Abstract
Atherosclerosis is a chronic inflammatory disease of the vasculature. There are various methods to study the inflammatory compound in atherosclerotic lesions. Mouse models are an important tool to investigate inflammatory processes in atherogenesis, but these models suffer from the phenotypic and functional differences between the murine and human immune system. In vitro cell experiments are used to specifically evaluate cell type-dependent changes caused by a substance of interest, but culture-dependent variations and the inability to analyze the influence of specific molecules in the context of the inflammatory compound in atherosclerotic lesions limit the impact of the results. In addition, measuring levels of a molecule of interest in human blood helps to further investigate its clinical relevance, but this represents systemic and not local inflammation. Therefore, we here describe a plaque culture model to study human atherosclerotic lesion biology ex vivo. In short, fresh plaques are obtained from patients undergoing endarterectomy or coronary artery bypass grafting and stored in RPMI medium on ice until usage. The specimens are cut into small pieces followed by random distribution into a 48-well plate, containing RPMI medium in addition to a substance of interest such as cytokines or chemokines alone or in combination for defined periods of time. After incubation, the plaque pieces can be shock frozen for mRNA isolation, embedded in Paraffin or OCT for immunohistochemistry staining or smashed and lysed for western blotting. Furthermore, cells may be isolated from the plaque for flow cytometry analysis. In addition, supernatants can be collected for protein measurement by ELISA. In conclusion, the presented ex vivo model opens the possibility to further study inflammatory lesional biology, which may result in identification of novel disease mechanisms and therapeutic targets.
Keywords: Medicine, Issue 87, ex vivo model, human, tissue culture, atherosclerosis, immune response, inflammation, chronic inflammatory disease
Introduction
Atherosclerosis as a chronic inflammatory disease is one of main causes of death in industrialized nations1-2. Complications of atherosclerosis, especially acute coronary syndromes, have been linked to rupture of vulnerable lesions, causing atherothrombosis and vessel occlusion3. Innate and adaptive immunity seem to be involved during all steps of atherogenesis2,4-5. Although significant progress has been made in the treatment of myocardial infarction, effective prevention of atherosclerosis and adverse cardiovascular events are still unresolved. Thus, studying lesional biology is essential for increasing our knowledge on the pathophysiology of atherosclerosis and to allow identification of novel therapeutic targets and development of novel therapies.
In many cases, murine models are used to investigate the pathophysiology of specific diseases. However, studying atherogenesis using mouse models is accompanied by several limitations: (1) Usually, atherosclerotic mice receive a high cholesterol diet. The cholesterol levels in these models cannot be compared with those in patients with elevated cholesterol serum levels6. (2) There are substantial differences between the murine and human immune system; thus foxp3 is a specific marker of murine regulatory T cells, whereas human foxp3 expression in human T cells does not necessarily confer a regulatory phenotype7. Also, the Th1/Th2 paradigm as defined in humans is not fully transferable to murine T cells. (3) A number of markers that are used to identify murine monocytes and macrophages such as F4/80 and markers of classical (M1) vs. alternative (M2) activation patterns does not exist in human myeloid cells8. (4) Gene expression of murine and human peripheral blood monocytes has been found to be substantially different9.
Thus, in order to increase our understanding of chronic inflammatory processes in human atherosclerosis, we need to make use of models working with human tissues, blood or cells. Here, we describe a model of human plaque tissue culture, which allows investigation of potential novel substances in the concept of human inflammatory lesional biology.
Protocol
1. Prepare medium as follows
Culture Medium: RPMI medium.
Add 10% fetal calf serum (FCS).
Add 100 U/ml penicillin G, and 100 g/ml streptomycin.
2. Storage of fresh plaque cylinder until use
The carotid endarterectomy operation of patients with or without ischemic symptoms (stroke, transitory ischemic attack) with a significant carotid artery stenosis will be done by vascular surgeons and coronary artery endarterectomy during coronary artery bypass grafting by heart surgeons. Carotid / coronary plaques need to be removed en bloc to preserve the plaque structure as described previously5.
