Abstract
Eisenmenger's physiology has significant anesthetic implications. The symptamology, in the early course of disease can be subtle at times and missed on regular PAC. Pulse oximetry, in our patient detected differential saturations. The possibility of underlying congenital cardiac illness was assumed, rescheduling of case was debated and finally the abnormal cardiac lesions were identified in ECHO in immediate postoperative period.
Keywords: Cleft lip-palate, congenital heart disease, differential cyanosis, eisenmenger
INTRODUCTION
Anesthesiologists who routinely do cleft surgeries, do encounter children with associated cardiac diseases. The incidence of cardiovascular malformations in cleft lip and palate (CL/P) has been estimated to be 16-fold higher than that in the general pediatric population.
The association of congenital heart disease with CL/P patients has been studied by Liang et al.[1] in 1148 children. The overall prevalence was 5.4% with atrial septal defect (ASD) and ventricular septal defect (VSD) defects being the two most common cardiac anamolies.
A recently published review[2] in 329 cleft patients had addressed the frequency and distribution of specific cardiovascular malformations in CL/P patients. Their incidence of cardiac involvement is 10% with cardiac septal defects (91%), followed by aberrant or defective structures in central vasculature (58%) and valvular malformation (24%).
Eisenmenger syndrome is an uncommon complication of many congenital heart diseases, simple such as VSD and ASD, as well as complex, such as single ventricle.
Apart for individual case reports[3,4] of anesthetic management of these patients for caesarean section, pubmed search revealed a review[5] of 48 patients with eisenmengers physiology for non-cardiac surgery (NCS), none posted for CL/P procedure. A recent case series[6] highlighted the usability of dexmeditomidine – ketamine for inducing 5 adolescents for NCS other than cleft reparative surgery, without causing any additional morbidity. As per the existing literature on facial clefts with associated cardiovascular malformation, the present case report should be the first published article of eisenmenger physiology for palate surgery. Cleft surgeries are performed earlier in life and hence, the possibility of increased pulmonary vascular resistance (PVR) and subsequent shunt reversal is virtual prior to this surgical exercise. Our patient also, had previous two cleft surgeries at a younger age which were completely uneventful. There was in addition no history suggestive of cardiac involvement in the pre anesthetic evaluation. However, once inside the theater, his saturations in toes were less than in fingers (85 vs. 97%). The possibility of either a preductal coarctation of aorta with patent ductus arteriosus (PDA) or a Persistent ductus arteriosus with pulmonary hypertension were assumed. We discussed the option of deferring the case for a diagnostic ECHO with the surgical colleague. The need to perform cleft surgery prior to cardiac surgery to improve the general well-being of the child was also obvious to us. Hence, we tailored our anesthetic to child's clinical condition. He was induced with fentanyl (1.5 ug/kg) and thiopentone (3 mg/kg) and intubated with vecuronium (0.1 mg/kg) and maintained with 100% O2 with neostigmine (0.5 mg/kg) and glycopyrrolate (20 ug/kg). Except for a single episode of first degree atrioventricular (AV) block, which responded to atropine just before reversal, the surgery was uneventful. The child was shifted to cardiac lab for diagnostic echocardiography (ECHO) under anesthesia supervision immediately in post-operative period. The diagnosis was large VSD, large ASD with PDA with bidirectional shunt. He was discharged on day 3 with appropriate instructions.
CASE REPORT
Our institute is a recognized center for Smile Train program and annually, we anesthetise approximately 500 cases for CL/P procedures. This particular child was fifth on the list, wheeled inside the theatre at 1430 h and posted for redopalatoplasty for a small palatal fistula. He was fasting since 0400 h. The anesthetic plan was General anesthesia with cuffed south polar Ring-Adair-Elwyn (RAE) tube. An intravenous (i.v) line with Ringer lactate was started in pre-operative holding area. In the theater, he was attached to Datex Ohmeda S5 monitor and premedicated with 0.1 mg glycopyrrolate and 30 ug fentanyl i.v. His heart rate was 73 regular, blood pressure 104/47 mmHg and SpO2 read 85% on right lower limb first toe with good plethismographic waveform. We reapplied the probe to right upper limb index finger. It read 97%. We repeated probe at lower limb, arranged another oximeter and the differential saturations were evident [Figure 1]. The case was discussed with surgeons and possibility of PDA with pulmonary hypertension or preductal coarctation of aorta with PDA was thought of. The need for an urgent ECHO was debated as previous two anesthetic exposures for primary lip and palate surgery at 4 and 9 years of age were uneventful. There was no heart murmur, no additional heart sounds, no parasternal heave presently. Although, a grade 2 clubbing in the toes was noted now which was incidentally missed in initial evaluation. We took the decision to anesthetise the child and induced with 60 mg thiopentone and intubated with 5.5 cuffed RAE tube after administration of 2 mg vecuronium i.v. The anesthesia was maintained with 0.6% isoflurane in 100% O2 at a tidal volume of 200 ml and a respiratory rate of 18/min. Antibiotic prophylaxis with novaclox 250 mg and amikacin 125 mg was given. In the meantime, another anesthetist interviewed the parents again. The mother told about two major respiratory infections during past 1 year, which were managed with antibiotics from a local practitioner. History of leg claudication pain was denied. He was a schoolgoing child and used to participate in outdoor games. The surgeons infilterated the palate with 1% lignocaine without adrenaline. The surgery was over in 45 min. Anesthesia was reversed with 1.25 mg neostigmine and 0.2 mg glycopyrrolate after clinical observation of good spontaneous respiratory efforts and trachea was extubated. During the extubation process, he developed first degree AV conduction block on electrocardiography which responded to 0.2 mg atropine i.v. Under anesthesia supervision, child was shifted to ECHO room for a detailed report on the cardiac anamoly. The cardiologist reported a large ASD, VSD and PDA with bidirectional shunt without coarctation of aorta. He was advised transesophageal ECHO at a later visit and started on oral diltiazem. Child was discharged from hospital on the post-operative day 3 with appropriate instructions.
