes can’t tolerate such anesthesia. Regional anesthesia namely segmental thoracic spinal anesthesia may be beneficial in such patients.
Materials and Methods:
A total of 60 patients classified according to American Society of Anesthesiology (ASA) as class II or III undergoing surgeries for abdominal malignancy, like colonic or gastric carcinoma, divided into two groups, 30 patients each. Group G, received general anesthesia, Group S received a segmental (T9-T10 injection) thoracic spinal anesthesia with intrathecal injection of 2 ml of hyperbaric bupivacaine 0.5% (10 mg) and 20 ug fentanyl citrate. Intraoperative monitoring, postoperative pain, complications, recovery time, and patient satisfaction at follow-up were compared between the two groups.
Results:
Spinal anesthesia was performed easily in all 30 patients, although two patients complained of paraesthesiae, which responded to slight needle withdrawal. No patient required conversion to general anesthesia, six patients required midazolam for anxiety and six patients required phenylephrine and atropine for hypotension and bradycardia, recovery was uneventful and without sequelae. The two groups were comparable with respect to gender, age, weight, height, body mass index, ASA classification, preoperative oxygen saturation and preoperative respiratory rate and operative time.
Conclusion:
This preliminary study has shown that segmental thoracic spinal anesthesia can be used successfully and effectively for open surgeries for abdominal malignancies by experienced anesthetists. It showed shorter postanesthesia care unit stay, better postoperative pain relief and patient satisfaction than general anesthesia.
Keywords: Abdominal, anesthesia, general, malignancy, spinal, thoracic
INTRODUCTION
General anesthesia is the traditional anesthetic technique used for the majority of open abdominal surgical procedures, and regional anesthesia is preferred only for patients who are at high-risk while under general anesthesia.[1] Among the drawbacks of general anesthesia are the negative side-effects of the drugs, relatively longer recovery times, contraindications for certain patients (elderly or those with heart conditions), cost and safety, all limit the usefulness of general anesthesia.[1,2,3,4] Some studies indicate that time under anesthetic induced sleep may be important indicator of postoperative mortality.[5] Nowadays, there is significant and renewed interest in the use of regional anesthesia techniques for a number of common surgeries.[6,7,8] Anesthesiologists are afraid to perform spinal anesthesia above the termination of the spinal cord due to fear of injuring the spinal cord from the needlepoint. However, in the past neurologists performed subarachnoid myelographic injections at thoracic and cervical levels.[9] Even high spinal anesthesia has been employed for craniotomies.[10] Magnetic resonance imaging (MRI) and other imaging techniques replace these myelographic procedures. Intrathecal injection of anesthetic drugs into the desired height of the body, even above the termination of the spinal cord, has been shown to be potentially very valuable.[11,12] Since, there is minimal blockade of the lower extremities, a significantly larger portion of the body experiences no venal dilation, and may offer a compensatory buffer to adverse changes in blood pressure intraoperatively. Further, the dosing of the anesthetic is exceedingly low, so hemodynamic consequences would be minimal. Results from MRI studies indicate that the spinal cord lies anteriorly within its thecal boundaries in the apex of the thoracic curve. Intrathecal injections, therefore, at thoracic levels may have a safety minimal distance before spinal needle contact with neural tissue. Safe administration of thoracic combined spinal epidural (CSE) anesthesia may facilitate the option that common surgeries, usually requiring prolonged hospital stays, may be performed on an outpatient basis or high-risk patients can be more safely anesthetized.[12] Open surgeries for abdominal malignancies are usually done under general anesthesia, but many patients presented to this type of surgeries have major medical problems. Thus, the aim of this study is to compare discharge time and patient satisfaction between two groups of patients (segmental thoracic spinal anesthesia and general anesthesia) submitted to open surgeries for abdominal malignancies.
