Abstract
Purpose
Dry eye condition is an extrahepatic manifestation associated with chronic hepatitis C virus (HCV) infection. Since conjunctival inflammation can contribute to the dry eye condition, in the present study we analyzed the conjunctival inflammatory response to HCV core and NS3 proteins.
Methods
We used primary human conjunctival fibroblasts for our study. Cytokines were measured with enzyme-linked immunosorbent assay (ELISA). Toll-like receptor (TLR) and cell adhesion molecule gene expression patterns were analyzed with semiquantitative reverse transcription (RT)–PCR. Immunofluorescence staining was performed for the MyD88, nuclear factor-kappa B (NF-kB), and inducible nitric oxide synthase (iNOS) proteins. Nitric oxide (NO) was measured with the Griess assay; terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were performed for apoptosis and cell viability, respectively.
Results
When exposed to the HCV core and NS3 proteins, the conjunctival fibroblasts secreted interleukin-8 (IL-8), IL-6, tumor necrosis factor-alpha (TNF-α), and IL-10 in a dose-dependent manner. Various TLRs were involved in the innate immune response via MyD88 signaling without NF-kB involvement. The gene expression of cell adhesion molecules such as CD44 and ICAM-1 was upregulated, and the cells secreted NO via iNOS. As the sum of these stress responses, the cells underwent apoptosis, which eventually lead to cell death.
Conclusions
HCV core and NS3 proteins induced conjunctival inflammation that may form the pathogenesis of dry eye condition.
Introduction
Hepatitis C virus (HCV), a positive-stranded RNA virus in the Flaviviridae family, induces liver inflammation and cirrhosis [1]. About 80% of infected individuals tend to develop chronic infection [2]. Chronic HCV infection may lead to many extrahepatic manifestations including dry eye [3-5]. Dry eye syndrome is a multifactorial disease condition that leads to tear film instability, lacrimal gland dysfunction, and ocular surface damage. The pathology of dry eye syndrome includes immune reactions associated with autoimmune conditions or inflammation associated with other microbial infections [6,7]. Chronic inflammation plays a major role in mediating some of the signs and symptoms associated with dry eye, including structural alterations, functional paralysis of lacrimal glands, etc. [8,9]. Experimentally induced dry eye in mice models has induced the apoptosis of various cell types in the cornea and the conjunctiva followed by inflammatory cytokine secretion via the mitogen-activated protein kinase (MAPK) signaling pathway [10,11]. Case studies on HCV infection and associated ocular manifestations such as Mooren’s ulcer have been reported [12,13]. HCV RNA has been detected in the tear fluid of patients with chronic HCV [14]. HCV core is a structural protein known to be involved in inducing hepatocellular carcinoma, liver steatosis, and cytotoxic T-lymphocyte responses [15,16]. HCV NS3 is a non-structural protein and is known to elicit interleukin (IL)-8 secretion and interferon (IFN)-γ production in monocytes [17]. Toll-like receptors (TLRs) are pattern recognition receptors that participate in the innate immune response, activate cell signaling pathways, and induce the production of proinflammatory and anti-inflammatory cytokines when being stimulated by the respective ligands. MyD88 is an adaptor protein that transfers the intracellular signals generated by TLRs, which trigger NF-kB, p38 MAPK, and other signaling molecules involved in producing proinflammatory cytokines [18]. In addition to TLRs, many cell adhesion molecules such as cluster of differentiation 44 (CD44), integrin β1 (INTGβ1), and intercellular adhesion molecule 1 (ICAM-1) have been associated with various inflammatory conditions [19-21]. In the present study, we focused on conjunctival inflammation mediated by HCV core and NS3 proteins.
Methods
Ethics statement
All the experiments were performed with fibroblasts established from a male patient with age of 48 years recruited from the ophthalmic clinic, Sankara Nethralaya, undergoing treatment for ocular surface reconstruction following chemical injury. The patient was healthy with no known systemic infection at the time of recruitment. The conjunctival tissue specimens were collected from adult patients with written informed consent. The study was approved by the Vision Research Foundation institutional ethical committee board of Sankara Nethralaya, Chennai. All the experiments were performed according to the Declaration of Helsinki. The study adhered to the ARVO statement on human subjects.