After plaque extirpation place the specimen in a medium filled tube and store it on ice (plaque needs to be completely covered with medium) until use.
3. Plaque processing
Use an adequate cell culture dish (e.g. 60 mm) and add 5 ml RPMI medium.
Place the plaque tissue into the culture dish (plaque should be completely covered with medium).
Hold the plaque tissue carefully at the edges of the tissue by using sterile forceps.
Cut off the edges of the plaque sample by using a sterile scalpel.
Divide plaque tissue in half.
Assess the lesion morphology macroscopically (calcified, lipid rich, ruptured, thrombus, fibrosis).
Analyze the exact plaque morphology after the ex vivo experiment by immunohistochemistry. Use the AHA classification 10.
Discard plaques with severe calcification or fibrosis.
Cut the plaque tissue into homologous small pieces (3 x 3 x 3 mm).
Shock freeze two plaque pieces and store them in liquid nitrogen for basic values of the lesion until use.
Prepare a 48-well plate.
Add 500 µl RPMI medium to each well used for the experiment.
Please use at least two plaque pieces for each group.
Add the substance of interest (e.g. specific cytokines and chemokines).
Use unstimulated plaque pieces as controls.
Randomly plant the appropriated number of plaque pieces into the wells.
Culture the plaque pieces for indicated time points.
- For the plaque tissue stimulation experiment with Lipopolysaccharide (LPS)
- Use the following time points: 3 hr, 8 hr and 24 hr.
- Use 2 plaque pieces for the LPS and two for the unstimulated group for each time point respectively.
- Add 1 µg/ml of Lipopolysaccharide.
During the incubation, maintain the 48-well plate at 37 °C in humidified air containing 5% CO2.
4. After indicated time harvest plaque tissue pieces
Shock freeze the plaque pieces for mRNA isolation and cDNA synthesis (for detailed information-see protocol section 5).
Collect the supernatant and stored it at -20 °C for ELISA analysis.
For western blotting, smash and lyse plaque tissue. Filter the lysate through a 0.65 µm and 0.1 µm centrifugal filter device.
Embed plaque tissue in tissue-tec or paraffin for immunohistochemistry stainings.
5. RNA extraction from cultured plaque pieces
Use a TissueLyser for homogenization.
Isolate the RNA by using the kit (see materials table) according to manufacturer's instructions.
Determine the RNA quantity and quality of the samples with spectrophotometer.
Use the Boehringer cDNA kit for reverse transcription according to manufacturer's instructions.
For quantitative PCR, use for instance the Roche real-time PCR kit with SYBR Green.
Representative Results
Here we present a number of figures that demonstrate results of the ex vivo plaque culturing. To assess changes in the inflammatory milieu in response to the agent of interest in the ex vivo model experiment, we measure different molecules which are known to be primarily involved in atherogenesis. As representative pro-atherogenic cytokines we choose TNFa, IL6 and IFNg2,11. In addition, we use von Willebrand factor and tissue factor to evaluate pro-thrombotic changes. Furthermore, to address the development of plaque instability induced by an agent of interest, matrix metallopeptidase 9 (MMP9) levels will be analyzed. Chemoattractance is also an important step in plaque progression and regression11, thus, we investigate the levels of CCL2, also referred to as monocyte chemotactic protein-1 (MCP-1), principle chemoattractant for monocytes/macrophages. After cell attraction cell rolling and adhesion are the following steps. Because of that we choose ICAM-1. By further analyzing the signaling pathways influenced by the substance of interest we use extracellular-signal-regulated kinases (ERK1/2), widely expressed and activated by many different stimuli such as cytokines, apoptosis, differentiation, virus infection and ligands for heterotrimeric G protein-coupled receptors12.
