Abstract
We report on a challenging case of a 34-year-old male patient with giant cell myocarditis (GCM) and fulminant relapse after discontinuing immunomodulatory therapy 2 years after the initial event. Specific combined immunosuppressive therapy with antithymocyte globulin (ATG), cyclosporine and high-dose glucocorticoids combined with guideline-based heart failure medication led to the recovery of GCM, improvement of systolic left ventricular function and clinical remission. This case report emphasises the importance of an immunosuppressive therapy for the prognosis and outcome and the risk of discontinuation. Most importantly, ATG seems to be one new possible potential treatment option for patients with acute GCM.
Background
Giant cell myocarditis (GCM) is a rare and fulminant cardiac disease which mostly affects young patients presenting with congestive heart failure, arrhythmias, heart block and a rapidly fatal progression.1 Histopathological findings and immunohistochemistry reveal multinucleated giant cells, multifocal interstitial infiltrations of T-lymphocytes and often necrosis followed by fibrosis.2 For diagnosing GCM taking endomyocardial biopsies (EMB) early is essential. Only after that, immunosuppressive treatment, which is important for outcome, can be started.
Case presentation
In January 2011, a 32-year-old man was admitted after successful cardiopulmonary resuscitation because of ventricular fibrillation. Laboratory findings showed elevated cardiac markers as well as D-dimers. The following coronary angiography excluded obstructive coronary artery disease and CT excluded aortic dissection and/or fulminant pulmonary embolism. Estimated ejection fraction (EF; laevocardiography and echocardiography) was reduced to 45%. ECG showed an atrioventricular (AV) block I and a right bundle branch block, followed later by an AV block II. After clinical recovery from the resuscitation but still NYHA III, further tests including cardiac MRI led to the suspected diagnosis of acute myocarditis. After episodes of Torsade de pointes tachycardia, an electrophysiological study led to the implantation of a cardioverter defibrillator. EMB procedure from the right ventricle was performed but initially revealed no pathological findings. Since myocarditis was likely with acute cardiac failure and arrhythmias (all known to be associated with GCM) and no other cause for ventricular fibrillation with ongoing Torsade de pointes could be found, another EMB procedure was performed from the left ventricle (LV) during the hospital stay. The second EMB procedure revealed fulminant GCM with T-lymphocyte infiltration and giant cells. Thus, an effective immunosuppressive regimen with cyclosporine (120–140 µg/L) and methylprednisone (weight-adapted) was started, combined with conventional heart failure therapy. At this point, echocardiography showed reduced systolic LV function (EF 47%), which increased gradually in the following month after the beginning of medical therapy to normal values (up to EF 62%).
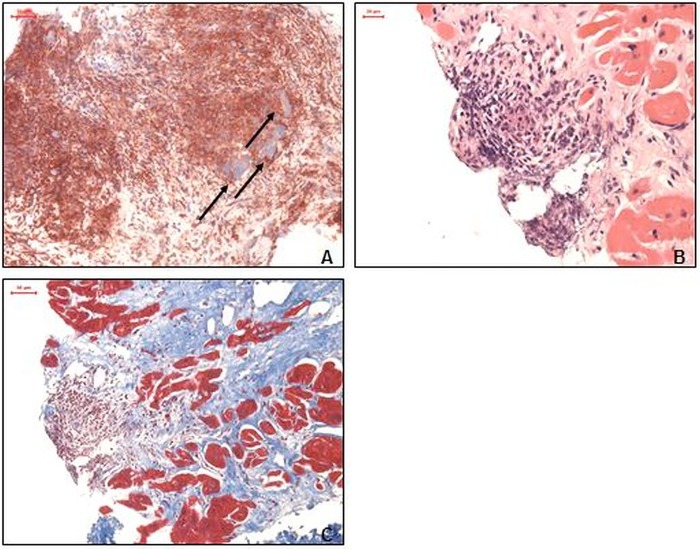
After 2 years of mono-immunosuppressive therapy with cyclosporine, the patient remained asymptomatic and in a stable sinus rhythm. The immunomodulatory treatment was discontinued after 2 years due to side effects leading to a fatal relapse with acute cardiogenic shock, third degree AV block and deterioration of the LV systolic function. Recurrence of GCM was confirmed by a LV EMB procedure with giant cells and highly increased inflammatory cell infiltration. Severe fibrosis could also be documented in the LV (figure 1A–C). AV block III aggravated the clinical situation and therapy with orciprenalin and dobutamin was started to treat cardiogenic shock with organ failure.
Figure 1.
Endomyocardial biopsies after relapse of giant cell myocarditis. (A) Proof of giant cells and immunohistological staining of extensive macrophages (CD11b) infiltration (magnification ×100). (B) H&E staining with massive cellular infiltration close to the endocardium (magnification ×100). (C) Azan staining with a significantly increased extracellular matrix (blue; magnification ×200).