Cell culture and treatments
The conjunctival tissue was washed with 1X PBS (pH 7.4; TL1101; 154 mM NaCl, 1 mM KH2PO4, 5 mM Na2HPO4, HiMedia Laboratories, Mumbai, India) containing 100 units/ml penicillin G and 100 units/ml streptomycin (Invitrogen, Carlsbad, CA). The tissue was minced into 2 mm pieces and plated on 35 mm dishes. Dulbecco’s Modified Eagle Medium (DMEM from Gibco, Carlsbad, CA) and 10% fetal bovine serum (FBS; HiMedia Laboratories) was added such that the tissue was maintained in an air/liquid interface. After 3 days, 2 ml of the complete growth medium with antibiotics was added to the dishes and maintained until the cells became confluent. The confluent cultures were trypsinized and seeded onto T25 flasks. The cells used for the experiments were from the 2nd to the 5th passage, and each time, the expression of vimentin, a conjunctival fibroblast marker, was confirmed with immunofluorescence staining and PCR. One hundred percent of the cells expressed vimentin. The cells were seeded on six-well plates with or without 11 mm coverslips. After 24 h post-seeding, the cells were treated with recombinant HCV core (95% pure) and NS3 proteins (90% pure) for different time points; β-galactosidase (β-Gal), which was expressed and purified similarly to core and NS3, was used as a control where indicated. All recombinant proteins were from Sigma-Aldrich. The proteins were free of endotoxin (<0.005 EU/ml) as determined with the limulus amebocite lysate (LAL) assay.
ELISA
The IL-8, IL-6, TNF-α, and IL-10 enzyme-linked immunosorbent assay (ELISA) kits were from e-Bioscience (San Diego, CA). After 8 h of exposure to HCV core and NS3 and β-Gal, the cell culture medium was collected, and ELISA was performed according to the manufacturer’s instructions.
RNA extraction and RT–PCR
After 24 h of viral protein treatment, total RNA was extracted using the Qiagen RNase mini kit and 5 µg of total RNA was used for cDNA conversion using the Sensiscript RT kit (Qiagen, Hilden, Germany) and oligo-dT primers (Fermentas, Hanover, MD). RT–PCR was performed for all ten TLR genes, CD44, ICAM-1, INTGβ1, and GAPDH using equal amounts of cDNA. All PCR reagents were purchased from Fermentas; unless otherwise stated, all the primers except GAPDH were from Hysel Biotech, India, and the GAPDH primers were from Genei, India. All primers and the product sizes are listed in Table 1. The PCR cycling conditions were as follows: 95 °C for 10 min, followed by 35 cycles of 94 °C for 1 min, 55 °C for 1 min for all TLRs and 63 °C for GAPDH and 72 °C for 1 min, with a final extension of 72 °C for 10 min. Equal volumes of the PCR products were loaded on a 2% agarose gel with 0.5 µg/ml ethidium bromide, and images were captured.
Table 1. List of primers.
| Gene | Primer (5′-3′) | Size (bp) |
|---|---|---|
| GAPDH-F |
GCCAAGGTGATCCATGACAAC |
498 |
| GAPDH-R |
GTCCACCACCCTGTTGCTGTA |
|
| TLR1-F |
GGTCTTGCTGGTCTTAGGAGAGAC |
372 |
| TLR1-R |
CTGAAGTCCAGCTGACCCTGTAGCTTCACG |
|
| TLR2-F |
GGCCAGCAAATTACCACCTGTGTG |
637 |
| TLR2-R |
CTGAGCCTCGTCCATGGGCCACTCC |
|
| TLR3-F |
CGGGCCAGCTTTCAGGAACCTG |
400 |
| TLR3-R |
GGCATGAATTATATATGCTGC |
|
| TLR4-F |
TGCAATGGATCAAGGACCAGAGGC |
449 |
| TLR4-R |
GTGCTGGGACACCACAACAATCACC |
|
| TLR5-F |
CCTCATGACCATCCTCACAGTCAC |
355 |
| TLR5-R |
GGCTTCAAGGCACCAGCCATCTC |
|
| TLR6-F |
CCAAGTGAACATATCAGTTAATACTTTAGGGTGC |
358 |
| TLR6-R |
CTCAGAAAACACGGTGTACAAAGCTG |
|
| TLR7-F |
CTCCCTGGATCTGTACACCTGTGAG- |
551 |
| TLR7-R |
CTCCCACAGAGCCTTTTCCGGAGCT |
|
| TLR8-F |
GTCCTGGGGATCAAAGAGGGAAGAG |
581 |
| TLR8-R |
CTCTTACAGATCCGCTGCCGTAGCC |
|
| TLR9-F |
GCGAGATGAGGATGCCCTGCCCTACG |
510 |
| TLR9-R |
TTCGGCCGTGGGTCCCTGGCAGAAG |
|
| TLR10-F |
CAGAGGTCATGATGGTTGGATGG |
256 |
| TLR10-R |
GACCTAGCATCCTGAGATACCAGGGCAG |
|
| CD44-F |
GACCTAGCATCCTGAGATACCAGGGCAG |
471 |
| CD44-R |
GCAAACTGCAAGAATCAAAGCC |
|
| ICAM1-F |
CCGGAAGGTGTATGAACTG |
319 |
| ICAM1-R |
TCCATGGTGATCTCTCTCCTC |
|
| INTGβ1-F |
GAAGGGTTGCCCTCCAGA |
107 |
| INTGβ1-R | GCTTGAGCTTCTCTGCTGTT |
The gel optical density (OD) values of the TLRs and cell adhesion molecule genes were measured with ImageJ software and normalized regarding the corresponding GAPDH OD values. The fold change in gene expression for the HCV core and NS3-treated cells was calculated regarding the untreated control.