First, we show time dependent changes of the inflammatory compound of unstimulated cultured atherosclerotic lesions, analyzed by quantitative real time-polymerase chain reaction (qRT-PCR)(Figure 1). During plaque culturing, an increase of the activity of the inflammatory lesional milieu can be observed. No difference was found after 3 hr vs. no culture (data not shown). After 8 hr of plaque culture, we found only a slight upregulation of some molecules such as IL6, but not of TNFa and IFNg, whereas after 24 hr, there was an upregulation of various molecules such as TNFa, IL6 and IFNg. Notably, the longer the time of plaque culture the higher the variation of molecule expression are.
Second, to demonstrate the feasibility of influencing the inflammatory milieu in atherosclerotic lesions, we present results of plaque pieces incubated with 1 µg/ml LPS for 3 hr, measured by qRT-PCR (Figure 2). Unstimulated plaque pieces served as a negative control. We found that LPS induced a marked increase of the grade of activation of the inflammatory compound inside atherosclerotic lesions. Various cytokines (IL6, TNFa etc., Figure 2A-B), chemokines (CCL2 etc., not shown), adhesion (ICAM1, not shown), plaque destabilizing (Matrix metallopeptidase 9 (MMP9), not shown) and pro-thrombotic molecules (von Willebrand factor, Figure 2C, tissue factor, not shown) were up regulated by LPS incubation.
Third, to evaluate protein abundance, supernatants of cultured lesions were analyzed by ELISA and smashed plaque tissues by western blotting (Figure 3D). In Figure 3A-C, we show ELISA analysis of IFN-g, MCP-1 and TNF-a in the supernatant of cultured specimens incubated with LPS or control for 8 hr. In addition, western blot analysis for (phosphorylated) ERK 1/2 of smashed and lysated plaque tissues, either stimulated with LPS or control, is demonstrated in Figure 3D.
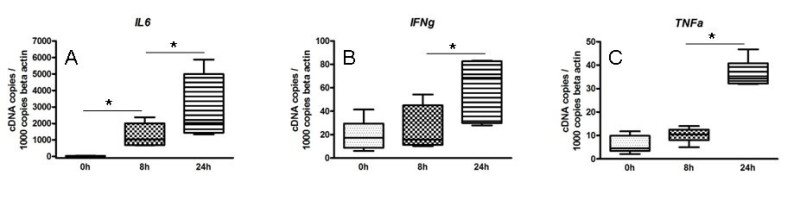 Figure 1. Effect of plaque culturing on the inflammatory lesional compound over time. Atherosclerotic plaque pieces were immediately shock frozen or cultured for 8 or 24 hr and expression of indicated cytokines IL6 (A), IFNg(B) and TNFa
(C) was analyzed by qRT-PCR. The results shown represent the average of five independent experiments. Five atherosclerotic lesion pieces for each group were used for indicated time point. Values are normalized to b-actin and expressed as cDNA copies / 1,000 b-actin copies. Results are shown as box plots displaying mean and 25th and 75th percentiles as boxes and 10th and 90th percentiles as whiskers. *: p < 0.01.
Figure 1. Effect of plaque culturing on the inflammatory lesional compound over time. Atherosclerotic plaque pieces were immediately shock frozen or cultured for 8 or 24 hr and expression of indicated cytokines IL6 (A), IFNg(B) and TNFa
(C) was analyzed by qRT-PCR. The results shown represent the average of five independent experiments. Five atherosclerotic lesion pieces for each group were used for indicated time point. Values are normalized to b-actin and expressed as cDNA copies / 1,000 b-actin copies. Results are shown as box plots displaying mean and 25th and 75th percentiles as boxes and 10th and 90th percentiles as whiskers. *: p < 0.01.