Figure 1.
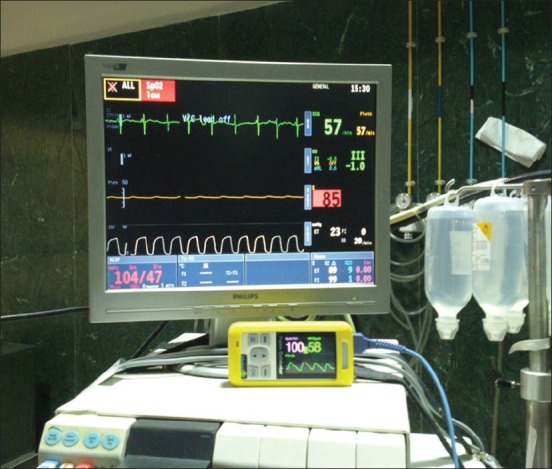
Differential saturations on two oximeters
DISCUSSION
Mostly, the cyanotic congenital heart diseases are evident in earlier childhood and a palliative or corrective cardiac surgery is undertaken. Whereas, Acyanotic congenital heart diseases may be relatively asymptomatic for few years of life due to the balance between the systemic and pulmonary circulation. As the PVR increases over years with reversal in shunt direction, the patients develops Cyanotic congenital heart disease. The difficulty to access surgical facility and non-recognition of cardiac problem in childhood can be the two main reasons of Grown up congenital heart diseases, left untreated or partially treated. These patients are increasingly being presented to anesthesiologists for NCS. The safety profile of anesthetic drugs and procedures has significantly improved and anaesthesiologists are now more and more getting regarded as Perioperative physicians. Pre-operative investigations are getting need based. Patients are being managed with anesthesia tailor made to a specific situation rather than pushing the patient for extensive and expensive investigations and thereafter, doing the same anesthetic as otherwise.
Eisenmenger's syndrome is defined as obstructive pulmonary vascular disease that develops as a consequence of a large pre-existing left to right shunt causing pulmonary artery pressures to increase and approach systemic levels, such that the direction of blood flow then becomes bidirectional or Right to Left. Differential cyanosis, meaning cyanosis and clubbing of the lower extremities with normal upper extremity nailbeds, is diagnostic of PDA with pulmonary hypertension. Desaturated blood from the ductus enters the aorta distal to the left subclavian artery, sparing the brachiocephalic circulation. The oral mucosa and tongue receive saturated blood and are not cyanotic. In some cases, ductal flow will enter the left subclavian artery and cause clubbing of the left hand as well as the feet.
This patient had a large ductus arteriosus with VSD and ASD along with PVR exceeding systemic resistance. The aortic and pulmonary arterial pressures are almost identical, but the course of least resistance for the pulmonary arterial blood is through the ductus to the descending aorta, as in fetal circulation. The diagnosis of PDA can be challenging in the patient with Eisenmenger syndrome, when systemic level pulmonary artery pressure effectively abolishes the usual “machinery” murmur. Differential clubbing and cyanosis is a clue to the diagnosis of PDA related to Eisenmenger syndrome.
A heart murmur is moderately sensitive for detecting cardiovascular malformation. In the absence of murmur, the cleft team should be vigilant of other signs of cardiac disease, such as cyanosis or failure to thrive. Our patient was an active school going child. Apart from stunted growth, he had no other obvious cardiac morbidity. Ammash et al.[5] have proposed that NCS can be undertaken in eisenmenger population without substantial morbidity and with current anesthetic techniques, risk of death is less than previously thought. Diller et al.[7] studied 171 pediatric patients of Eisenmengers physiology and concluded that functional class, signs of heart failure, history of clinical arrhythmias, QRS duration and QTc interval and low serum albumin and potassium levels are pointers to increased morbidity. Perioperative risk and outcome depends on urgency, duration of surgery, anesthesia used and underlying pathology.