Immunofluorescence staining
Cells were seeded on 11 mm coverslips in 35 mm dishes. After the treatment, the cells were washed with 1X PBS, and fixed with 4% paraformaldehyde for 10 min followed by incubation in 10% FBS + 0.01% Triton X-100 in 1X PBS for 15 min. The cells were then probed with primary antibodies (1:500; Santa Cruz Biotechnology, Heidelberg, Germany) and incubated overnight in a humidified chamber at 4 °C. After washing with 1X PBS, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (1:300; Dako, Glostrup, Denmark) for 2 h. After the final washing, the coverslips were mounted with 4',6-diamidino-2-phenylindole (DAPI). The fluorescent micrographs were taken with a Zeiss Axiovert microscope at 20X magnification.
Nitric oxide measurement and apoptosis assay
The stable end product of NO, nitrite, was measured with the Griess reagent assay kit (Invitrogen Molecular Probes), and the assay was performed according to the manufacturer’s instructions. After the protein treatment, the cells were washed with 1X PBS and then fixed with 4% paraformaldehyde for 10 min. The apoptotic nuclei were detected with terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) assay by using a commercial kit (TACS 2 TdT-Fluor In situ Apoptosis Detection Kit) according to the manufacturer’s protocol. The fluorescent images were taken with a Zeiss Axiovert microscope at 20X magnification.
MTT cell viability assay
Cytotoxicity was detected with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. After the protein treatment, the cell culture medium was removed, and the cells were rinsed with 1X PBS and incubated with 0.5 mg/ml MTT diluted in complete DMEM for 2 h at 37 °C. After incubation, DMEM with MTT was removed, formazan crystals were solubilized in 100 µl dimethyl sulfoxide (DMSO), and absorbance was read at 570 nm.
Statistics
All the experiments were performed three times independently unless otherwise stated. The Student t test was performed with variance as standard error mean (SEM), and a statistical p value <0.05 was considered significant.
Results
Human conjunctival fibroblasts released cytokines upon exposure to HCV core and NS3 proteins
Human conjunctival fibroblasts were exposed to 2 ng/ml and 20 ng/ml of core, NS3, and β-Gal. IL-8, IL-6, TNF-α, and IL-10 were detected at picogram levels in the cell culture media exposed to the core and NS3 proteins whereas this was absent with the β-Gal treatment. IL-8 was secreted at much higher concentrations (Figure 1A) followed by IL-6 (Figure 1B) and IL-10 (Figure 1C).
Figure 1.
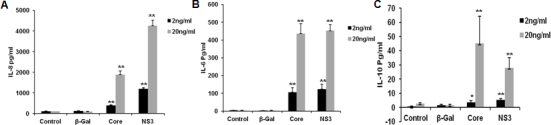
Hepatitis C virus core and NS3-induced cytokine production. Human conjunctival fibroblasts were exposed to 2 ng/ml and 20 ng/ml of core, NS3, and β-galactosidase (β-Gal) proteins for 8 h. Enzyme-linked immunosorbent assay (ELISA) was performed to detect cytokines in the cell culture supernatants. A–C: Core and NS3 induced interleukin (IL)-8, IL-6, tumor necrosis factor-alpha (TNF-α), and IL-10 secretion at a significant level. Data are represented as mean ± standard error of the mean (SEM) over the untreated control group. *p<0.05, ** p<0.001.