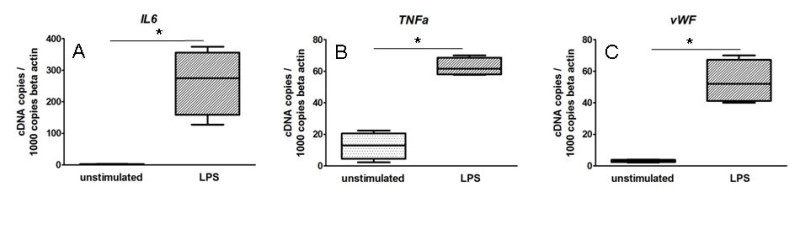 Figure 2. LPS stimulation of cultured plaque pieces induced an increase of various inflammatory molecules. Plaque pieces were incubated with 1 µg/ml LPS or unstimulated for 3 hr and analyzed by qRT-PCR. The results shown represent the average of five independent experiments. Two pieces for each group were used. Values are normalized to b-actin and expressed as cDNA copies / 1,000 b-actin copies. Results are shown as box plots displaying mean and 25th and 75th percentiles as boxes and 10th and 90th percentiles as whiskers. *: p < 0.01.
Figure 2. LPS stimulation of cultured plaque pieces induced an increase of various inflammatory molecules. Plaque pieces were incubated with 1 µg/ml LPS or unstimulated for 3 hr and analyzed by qRT-PCR. The results shown represent the average of five independent experiments. Two pieces for each group were used. Values are normalized to b-actin and expressed as cDNA copies / 1,000 b-actin copies. Results are shown as box plots displaying mean and 25th and 75th percentiles as boxes and 10th and 90th percentiles as whiskers. *: p < 0.01.
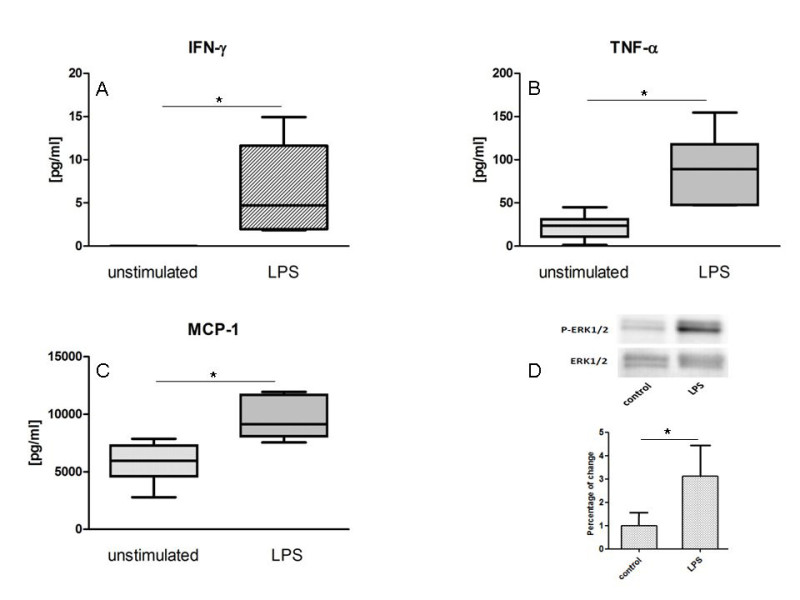 Figure 3. Protein expression in the supernatant of cultured plaque. Representative ELISA measurements of cytokines IFN-g (A), TNF-a (B) and MCP-1 (C) in the supernatant of plaque pieces treated with LPS or unstimulated for 8 hr are shown. The results shown represent the average of five independent experiments. Results are shown as box plots displaying mean and 25th and 75th percentiles as boxes and 10th and 90th percentiles as whiskers. In addition, representative western blotting for (phosphorylated) ERK 1/2 of LPS stimulated or control cultured atherosclerotic lesions after 3 hr is demonstrated in Figure 3D. The column bar graphs displaying mean + SEM as whiskers represent the average of five independent experiments. *: p < 0.01.
Figure 3. Protein expression in the supernatant of cultured plaque. Representative ELISA measurements of cytokines IFN-g (A), TNF-a (B) and MCP-1 (C) in the supernatant of plaque pieces treated with LPS or unstimulated for 8 hr are shown. The results shown represent the average of five independent experiments. Results are shown as box plots displaying mean and 25th and 75th percentiles as boxes and 10th and 90th percentiles as whiskers. In addition, representative western blotting for (phosphorylated) ERK 1/2 of LPS stimulated or control cultured atherosclerotic lesions after 3 hr is demonstrated in Figure 3D. The column bar graphs displaying mean + SEM as whiskers represent the average of five independent experiments. *: p < 0.01.