MATERIALS AND METHODS
After approval of the Medical Ethical Committee, and informed consent were obtained from 60 patients submitted to various open surgeries for abdominal malignancies (like gastric, pancreatic, intestinal or colonic carcinoma using stapler technique) at Medical Research Institute, Alexandria University. Inclusion criteria of the patients are American Society of Anesthesiology (ASA) physical status classification Groups II or III, and ages 20–70 years, and exclusion criteria of body mass index above 35 kg/m2, surgery time more than 150 min, and the presence of any contraindication to spinal anesthesia. A study to determine the size of the study groups showed that 30 patients in each group is appropriate with power analysis of 81%. All patients signed an informed consent after the thoracic spinal procedure was explained to them. After informed consent, patients were randomized by sealed envelopes to receive either general (Group G) or segmental thoracic spinal anesthesia (Group S). Numbered and sealed envelopes were placed in the operating room and only opened at the patients’ arrival there. Patients’ preoperative evaluation and preparation were standardized. Both anesthesia and surgery were performed in all patients by the same anesthetic and surgical team. On patients’ arrival in the operating room, after establishing noninvasive monitoring (electrocardiogram, arterial blood pressure, and pulse oximetry), 500–1000 ml of Ringer acetate solution was commenced intravenously (IV). A complete preanesthetic evaluation was performed. Every patient was submitted to 8 h fasting period prior to the procedure. Before the anesthesia, a peripheral venous access was placed with a 16G or 18G catheter for hydration with Ringer's Lactate and administration of medication during the procedure. Electrocardiogram, pulse oximetry and noninvasive blood pressure were monitored, and oxygen at 5 L/min was given to patients of Group S through a face-mask during the procedure. Before the blockade, patients received IV 1 mg midazolam. All patients were IV administered 1 mg of granisetron hydrochloride, 50 mg of ranitidine hydrochloride, and 10 mg metoclopramide before the induction of anesthesia. Patients randomized to segmental thoracic spinal anesthesia were positioned at the sitting position and under full aseptic technique, a subarachnoid puncture was done between the 9th and 10th thoracic interspace using 27G Quincke type spinal needle using a paramedian approach. The advancement of the spinal needle was very slow and cautious, the dura was then pierced, once flow of clear cerebrospinal fluid (CSF) began, 2 ml of hyperbaric bupivacaine 0.5% (10 mg) plus 20 ug fentanyl injected intrathecally. After placement of the subarachnoid block, all patients were placed in 5–10° Trendelenburg position until the level of sensory anesthesia (tested by pinprick at 1-min intervals) reached T4. As soon as the sensory block level reached T4 dermatome level, the procedure was begun. Hemodynamic parameters were recorded every 2 min for 10 min then every 5 min thereafter. Sensory loss was confirmed by pinprick determining its upper and lower level. Motor block was confirmed by using modified Bromage scale: (0) Able to lift extended legs; (1) just able to flex knees, full ankle movement; (2) no knee movement, some ankle movement; (3) complete paralysis. Sensory and motor block were recorded just before the start of surgery and after the completion of surgery. Surgeon was allowed to start his incision once the block considered adequate (T4-T12 sensory block). Intravenous drugs were given to control patient anxiety, hypotension, and bradycardia (1 mg midazolam increments for anxiety, phenylephrine infusion for hypotension or atropine for bradycardia). In patients randomized to receive general anesthesia, anesthesia was induced with propofol (1–2 mg/kg), fentanyl citrate (2 μg/kg), and cis-atracurium besylate (0.15 mg/kg). Balanced anesthesia was continued with sevoflurane, 1–2% in 50% oxygen. After intubation of the trachea, the lungs were ventilated with 50% oxygen in air using a semi-closed circle system. Ventilation was controlled with a tidal volume of 6–8 ml/kg and the ventilatory rate was adjusted to maintain a PaCO2 value of 35–40 mmHg. Residual neuromuscular block was antagonized with 2.5 mg neostigmine and 1 mg atropine sulfate at the end of surgery. All patients were monitored by electrocardiogram, heart rate, noninvasive arterial blood pressure, respiratory rate, and pulse oximetry and were recorded at 5-min intervals. Operative time as well as any intra-operative adverse effects such as bradycardia, hypotension, nausea, vomiting, and abdominal discomfort were recorded. Drug consumption and fluid intake were also recorded. Discharge time and patient satisfaction were recorded by an observer using an objective scale for recovery assessment and a verbal rating scale for satisfaction (1/5 very dissatisfied, 2/5 dissatisfied, 3/5 neutral, 4/5 satisfied, and 5/5 very satisfied). All patients were transferred to the postanesthesia care unit (PACU). Discharge time was recorded as the time from admission to PACU until the patient met all discharge criteria from it. These included mental alertness, stable vital signs, absence of nausea, vomiting and control of pain. Side-effects measured were the incidence of hypotension, bradycardia, nausea, vomiting, abdominal pain (severe enough to require IV narcotics), and pruritis during PACU stay. Postoperatively, all patients received IV analgesia (1000 mg of acetaminophen every 8 h, and supplementary opioids on demand). Postoperative pain was assessed at relaxed conditions by using the visual analog scale (VAS) at the completion of surgery, 4, 8, 12, and 24 h after completion of the surgery. Other postoperative events such as abdominal discomfort, nausea, vomiting, pruritus, and neurologic sequelae, were also recorded.