TLRs were involved in HCV core and NS3-mediated inflammation
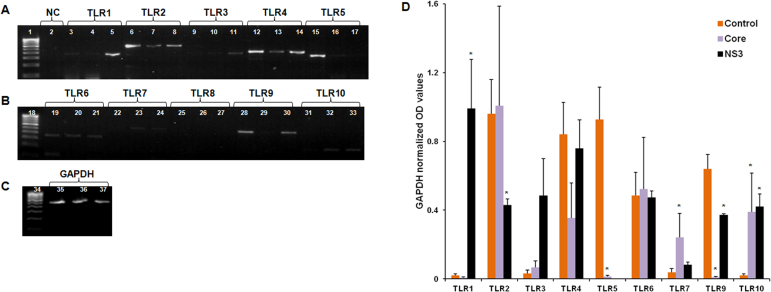
We hypothesized the involvement of TLRs in the HCV core and NS3-mediated inflammatory response. Semiquantitative RT–PCR was performed to measure the TLR gene expression. HCV core exposure downregulated TLR5 and TLR9 gene expression whereas TLR7 and TLR10 gene expression was significantly upregulated (Figure 2). The HCV NS3-treated cells showed significantly upregulated expression for the TLR1 and TLR10 genes, and the TLR2, TLR5, and TLR9 genes were significantly downregulated (Figure 2). We could not detect TLR8 gene expression.
Figure 2.
Hepatitis C virus core and NS3 induced toll-like receptor activation. Human conjunctival fibroblasts were exposed to 20 ng/ml of core and NS3 proteins for 6 h. Semiquantitative reverse transcription (RT)–PCR was performed to measure the TLR gene expression. A–C: Lanes 1, 18, and 34 represent the 100 bp DNA ladder; lane 2 represents PCR negative control; all the TLRs and GAPDH include PCR products from the control, core, and NS3-treated groups in that order. Data are representative of three independent experiments. D: During the core protein treatment, TLR7 and TLR10 were upregulated, and TLR5 was downregulated. During the NS3 protein treatment, TLR1, TLR9, and TLR10 were upregulated; TLR2 was downregulated, and TLR8 expression was not detected. Data are represented as mean ± standard error of the mean (SEM) over the untreated control group. *p<0.05.
Cell signaling mechanism involved in TLR-mediated immune response
Immunofluorescence staining was performed to detect MyD88 protein expression and nuclear translocation of NF-kB. Fluorescent micrographs show that MyD88 protein expression was upregulated in the core and NS3-exposed cells at the 8 h time point, but the same was not detected at the 24 h time point (Figure 3A,B). We could not detect NF-kB nuclear translocation at the 8 and 24 h time points (Figure 3C,D).
Figure 3.
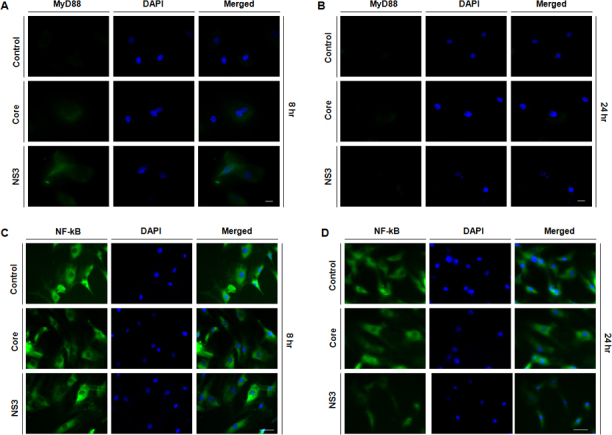
Toll-like receptors signaled via MyD88. Human conjunctival fibroblasts were exposed to 20 ng/ml of core and NS3 proteins for 6 h. Immunofluorescence staining was performed for MyD88 and nuclear factor-kappa B (NF-kB). A: At the 8 h time point, MyD88 fluorescent intensity increased in the core and NS3-treated cells compared to the untreated control. B: At the 24 h time point, the fluorescent signal for the core and NS3-treated cells was similar to that of the control cells. C–D: At the 8 and 24 h time points, NF-kB nuclear translocation was not observed for the core and NS3-treated cells. Data are representative of three independent experiments. Scale bar=50 μm.
Involvement of cell adhesion molecules in inflammation
We analyzed the transcript-level expression of CD44, INTGβ1, and ICAM-1 in the core and NS3-treated cells with semiquantitative RT–PCR. CD44 and ICAM-1 relative gene expression was significantly upregulated in the core and NS3 exposed cells (Figure 4A(a), 4B(a)). The HCV protein treatments had no effect on INTGβ1 gene expression (Figure 4C(a)).
Figure 4.