Discussion
Here we present an ex vivo plaque culture model to investigate the influence of potentially relevant substances on atherosclerotic lesion biology. The major advantage of this ex vivo method is the ability to evaluate the influence of indicated substances on inflammatory cells and their cellular interplay as well as inflammatory pathways and cascades within human atherosclerotic lesions. Several usable methods (e.g. RT-PCR, western blot, immunohistochemistry, flow cytometry, ELISA) help to provide a comprehensive analysis of the impact of the substance of interest on atherosclerotic lesion inflammation.
The inflammatory processes in murine atherosclerosis are well understood, also thanks to mouse models over-expressing or lacking certain genes of interest11. However, the findings do not always closely correspond to humans6-9,13. Atherosclerotic mice usually receive a pathologic high cholesterol diet6. In addition, it is known that the immune systems between humans and mice show substantial differences7-9. Here, we present an ex vivo plaque culture model, which allows studying the influence of a substance of interest on the inflammatory compound in human atherosclerotic lesions as shown by Niessner et al.14. Moreover, various additional methods can be used to analyze the results of a plaque culture experiment, comparable to murine studies. Therefore, the ex vivo model opens the possibility to replace murine in vivo studies in some cases and may represent an excellent bridging method between a putative atherosclerosis promoting molecule in the murine system and translation to human biology.
The human ex vivo model for atherosclerosis is not comparable with in vitro cell experiments. Cell lines can be easily stored and cultured, but are phenotypically and functionally not identical to primary cells. Fresh cells can be obtained by regular blood draws, but it is occasionally very difficult to culture them and culture is subjected to many factors. In addition, by using in vitro cell culture experiments, it is not possible to investigate the influence of specific molecules on the inflammatory compound in atherosclerotic lesions. Thus, in vitro experiments are important for initial (translational) step for human biology investigations, but fail to further analyze the role of the molecule of interest in the concept of human atherosclerosis, especially cellular interplay and influence on inflammatory cascades and pathway within atherosclerotic lesions.
Monaco et al. established an important short-term culture system of cells isolated from human atherosclerotic tissue15. They have used an enzymatic mixture of collagenase, elastase and DNase. This method allows investigation of lesional cell-cell experiments, signaling pathway analysis and gene transfer studies with adenoviral vectors. But it remains unknown how the enzymatic mixture influences the cells, i.e. grad of activation, surface marker expression etc. In addition, it can be questioned whether the results of these experiments reflect the real processes inside atherosclerotic lesions. Furthermore, the original location of the cells will be destroyed by the enzymatic mixture and the short-term culture system has the same limitations as the in vitro cell experiments.
For specific cell or cell population studies inside an atherosclerotic lesion, it is possible to use the laser capture micro dissection method. The cell or cell compound of interest will be cut out of the tissue by using an infrared- or UV-Laser and RNA, DNA or protein analysis can be performed. The laser capture micro dissection does not seem to damage the cells, the cell surface receptor expression etc. The advantage of the method is the analysis of a cell or a location of interest inside an atherosclerotic lesion. But it is not possible to further evaluate the cells such as in vitro studies.
The ex vivo model implicates a wide range of possible methods to measure and analyze the findings. Real time polymerase chain reaction can be performed after RNA isolation and cDNA synthesis to investigate the gene expression level as shown by Niessner et al.14. Immunohistochemistry is an established tool to evaluate the protein level in order to prove the protein expression and the cellular origin inside atherosclerotic lesions. Western blotting can be used to analyze signal pathways. In addition, cell isolation by digestion in an enzyme solution opens the opportunity to perform flow cytometry analysis to further analyze for instance cell type, cellular subtypes and grade of activation. Furthermore, after cell isolation spectratyping represent a good method to further analyze T cell receptor variability. Moreover, after digestion magnetic cell isolation is a proven high-quality method of cell separation of specific cellular subtypes for further in vitro studies or microarray analysis. At last, supernatants can be collected for protein measurement by ELISA. In conclusion, the ex vivo model is a valuable tool to investigate the exact changes in the inflammatory milieu in human atherosclerotic lesions.