Statistical analysis
Statistical analysis was performed with analysis of variance and standard deviation (Chi-square test [χ2]). For data analysis, discharge time was considered the primary outcome variable, with a difference considered significant at the P < 0.05 level. The secondary variable was the patient satisfaction; the two variables were estimated by an observer using an objective scale for recovery assessment and a verbal rating scale for satisfaction. These were compared by analysis of variance for the two groups and confirmed with Wilcoxon's nonparametric test. The frequency of side-effects among the groups was compared by χ2 analysis with Fisher's exact test, 0.05 level with 80% power.
RESULTS
Sixty patients were recruited in a span of 2 years, the segmental thoracic spinal technique was successful in all patients of Group S and there was no anesthetic conversion. The two groups were comparable with respect to gender, age, weight, height, body mass index, ASA classification, preoperative oxygen saturation, and preoperative respiratory rate [Table 1].
Table 1.
Personal characteristics of the studied cases of both groups
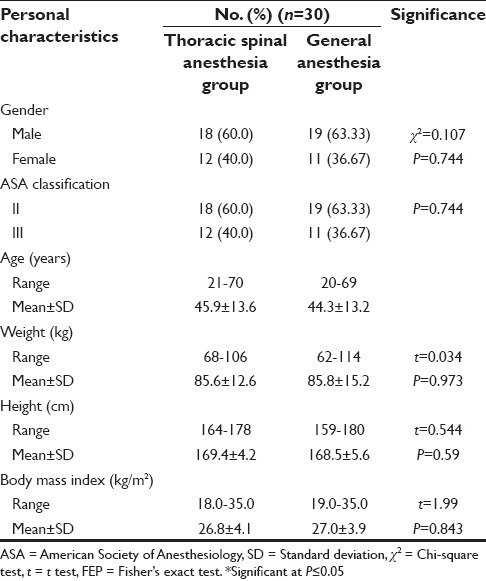
Regarding the operative time, there was no significant difference between groups, as it ranged from 92 to 149 min, with a mean of 118.6 ± 26.6 in Group G, while it ranged from 95 to 150 min, with a mean of 121.3 ± 24.3 in Group S.
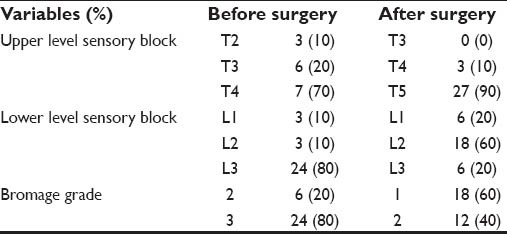
In the thoracic spinal anesthesia group, during insertion of the spinal needle, two patients developed paraesthesia (6.6%) which responded to immediate withdrawal of the stylet of the needle with good effect. No patient experienced problems during injection of the anesthetic solution. An effective sensory block (range: upper T2-T3; lower L1-L3) developed within 15 min in every patient. Modest amounts of lower limb motor block developed before the start of surgery in most of the patients. The mean time to full block regression was 248.9 min [Table 2].