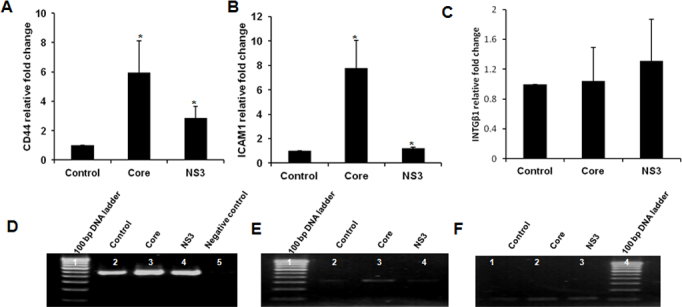
Cell adhesion molecule expression. Human conjunctival fibroblasts were exposed to 20 ng/ml of core and NS3 proteins for 6 h. Semiquantitative reverse transcription (RT)–PCR was performed for the CD44, ICAM-1, and INTGβ1 genes. A–C: Relative gene expression for CD44 and ICAM-1 was high in the core and NS3-treated cells; there was no difference in INTGβ1 gene expression. Data are represented as mean ± standard error of the mean (SEM) over the untreated control group. *p<0.05. PCR gel images for CD44 (D), ICAM-1 (E), and INTGβ1 (F). Data are representative of three independent experiments.
HCV core and NS3 induced nitric oxide production via inducible nitric oxide synthase
Core and NS3 exposure induced NO production in conjunctival fibroblasts at the µM concentration (Figure 5A). iNOS protein expression was detected in the core and NS3 exposed cells but was not detected in the untreated control cells (Figure 5B–D).
Figure 5.
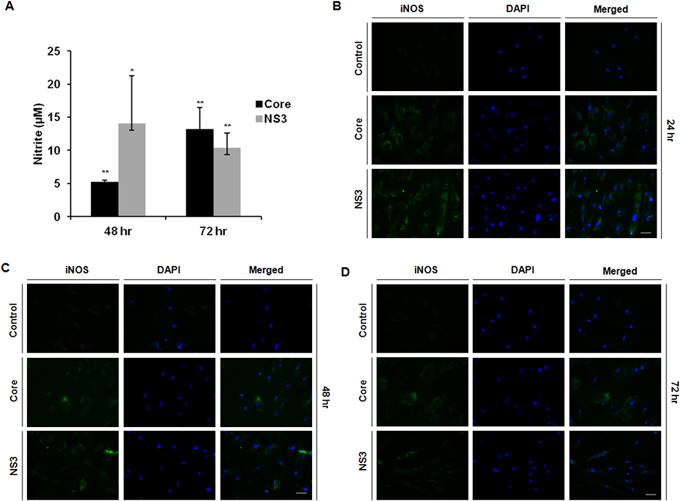
Conjunctival fibroblasts induced nitric oxide production via inducible nitric oxide synthase. Human conjunctival fibroblasts were exposed to 20 ng/ml of core and NS3 proteins. Nitrite was measured with the Griess assay. Immunofluorescence was performed for inducible nitric oxide synthase (iNOS). A: Core and NS3 protein-exposed cells synthesized nitric oxide (NO) at 48 and 72 h. Data are represented as mean ± standard error of the mean (SEM) over the untreated control group. *p<0.05, **p<0.01. B–D: iNOS protein fluorescent intensities were comparatively high in the core and NS3-treated cells at 24, 48, and 72 h. Data are representative of three independent experiments. Scale bar=50 μm.
HCV core and NS3 mediated apoptosis and cell death
Core and NS3 exposure induced apoptosis in conjunctival fibroblasts. Apoptotic cells were detected at 24, 48, and 72 h exposure to core and NS3 (Figure 6A–C). At the 24 h time point, we could not detect a significant decrease in cell viability; however, at later time points, cell viability decreased in a time-dependent manner (Figure 6D).
Figure 6.
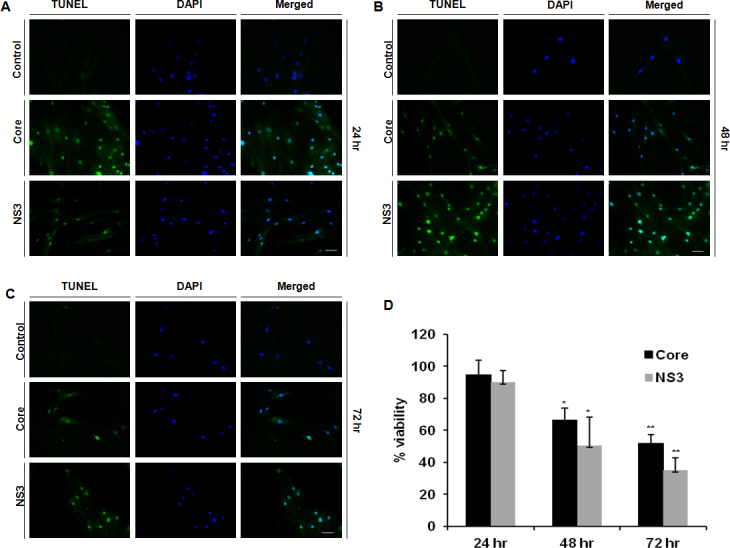
Hepatitis C virus core and NS3 induced apoptosis and cell death. Human conjunctival fibroblasts were exposed to 20 ng/ml of core and NS3 proteins. The terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) assay was performed to detect apoptosis. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to measure cell viability. A–C: Cells stained positive for apoptotic nuclei at all time points during the core and NS3 treatment, and this was absent in the control cells. Data are representative of three independent experiments. Scale bar=50 μm. D: During the core and NS3 treatment, cell viability significantly decreased at 48 and 72 h. Data are represented as mean ± standard error of the mean (SEM) over the untreated control group. *p<0.05, **p<0.01.