The requirements of anesthesia in this subgroup of patients are illustrated in Figure 2. The response to O2 administration can be a reliable guide to assess the pulmonary vasculature, a fall in PVR with an increase in saturations being a positive sign. There are limited data[8] on which to base a definition of a safe upper limit for PVR when considering surgical repair, although <6 Wood units after vasoreactivity testing is generally accepted. Single lung transplantation or Heart-lung transplantation is definitive treatment in later stages.
Figure 2.
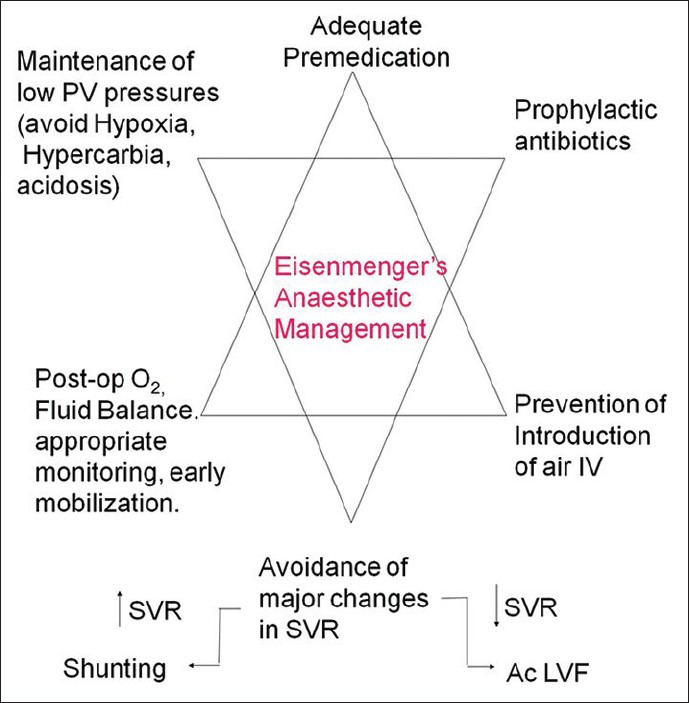
Anesthetic challenges in eisenmengers syndrome
CONCLUSION
Pulse oximetry has proved beneficial in detecting congenital cardiac problems, especially the cyanotic ones. ASA endorses a continuous oxygen saturation monitoring for each anesthetic. A step forward could be inclusion of pulse oximetry in PAC clinics. As far as possible, it is essential to recognize a cardiac comorbidity in cleft patients pre-operatively.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Liang CD, Huang SC, Lai JP. A survey of congenital heart disease in patients with oral clefts. Acta Paediatr Taiwan. 1999;40:414–7. [PubMed] [Google Scholar]
- 2.Harry BL, TeBockhorst S, Deleyiannis FW. The impact of congenital cardiovascular malformations on the assessment and surgical management of infants with cleft lip and/or palate. Cleft Palate Craniofac J. 2013;50:323–9. doi: 10.1597/12-131. [DOI] [PubMed] [Google Scholar]
- 3.Solanki SL, Vaishnav V, Vijay AK. Non Cardiac Surgery in a Patient with Eisenmenger Syndrome-Anaesthesiologist's Challenge. J Anaesthesiol Clin Pharmacol. 2010;26:539–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Collins-Nakai RL, Rabinovitch M. Pulmonary vascular obstructive disease. Cardiol Clin. 1993;11:675–87. [PubMed] [Google Scholar]
- 5.Ammash NM, Connolly HM, Abel MD, Warnes CA. Noncardiac surgery in Eisenmenger syndrome. J Am Coll Cardiol. 1999;33:222–7. doi: 10.1016/s0735-1097(98)00554-3. [DOI] [PubMed] [Google Scholar]
- 6.Goyal R, Singh S, Bangi A, Singh SK. Case series: Dexmedetomidine and ketamine for anesthesia in patients with uncorrected congenital cyanotic heart disease presenting for non-cardiac surgery. J Anaesthesiol Clin Pharmacol. 2013;29:543–6. doi: 10.4103/0970-9185.119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diller GP, Dimopoulos K, Broberg CS, Kaya MG, Naghotra US, Uebing A, et al. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: A combined retrospective and case-control study. Eur Heart J. 2006;27:1737–42. doi: 10.1093/eurheartj/ehl116. [DOI] [PubMed] [Google Scholar]
- 8.Gatzoulis MA. The management of Eisenmenger syndrome in the modern treatment era: A case report. Eur Respir Rev. 2011;20:293–6. doi: 10.1183/09059180.00006211. [DOI] [PMC free article] [PubMed] [Google Scholar]