Table 2.
Characteristics of thoracic spinal anesthesia among the studied cases (n=30)
In Group S, six patients (20%) developed hypotension following induction of spinal anesthesia, they received phenylephrine infusion, in the same group, another six patients (20%) developed bradycardia and they were given increments of atropine, while in Group G, no patient developed hypotension or bradycardia. In Group S, six patients (20%) received increments of midazolam 1 mg for anxiety and three patients (10%) experienced intraoperative nausea and vomiting. No patient showed overt evidence of respiratory depression; the respiratory rate was more than eight breath/minute in all patients and oxygen saturation being 97–98% throughout. As regards the intraoperative fluids, patients of Group S were given a mean of 4450 ml fluids which was significantly greater than patients of Group G (3225 ml).
Postanesthesia care unit complications (hypotension, bradycardia, nausea, vomiting, pruritus and abdominal pain severe enough to require IV narcotics) showed insignificant difference between both groups except for nausea and vomiting (15 patients [50%] in Group G, while three patients [10%] in Group S developed nausea and vomiting) and abdominal pain, where 21 patients (70%) in Group G required PACU opioid administration, while in Group S, three patients (10%) only.
The mean discharge time from PACU in Group S was 141 min, which was significantly less than in Group G (224.9 min).
The mean postoperative VAS score at 4, 8, 12, and 24 h was significantly less in Group S, when compared with Group G [Table 3].
Table 3.
Postoperative VAS and patient satisfaction among both studied groups
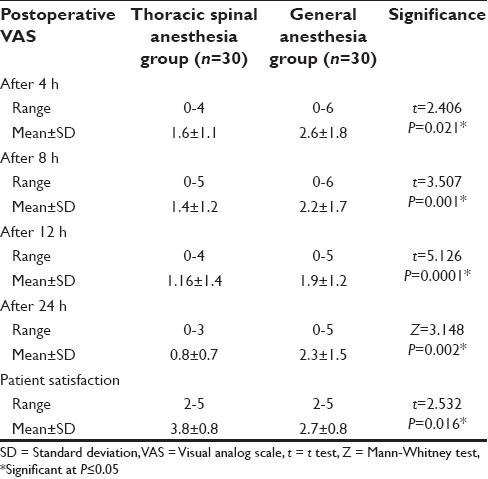
Patients of Group S gave a mean satisfaction score of 3.6, which was significantly more than patients of Group G, whose mean satisfaction score was 2.9 [Table 3].
DISCUSSION
Patients who received segmental thoracic spinal anesthesia, showed significantly shorter discharge time (141 vs. 224.9 min) and better satisfaction (3.6 vs. 2.9) than patients who received general anesthesia. Surgery creates a profound perioperative stress that manifests in neural, endocrine, metabolic, inflammatory, and immunological changes. These changes result in significant immunosuppression. Meanwhile, tumor cells are released into circulation during surgery. This double punch makes perioperative period highly conducive to tumor metastasis. General anesthesia suppresses cerebral and thalamus functions while preserving the function of low brain and spinal circuits.[13] In contrast, regional anesthesia, due to direct nerve block, attenuates or abolishes the reflex circuit between noxious afferents and sympathetic efferents at the surgical level and thus attenuates the surgical stress and immunosuppression. Blockade of sympathetic activity can produce a similar effect. Retrospective human studies suggest that regional anesthesia is an independent beneficial factor for tumor recurrence.[14,15] Regional anesthesia offers superior analgesia over opioid-based analgesia, and a significant reduction in postoperative pain is still a worthwhile outcome. Recent developments in technical aspects of regional anesthesia have the potential to provide significant advantages for many patients in all age groups. Moreover, studies focusing on specific outcomes have shown benefits for regional anesthesia used for surgery and postoperative analgesia.[16] Parker et al.[17] investigated in a Cochrane meta-analysis 22 clinical trials involving 2567 patients where neuraxial (mainly spinal) anesthesia was compared with general anesthesia. Despite the fact that all included trials had methodical problems, the authors found a reduced risk for postoperative deep venous thrombosis (30% compared with 47%) and acute postoperative confusion (9.4% compared with 19.2%) in patients treated with neuraxial (mainly spinal) anesthesia compared with general anesthesia. There was no evidence for reduced perioperative mortality or other outcome parameters (myocardial infarction, pulmonary embolism, etc.) Luger et al.[18] investigated 18,715 patients from 34 randomized controlled trials in which spinal anesthesia was compared with general anesthesia for hip surgery in elderly patients. Their conclusions are much more optimistic compared with the Cochrane meta-analysis above with a clear statement to use regional (spinal) anesthesia for hip surgery in elderly patients.