Discussion
HCV primarily affects the liver; however, chronic infection leads to many extrahepatic complications such as dry eye. Direct association between dry eye syndrome and HCV infection is well established. Patients with chronic HCV with advanced stages of liver fibrosis are more likely to exhibit ocular surface damage, and clinical studies have correlated the presence of HCV RNA in tear fluid and dry eye [4,14]. Immune-based inflammation has been observed as a common feature associated with dry eye syndrome. A strong association has been suggested between inflammatory pathways and apoptosis directly affecting total cellular turnover in the dry eye condition [22]. In this context, we examined the inflammatory potential of two major HCV antigens, core and NS3 proteins, in mediating conjunctival inflammation and the associated innate immune response via TLRs. We found that conjunctival fibroblasts responded to the core and NS3 proteins by secreting IL-8, IL-6, and IL-10. IL-8 is a potent chemokine and can extend inflammation by recruiting eosinophils to the ocular surface. IL-6 has been reported as a common inflammatory marker associated with conjunctival inflammation as well as the dry eye condition [23-25]. Earlier clinical studies documented IL-10 secretion in the tear fluid of patients with dry eye [26]. In our study, conjunctival fibroblasts secreted IL-10; however, the concentration was low compared to IL-8 and IL-6. Though the dry eye condition has been linked with the overexpression of various cytokines, the exact role of each cytokine in the disease pathogenesis must be elucidated. In our study, core and NS3 exposure induced cytokine production and at the 2 ng/ml concentration, which is comparable to the serum concentration of one of the HCV antigens [27].
TLR receptors respond to many structurally unrelated ligands, and at the same time, one group of pathogens is recognized by multiple TLRs. Similarly, downregulation and upregulation of TLRs have been reported for ligand-specific stimulation. In our experimental condition, the core and NS3 proteins were not specific for a single TLR as for the core and NS3 protein treatment. The mRNA transcripts for multiple TLRs were either upregulated or downregulated. The upregulated response of the TLR transcripts can be explained by the innate immune mechanisms to eradicate the pathogen by mediating the release of proinflammatory cytokines. TLR downregulation could be a mechanism of immune evasion mediated by the HCV proteins. This is the first demonstration of TLR response in conjunctival fibroblasts against HCV viral proteins. This study shows that various TLRs respond via MyD88 in conjunctival fibroblasts. Future studies involving the evaluation of the effect of siRNA gene silencing of TLRs on the induction of innate immune responses by HCV and NS3 may provide insight into the identification of potential drug targets for reducing dry eye inflammation in patients who have hepatitis C.
Increased expression of CD44 and ICAM-1 gene transcripts along with IL-8 secretion further supports possible leukocyte infiltration in the later stages of inflammation. Though the inflammatory role of CD44 is well evidenced by literature, there has been recent reports on the anti-inflammatory role of this molecule via downregulation of TLR-mediated inflammation in which NF-kB activation is suppressed [28]. In our study, after 8 h of post-exposure to core and NS3, we did not detect nuclear translocation of NF-kB. We observed a similar response at the 24 h time point although we detected cytokine production within the 8 h exposure to the HCV proteins, which demonstrates the involvement of transcription factors other than NF-kB. Previous studies revealed the bifunctional role of the HCV core protein since this protein is known to suppress NF-kB activation and cyclooxigenase-2 expression by direct interaction with IkB kinase β [29], and another study stated the HCV core mediated NF-kB activation via tumor necrosis factor receptor associated factor [30]. Similarly, NS3/4A blocks HCV RNA-mediated induction of IL-28 via NF-kB activation [31]. Although we did not observe nuclear translocation of NF-kB as early as the 8 h time point, we cannot exclude the possibility of early phase activation of NF-kB and the involvement of transcription factors other than NF-kB. Our study results did not rule out the involvement of the NF-kB pathway during conjunctival fibroblast activation by HCV core and NS3 proteins.