Treatment
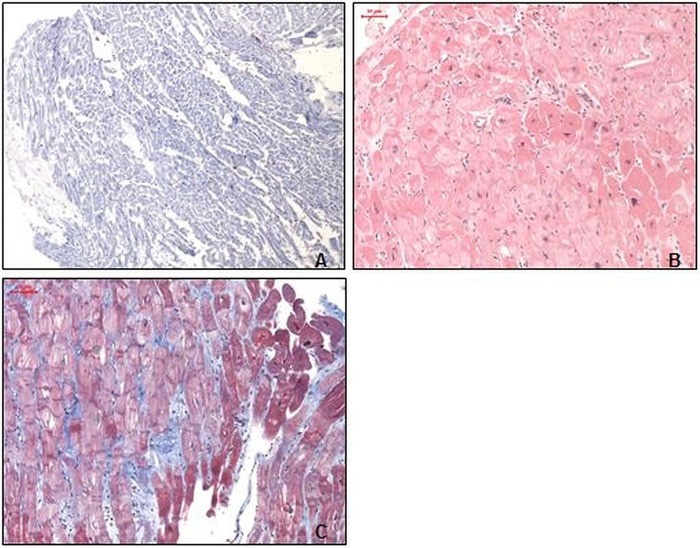
We initiated an immunomodulatory therapy with the alternative immunosuppressive agent antithymocyte globulin (ATG) and intravenous steroids combined with congestive heart failure therapy immediately. After 3 days of successful ATG therapy, cyclosporine (5 days dosage of 150 mg/day) was added and steroids oralised (methylprednisone 100 mg/day) according to protocols by Cooper et al.3 After 2 months of immunosuppressive therapy, the follow-up visit and additional EMB showed recovery without giant cells but severe fibrosis (figure 2A–C). Methylprednisone was reduced weekly in the following month (6 mg/day maintenance dose), and cyclosporine levels (150 µg/L) were maintained.
Figure 2.
Left ventricular endomyocardial biopsies after antithymocyte globulin treatment. (A) Normally distributed immunohistological staining of extensive macrophages (CD11b) infiltration (magnification ×100). (B) H&E staining with a largely inconspicuous myocardium, without infiltration of the endocardium (magnification ×100). (C) Azan staining with only a slightly increased extracellular matrix (blue; magnification ×100).
Outcome and follow-up
The patient has remained stable with no further relapse for half a year. Follow-up echocardiography revealed improvement of the LV systolic function as well as improvement of clinical symptoms.
Discussion
As presented in this case report, GCM is a cause for severe myocarditis associated with possible fatal outcome. GCM is an endomyocardial biopsy-guided diagnosis.4 Thus, EMB should be considered early in young patients with a sudden onset of congestive heart failure without other cause. However, false-negative EMB, as presented in this case, is not unusual because of a possible sampling error of any diagnostic test.5 Hence, a second EMB procedure should be considered in patients with highly suspected GCM, especially since EMB is associated with low risk. Growing evidence is accumulating that combined immunosuppressive treatment is the most important therapy to improve prognosis and outcome after GCM.6 There is a lack of data regarding the duration of immunosuppressive therapy. Complete withdrawal of immunosuppression, as shown in this case report, should be avoided or critically discussed because of the risk of fulminant recurrence of GCM, which is life threatening. The multicentre GCM Registry regimes and Kandolin et al7 showed that immunosuppressive regimens with cyclosporine, prednisone, azathioprins, etc. may improve clinical symptoms and progression of GCM. Some immunomodulatory regimes as steroids and/or cyclosporine are established. Other clinicalreports also suggest treatment with tacrolimus and with ATG, which is directed against T-cells.8 This case highlights the effectiveness of ATG as also shown recently by Grabmaier et al9 in acute heart failure therapy in GCM. While heart transplantation as a curative therapy should be considered in all cases without improvement of symptoms, life-threatening post-transplantation recurrence is not unusual. Several reports reveal a recurrence of GCM up to 20%.10 Also bridge to transplant with a ventricular assist device should be taken in consideration in fulminate cases, which do not improve despite medical therapy.11
Learning points.
This case report emphasises that discontinuation of immunosuppressive therapy reveals a risk for acute relapse of giant cell myocarditis (GCM).
Larger multicentre studies are needed to investigate different immunosuppressive regimens and the additional role of antithymocyte globulin (ATG) in GCM therapy protocols.
ATG seems to be one possible potential treatment option for patients with acute GCM.
Early diagnosis of GCM and individual surveillance is needed to recognise fatal recurrence.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cooper LT, Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis—natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med 1997;336:1860–6 [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ, Pomerance A, Teare RD. Idiopathic giant cell myocarditis—a distinctive clinico-pathological entity. Br Heart J 1975;37:192–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper LT, Jr, Hare JM, Tazelaar HD, et al. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol 2008;102:1535–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007;116:2216–33 [DOI] [PubMed] [Google Scholar]
- 5.Lassner D, Siegismund S, Stehr J, et al. Recent advances in molecular diagnostics and treatment of heart muscle diseases—from biopsy-focused to systemic diagnostics. J Anal Sci Methods Instrum 2013;3:89–109 [Google Scholar]
- 6.Cooper LT, Jr, Shabetai R. Giant Cell Myocarditis Study Group. Am Heart J 1995;130:1312. [DOI] [PubMed] [Google Scholar]
- 7.Kandolin R, Lehtonen J, Salmenkivi K, et al. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail 2013;6:15–22 [DOI] [PubMed] [Google Scholar]
- 8.Menghini VV, Savcenko V, Olson LJ, et al. Combined immunosuppression for the treatment of idiopathic giant cell myocarditis. Mayo Clin Proc 1999;74:1221–6 [DOI] [PubMed] [Google Scholar]
- 9.Grabmaier U, Brenner C, Methe H, et al. An alternative immunosuppressive regimen to prolong transplant free survival in a patient with giant cell myocarditis. Int J Cardiol 2013;168:27–8 [DOI] [PubMed] [Google Scholar]
- 10.Cooper DK, Schlesinger RG, Shrago S, et al. Heart transplantation for giant cell myocarditis. J Heart Lung Transplant 1994;13:555. [PubMed] [Google Scholar]
- 11.Murray LK, Gonzalez-Costello J, Jonas SN, et al. Ventricular assist device support as a bridge to heart transplantation in patients with giant cell myocarditis. Eur J Heart Fail 2012;14:312–18 [DOI] [PubMed] [Google Scholar]