The ex vivo model is based on endarterectomy samples. Using atherosclerotic plaques obtained from different individuals may result in a greater degree of differential cellular composition and thus higher variation of the levels of genes / proteins of interest. Thus, it is important to use lipid rich or complicated plaque samples rather than fibrosclerotic lesions10, because the inflammatory response is markedly lower in fibrosclerotic lesions. Therefore, it is of great interest to evaluate the plaque morphology before analyzing the results of plaque culture experiments.
In addition, each specimen sample includes different stages of the atherosclerotic lesion development / progression, from the initial endothelial dysfunction and lipid accumulation step, towards fatty streaks and development of a necrotic core to ruptured plaques. Thus, in order to minimize the heterogeneity of the plaque tissue, it is necessary to cut the edges, which generally represent early steps of atherogenesis.
It cannot be excluded that the cutting induces tissue injury responses. But our plaque tissue culture experiments showed comparable gene expression levels of the cultured plaque tissues to non-cultured plaque tissues. Thus, this underlines that the current method is a good tool to investigate the inflammatory milieu in atherosclerotic lesions.
The culture of plaque pieces is limited to a time period of up to one day. If plaque tissue is cultured more than a day, the variation of the results increases dramatically. In addition, after 3 days the plaque composition and morphology collapses.
There are limitations that need to be kept in mind when applying this model. The ex vivo model is a good method to study the inflammatory milieu in atherosclerotic lesions. Nevertheless, major components are missing in this context. No hemodynamic features are applicable and systemic influences are totally missing. In addition, with this method it is only possible to investigate short term changes, but lacks a long term analysis because of the collapse of the plaque morphology.
In conclusion, we present here a method that will be useful for researchers who are interested in the investigation of potential new molecules on human atherosclerotic lesion inflammation. The ex vivo plaque culture model does suffer from differences between the murine and human immune system, but it gives us the ability to analyze the cellular interplay in the context of atherosclerotic lesions and represent the opportunity to investigate local lesional inflammatory cascades and pathways. The ex vivo plaque culture method described here is easy to use and is reproducible and may help to identify and validate novel disease mechanisms and therapeutic targets.
Disclosures
The authors do not have any conflicts to disclose.
Acknowledgments
We thank Nadine Wambsganss for excellent technical assistance. This work was supported by the German Research Foundation (DFG) ER 682/2-1 and a research stipend from the German Society of Cardiology to C. Erbel as well as a research stipend from German Academic Service Heidelberg to L. Zhao.
References
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- Erbel C, et al. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res Cardiol. 2011;106:125–134. doi: 10.1007/s00395-010-0135-y. [DOI] [PubMed] [Google Scholar]
- Erbel C, et al. Functional profile of activated dendritic cells in unstable atherosclerotic plaque. Basic Res Cardiol. 2007;102:123–132. doi: 10.1007/s00395-006-0636-x. [DOI] [PubMed] [Google Scholar]
- Bentzon JF, Falk E. Atherosclerotic lesions in mouse and man: is it the same disease. Curr Opin Lipidol. 2010;21:434–440. doi: 10.1097/MOL.0b013e32833ded6a. [DOI] [PubMed] [Google Scholar]
- Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G, et al. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol. 2005;174:6561–6562. doi: 10.4049/jimmunol.174.11.6561. [DOI] [PubMed] [Google Scholar]
- Ingersoll MA, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:10–19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20:1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma T, Workman JL. MAP kinases and histone modification. J Mol Cell Biol. 2012;4:348–350. doi: 10.1093/jmcb/mjs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Niessner A, et al. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–2052. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]
- Monaco C, et al. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]