Later stages of conjunctival inflammation progressed with the production of nitric oxide via iNOS. Nitric oxide could contribute to the pathology of dry eye via oxidative stress–mediated tissue injury since this was observed in the significant decrease in cell viability during the core and NS3 protein treatments. Apoptosis was observed from the 24 h time point; however, since we could not detect NO secretion within 24 h, we presume that other factors such as inflammatory cytokines might have initiated apoptosis at the early time point, and NO secretion might have contributed to the same at the later time points. Although the role of NO in inducing tissue damage is well evidenced, NO production in many viral infections tends to increase the innate immune response, which downregulates virus replication [32].
Based on our observations, we have proposed a pathway (Figure 7) by which the HCV core and NS3 proteins can mediate inflammation in conjunctival fibroblasts, which, in turn, can form the pathogenesis of the dry eye condition observed in patients with chronic HCV. Since the study was conducted on one cell type in conjunctiva, in the real scenario, the inflammatory response could be complex and involve multiple pathways. However, HCV RNA has been detected in tear fluid, and thus far, no study regarding the presence of HCV antigens from tear samples of patients with chronic HCV has been performed. Studies on the role of HCV viral antigens in potentiating the dry eye condition observed in patients with chronic HCV might be insightful in understanding the pathology of this condition.
Figure 7.
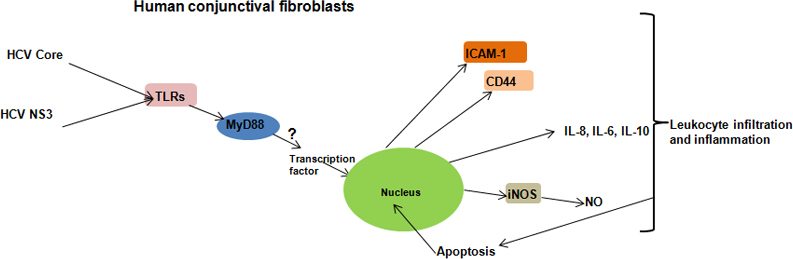
Schematic representation of a possible inflammatory pathway. The toll-like receptor (TLR) ligands identify core and NS3 proteins, and MyD88 transfers the signals to transcription factors, which, in turn, induce cytokine gene expression. Conjunctival fibroblasts increase cell adhesion molecule expression and synthesize nitric oxide (NO) via inducible nitric oxide synthase (iNOS). These stress responses induce apoptosis and cell death.
Acknowledgments
This work was supported by funding from Department of Biotechnology, Government of India (BT/PR1028/med/29/303/2011). ARR receiving Financial assistance from INSPIRE fellowship program (IF110775), Department of Science and Technology, Government of India.
References
- 1.Miller RH, Purcell RH. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus super groups. Proc Natl Acad Sci USA. 1990;87:2057–61. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szabó E, Lotz G, Paska C, Kiss A, Schaff Z. Viral hepatitis: new data on hepatitis C infection. Pathol Oncol Res. 2003;9:215–21. doi: 10.1007/BF02893380. [DOI] [PubMed] [Google Scholar]
- 3.Shepard CW, Finelli L, Alnter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 4.Gumus K, Yurci A, Mirza E, Arda H, Oner A, Topaktas D, Karakucuk S. Evaluation of ocular surface damage and dry eye status in chronic hepatitis C at different stages of hepatic fibrosis. Cornea. 2009;28:997–1002. doi: 10.1097/ICO.0b013e3181a0a3c5. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen CQ, Peck AB. Unravelling the Pathophysiology of Sjogren Syndrome-Associated Dry Eye Disease. Ocul Surf. 2009;7:11–27. doi: 10.1016/s1542-0124(12)70289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calonge M, Enríquez-de-Salamanca A, Diebold Y, González-García MJ, Reinoso R. Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm. 2010;18:244–53. doi: 10.3109/09273941003721926. [DOI] [PubMed] [Google Scholar]
- 7.Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocular Surf. 2004;2:124–30. doi: 10.1016/s1542-0124(12)70148-9. [DOI] [PubMed] [Google Scholar]
- 8.Lee SY, Han SJ, Nam SM, Yoon SC, Ahn JM, Kim TI, Kim EK, Seo KY. Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients. Am J Ophthalmol. 2013;156:247–53. doi: 10.1016/j.ajo.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–11. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 10.Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003;44:124–9. doi: 10.1167/iovs.02-0581. [DOI] [PubMed] [Google Scholar]
- 11.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 12.Wilson SE, Lee WM, Murakami C, Weng J, Moninger GA. Mooren-type hepatitis C virus-associated corneal ulceration. Ophthalmology. 1994;101:736–45. doi: 10.1016/s0161-6420(94)31291-7. [DOI] [PubMed] [Google Scholar]