The thoracic spinal puncture at T10 showed a rapid onset of action, decrease the incidence of hypotension with faster recovery of the blockade. General spinal analgesia of the upper dorsal puncture was primarily used in 1909.[10] Segmental spinal anesthesia of the lower thoracic spine was used in 1954.[19] As the results from MRI studies indicate that the spinal cord lies anteriorly within its thecal sac in the thoracic curve, intrathecal injections, therefore, at thoracic levels may have a safety minimal distance before spinal needle contact with neural tissue.[20,21,22] The risk of spinal cord injury is probably the most serious complication when performing thoracic spinal block, however, an anatomical explanation was proposed for the absence of spinal cord injury during an accidental perforation during attempted thoracic epidural.[21] This study confirmed this hypothesis, as no spinal cord injuries occurred in patients of Group S. The amount of CSF at thoracic levels is diminished compared to lumbar and cervical levels,[23] and the thoracic nerve roots are very slight compared to segments above and below,[24] resulting in less anesthetic dilution per segmental unit of distance from the site of injection, and the roots are easily blocked due to their small size, both of these factors predicting efficient blockade of these segments.
van Zundert et al.[12] published a case report in which a patient with severely abnormal respiratory function (chronic obstructive pulmonary disease with severe emphysema attributed to homozygote α-1-antitrypsine deficiency), requiring continuous oxygen therapy, had frequent respiratory infections and severe functional impairment.[12] Even with minimal activity the patient developed hypoxemia. Using the thoracic CSE technique, with a minute dose of local anesthetic, cholecystectomy could be performed successfully, with no problems. van Zundert et al.[11] also performed a feasibility study in 20 patients, using the CSE technique in the lower thoracic region, and concluded that segmental spinal anesthesia can be used successfully and effectively in laparoscopic surgery in healthy patients. Imbelloni et al.[25] performed thoracic spinal anesthesia in 636 patients subjected to different types of open and laparoscopic surgeries, they concluded that thoracic spinal anesthesia decreases latency time, motor block and cardiovascular changes.
Small amounts of local anesthetic injected in the subarachnoid space causes minimal hemodynamic changes. The level of hypotension is related to the level of the blockade. Hypotension is a frequent side-effect that can be seen in approximately 30% of patients during spinal anesthesia.[26] In this study, there were significant hypotension and bradycardia occurred after induction of anesthesia in Group S (20%), which might be due to relatively larger dose of local anesthetic (2 ml of hyperbaric bupivacaine). Paraesthesias occurred in two patients (6.6%) in this study, the frequency of paraesthesias was lower than the reported frequency of 13.6%.[27] A study of 300 patients undergoing thoracic spinal puncture reported a 6.6% incidence of paresthesia without neurological sequelae[28] similar to that found in this manuscript (6.6%). The paresthesias were transient and disappeared in the immediate postoperative period. There were no cases of neurological sequelae in all patients of Group S. Regional block has a lower incidence of nausea and vomiting, when compared with general anesthesia, which has been demonstrated in several procedures and studies.[29]
In the present study, the incidence of postoperative nausea and vomiting was only 10% in Group S, while 50% in Group G. This frequency in the general anesthesia group is comparable to that reported in other studies that used only general anesthesia and in those comparing general anesthesia and regional block.