- 13.Moazami G, Auran JD, Florakis GJ, Wilson SE, Srinivasan DB. Interferon treatment of Mooren's ulcers associated with hepatitis C. Am J Ophthalmol. 1995;119:365–6. doi: 10.1016/s0002-9394(14)71182-1. [DOI] [PubMed] [Google Scholar]
- 14.Mendel I, Muraine M, Riachi G, el Forzli F, Bertin C, Colin R, Brasseur G, Buffet-Janvresse C. Detection and genotyping of the hepatitis C RNA in tear fluid from patients with chronic hepatitis C. J Med Virol. 1997;51:231–3. doi: 10.1002/(sici)1096-9071(199703)51:3<231::aid-jmv15>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Tokushige K, Wakita T, Pachuk C, Moradpour D, Weiner DB, Zurawski VR, Jr, Wands JR. Expression and immune response to hepatitis C virus core DNA-based vaccine constructs. Hepatology. 1996;24:14–20. doi: 10.1002/hep.510240104. [DOI] [PubMed] [Google Scholar]
- 16.Chang ML, Chen JC, Yeh CT, Sheen IS, Tai DI, Chang MY, Chiu CT, Lin DY, Bissell DM. Topological and evolutional relationships between HCV core protein and hepatic lipid vesicles: studies in vitro and in conditionally transgenic mice. World J Gastroenterol. 2007;13:3472–7. doi: 10.3748/wjg.v13.i25.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479–87. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 18.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 19.Mrugacz M, Zak J, Bakunowicz-Lazarczyk A, Wysocka J, Kaczmarski M. ICAM1 Expression on Conjunctival Epithelial Cells in Patients with Cystic Fibrosis. Cytometry B Clin Cytom. 2007;72:204–8. doi: 10.1002/cyto.b.20159. [DOI] [PubMed] [Google Scholar]
- 20.Puré E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–21. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- 21.Zeidler A, Brauer R, Thoss K, Bahnsen J, Heinrichs V, Jablonski-Westrich D, Wroblewski M, Rebstock S, Hamann A. Therapeutic effects of antibodies against adhesion molecules in murine collagen type II-induced arthritis. Autoimmunity. 1995;21:245–52. doi: 10.3109/08916939509001943. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dastgheib K, Hikita N, Sredni B, Albeck M, Sredni D, Nussenblatt RB, Chan CC. Ocular inflammation stimulated by the immunomodulator AS101. Curr Eye Res. 1994;13:603–10. doi: 10.3109/02713689408999894. ammonium trichloro(dioxyethelene-O-O') tellurate. [DOI] [PubMed] [Google Scholar]
- 24.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci. 2002;43:737–43. [PubMed] [Google Scholar]
- 25.Taylor SR, Gurbaxani A, Sallam A, Lightman S. Topical prostaglandin analogues and conjunctival inflammation in uveitic glaucoma. Open Ophthalmol J. 2012;6:75–8. doi: 10.2174/1874364101206010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–7. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 27.Cividini A, Cerino A, Muzzi A, Furione M, Rebucci C, Segagni L, Gatti M, Barnaba V, Mondelli MU. Kinetics and Significance of Serum Hepatitis C Virus Core Antigen in Patients with Acute Hepatitis C. J Clin Microbiol. 2003;41:2144–6. doi: 10.1128/JCM.41.5.2144-2146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawana H, Haraki H, Higashi M, Miyazaki M, Hilberg F, Kitagawa M, Harigaya K. CD44 Suppresses TLR-Mediated Inflammation. J Immunol. 2008;180:4235–45. doi: 10.4049/jimmunol.180.6.4235. [DOI] [PubMed] [Google Scholar]
- 29.Joo M, Hahn YS, Kwon M, Sadikot RT, Blackwell TS, Christman JW, Hepatitis C. Virus Core Protein Suppresses NF-κB Activation and Cyclooxygenase-2 Expression by Direct Interaction with IκB Kinase β. J Virol. 2005;79:7648–57. doi: 10.1128/JVI.79.12.7648-7657.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida H, Kato N, Shiratori Y, Otsuka M, Maeda S, Kato J, Omata M. Hepatitis C virus core protein activates nuclear factor kappa B-dependent signalling through tumour necrosis factor receptor-associated factor. J Biol Chem. 2001;276:16399–405. doi: 10.1074/jbc.M006671200. [DOI] [PubMed] [Google Scholar]
- 31.Ding Q, Huang B, Lu J, Liu YJ, Zhong J. Hepatitis C virus NS3/4A protease blocks IL-28 production. Eur J Immunol. 2012;42:2374–82. doi: 10.1002/eji.201242388. [DOI] [PubMed] [Google Scholar]
- 32.Reiss CS, Komatsu T. Does nitric oxide play a critical role in viral infections? J Virol. 1998;72:4547–51. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]