The length of stay in the recovery room and in the hospital was shorter in Group S, together with better analgesia and patient satisfaction, when compared with Group G. The shorter PACU stay was proved in another study comparing recovery time between two groups of patients (one group received thoracic spinal anesthesia, and the other received general anesthesia) subjected to laparoscopic cholecystectomy.[30]
CONCLUSION
Single-dose segmental thoracic spinal anesthesia compared with general anesthesia was an adequate option for open abdominal surgeries in high-risk patients. Among its advantages, shorter recovery time, better patient satisfaction, better postoperative pain and lower incidence of nausea and vomiting with the consequent early hospital discharge.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Marik PE. Propofol: Therapeutic indications and side-effects. Curr Pharm Des. 2004;10:3639–49. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 2.Mingus ML. Recovery advantages of regional anesthesia compared with general anesthesia: Adult patients. J Clin Anesth. 1995;7:628–33. doi: 10.1016/0952-8180(95)00157-3. [DOI] [PubMed] [Google Scholar]
- 3.Rashiq S, Gallant B, Grace M, Jolly DT. Recovery characteristics following induction of anaesthesia with a combination of thiopentone and propofol. Can J Anaesth. 1994;41:1166–71. doi: 10.1007/BF03020655. [DOI] [PubMed] [Google Scholar]
- 4.Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: Results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 6.Hamad MA, El-Khattary OA. Laparoscopic cholecystectomy under spinal anesthesia with nitrous oxide pneumoperitoneum: A feasibility study. Surg Endosc. 2003;17:1426–8. doi: 10.1007/s00464-002-8620-5. [DOI] [PubMed] [Google Scholar]
- 7.McLain RF, Kalfas I, Bell GR, Tetzlaff JE, Yoon HJ, Rana M. Comparison of spinal and general anesthesia in lumbar laminectomy surgery: A case-controlled analysis of 400 patients. J Neurosurg Spine. 2005;2:17–22. doi: 10.3171/spi.2005.2.1.0017. [DOI] [PubMed] [Google Scholar]
- 8.Nakano M, Matsuzaki M, Narita S, Watanabe J, Morikawa H, Murata H, et al. Comparison of radical retropubic prostatectomy under combined lumbar spinal and epidural anesthesia with that under combined general and epidural anesthesia. Nihon Hinyokika Gakkai Zasshi. 2005;96:11–6. doi: 10.5980/jpnjurol1989.96.11. [DOI] [PubMed] [Google Scholar]
- 9.Robertson HJ, Smith RD. Cervical myelography: Survey of modes of practice and major complications. Radiology. 1990;174:79–83. doi: 10.1148/radiology.174.1.2294575. [DOI] [PubMed] [Google Scholar]
- 10.Jonnescu T. Remarks on general spinal anesthesia. Br Med J. 1909;2:1935. doi: 10.1136/bmj.2.2550.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Zundert AA, Stultiens G, Jakimowicz JJ, Peek D, van der Ham WG, Korsten HH, et al. Laparoscopic cholecystectomy under segmental thoracic spinal anaesthesia: A feasibility study. Br J Anaesth. 2007;98:682–6. doi: 10.1093/bja/aem058. [DOI] [PubMed] [Google Scholar]
- 12.van Zundert AA, Stultiens G, Jakimowicz JJ, van den Borne BE, van der Ham WG, Wildsmith JA. Segmental spinal anaesthesia for cholecystectomy in a patient with severe lung disease. Br J Anaesth. 2006;96:464–6. doi: 10.1093/bja/ael036. [DOI] [PubMed] [Google Scholar]
- 13.Kurata J. Anesthetic mechanisms revealed by functional brain imaging] Masui. 2011;60:566–73. [PubMed] [Google Scholar]
- 14.Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: The role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110:1636–43. doi: 10.1213/ANE.0b013e3181de0ab6. [DOI] [PubMed] [Google Scholar]
- 15.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kettner SC, Willschke H, Marhofer P. Does regional anaesthesia really improve outcome? Br J Anaesth. 2011;107(Suppl 1):i90–5. doi: 10.1093/bja/aer340. [DOI] [PubMed] [Google Scholar]
- 17.Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;4:CD000521. doi: 10.1002/14651858.CD000521.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Luger TJ, Kammerlander C, Gosch M, Luger MF, Kammerlander-Knauer U, Roth T, et al. Neuroaxial versus general anaesthesia in geriatric patients for hip fracture surgery: Does it matter? Osteoporos Int. 2010;21:S555–72. doi: 10.1007/s00198-010-1399-7. [DOI] [PubMed] [Google Scholar]
- 19.Frumin MJ, Schwartz H, Burns J, Brodie BB, Papper EM. Dorsal root ganglion blockade during threshold segmental spinal anesthesia in man. J Pharmacol Exp Ther. 1954;112:387–92. [PubMed] [Google Scholar]
- 20.Imbelloni LE, Quirici MB, Ferraz Filho JR, Cordeiro JA, Ganem EM. The anatomy of the thoracic spinal canal investigated with magnetic resonance imaging. Anesth Analg. 2010;110:1494–5. doi: 10.1213/ANE.0b013e3181d5aca6. [DOI] [PubMed] [Google Scholar]
- 21.Imbelloni LE, Gouveia MA. Low Incidence of neurologic complications during thoracic epidurals: Anatomic explanation. AJNR Am J Neuroradiol. 2010;31:E84. doi: 10.3174/ajnr.A2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee RA, van Zundert AA, Breedveld P, Wondergem JH, Peek D, Wieringa PA. The anatomy of the thoracic spinal canal investigated with magnetic resonance imaging (MRI) Acta Anaesthesiol Belg. 2007;58:163–7. [PubMed] [Google Scholar]
- 23.Hogan QH, Prost R, Kulier A, Taylor ML, Liu S, Mark L. Magnetic resonance imaging of cerebrospinal fluid volume and the influence of body habitus and abdominal pressure. Anesthesiology. 1996;84:1341–9. doi: 10.1097/00000542-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Hogan Q. Size of human lower thoracic and lumbosacral nerve roots. Anesthesiology. 1996;85:37–42. doi: 10.1097/00000542-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Imbelloni LE, Grigorio R, Fialho JC, Fornasari M, Pitombo PF. Thoracic spinal anesthesia with low doses of local anesthetics decreases the latency time, motor block and cardiovascular changes. Study in 636 patients. Anesthe Clinic Res. S11:001, doi:10.4172/2155-6148. [Google Scholar]
- 26.Casati A, Fanelli G, Berti M, Beccaria P, Agostoni M, Aldegheri G, et al. Cardiac performance during unilateral lumbar spinal block after crystalloid preload. Can J Anaesth. 1997;44:623–8. doi: 10.1007/BF03015446. [DOI] [PubMed] [Google Scholar]
- 27.Pong RP, Gmelch BS, Bernards CM. Does a paresthesia during spinal needle insertion indicate intrathecal needle placement? Reg Anesth Pain Med. 2009;34:29–32. doi: 10.2097/AAP.0b013e3181933eed. [DOI] [PubMed] [Google Scholar]
- 28.Imbelloni LE, Pitombo PF, Ganem EM. The incidence of paresthesia and neurologic complications after lower spinal thoracic puncture with cut needle compared to pencil point needle study in 300 patients. J Anesth Clin Res. 2010;1:106. [Google Scholar]
- 29.Borgeat A, Ekatodramis G, Schenker CA. Postoperative nausea and vomiting in regional anesthesia: A review. Anesthesiology. 2003;98:530–47. doi: 10.1097/00000542-200302000-00036. [DOI] [PubMed] [Google Scholar]
- 30.Ellakany M. Comparative study between general and thoracic spinal anesthesia for laparoscopic cholecystectomy. Egypt J Anaesth. 2013;29:375–81. [Google Scholar]


