Abstract
Difficulty in characterizing the relationship between climatic variability and climate change vulnerability arises when we consider the multiple scales at which this variation occurs, be it temporal (from minute to annual) or spatial (from centimetres to kilometres). We studied populations of a single widely distributed butterfly species, Chlosyne lacinia, to examine the physiological, morphological, thermoregulatory and biophysical underpinnings of adaptation to tropical and temperate climates. Microclimatic and morphological data along with a biophysical model documented the importance of solar radiation in predicting butterfly body temperature. We also integrated the biophysics with a physiologically based insect fitness model to quantify the influence of solar radiation, morphology and behaviour on warming impact projections. While warming is projected to have some detrimental impacts on tropical ectotherms, fitness impacts in this study are not as negative as models that assume body and air temperature equivalence would suggest. We additionally show that behavioural thermoregulation can diminish direct warming impacts, though indirect thermoregulatory consequences could further complicate predictions. With these results, at multiple spatial and temporal scales, we show the importance of biophysics and behaviour for studying biodiversity consequences of global climate change, and stress that tropical climate change impacts are likely to be context-dependent.
Keywords: climate change, biodiversity, tropics, biophysics
1. Introduction
While it has been suggested for decades that tropical species may have narrow thermal tolerance ranges relative to temperate species due to lower seasonality in the tropics [1], only in recent years has this pattern been demonstrated and recognized as important for understanding global change [2–4]. Specifically, while changes in temperature in future climates are projected to be smaller in the tropics than in temperate or polar regions [5], impacts of warming could be as great or greater for tropical species compared with species at higher latitudes [6–9]. However, there is a great deal of uncertainty in how warming might impact species globally as multiple evolutionary processes shape climatic adaption, including physiology, ecology and genetic diversity [10–13].
Intra-annual variation in temperature (i.e. seasonality) can have important implications for thermal tolerance, but thermal variation over other time scales and variation over spatial gradients also affect climate change vulnerability [2,14,15]. Habitat and behavioural factors can additionally influence a population's capacity to respond to climatic change at a regional, landscape or microclimatic scale [16–20]. For example, Huey et al. [21] showed that tropical forest lizards living in relatively homogeneous shaded habitats may be highly vulnerable to warming, while Logan et al. [22], using finer-spatial-scale thermal data, argued that forest lizards might not be so vulnerable due to high (and underestimated) spatial variance in temperature. Behavioural thermoregulation (e.g. shade-seeking) can also affect a species response to climate change [23–26]. Species interactions are critical such that extinction or distribution change of one species could result in the extinction of other members of that species interaction network [27].
Even before we are able to incorporate these additional factors, we must first understand the biophysical system and physiological ecology of a given species to effectively project how a regional warming trend is likely to affect it [28,29]. For example, Janzen's [1] hypothesis that lower intra-annual variation in temperature experienced by tropical organisms results in lower tolerance breadth has a largely untested, but critical, assumption that air temperature is equivalent to body temperature. If such variation is not equivalent (e.g. if body temperatures are largely overlapping between temperate and tropical species, in contrast to air temperature, as demonstrated by Janzen [1]), then the predicted high vulnerability of tropical species to warming might be overstated [30]. Incorporating biophysics and understanding variation in body temperature (rather than simply air temperature) are therefore important but frequently neglected components of climate change impact research [13,23,31].
Through modelling and analysis of an array of data (e.g. microclimate, morphology), we characterized the biophysics and thermal ecology of tropical and temperate populations of a single geographically widespread butterfly species, Chlosyne lacinia (Lepidoptera: Nymphalidae). Specifically, we used a biophysical model to incorporate local microclimatic variables (air temperature, ground temperature, wind speed and solar radiation) for body temperature simulations of the organisms in each population and used that model to examine geographical variation in body temperature. We integrated the biophysical model with a large-scale insect fitness model that approximates thermal tolerance (i.e. thermal performance curves) based on seasonal thermal variation [6,15] to explore potential impacts of climate change on temperate and tropical insect populations. Using the integrated biophysical fitness model, we also examined the role of morphology and behavioural thermoregulation in structuring climate change responses across latitude.
2. Material and methods
(a). Study sites and organism
We studied populations of C. lacinia in North and Central America. This species of the subfamily Nymphalinae was chosen due to its close relatedness to the well-studied checkerspot butterflies (e.g. Euphydryas editha and Melitaea cinxia) [32]. In addition, it is one of the most broadly distributed butterflies in the Americas, with populations ranging from Argentina to California and New Mexico. Where present, it tends to be locally abundant so that sufficient sample sizes could be found at each of the study sites.
What is known of C. lacinia natural history mostly comes from studies in Texas. Habitat requirements include open land, high food plant density and nectar sources, including a variety of host plants within Compositae [33]. Adults fly year round in the tropics, but only in the summer in North America. However, peak activity in Central America also typically occurs in the summer, usually beginning in July and lasting through November. Bonebrake et al. [34] explored the possibility that C. lacinia may actually represent multiple cryptic species, and found that while its evolutionary history is not straightforward, the phylogeny is consistent with the hypothesis that one single butterfly species is broadly distributed across temperate and tropical habitats.
We chose four main sites to conduct the study in 2007 and 2008: the Southwestern Research Station, Arizona (AZ; 31°53′ N, 109°13′ W, altitude 1700 m); Indio, California (CA; 33°43′ N, 116°12′ W, altitude –4 m); Santa Rosa National Park, Area de Conservacion Guanacaste, Costa Rica (CR; 10°48′ N, 85°36′ W, altitude 300 m); and Ahuachupan, El Salvador (ES; 13°59′ N, 89°11′ W, altitude 300 m). The ES and AZ sites underwent the most intensive sampling and serve as focal sites (see electronic supplementary material S1 for details on the sites and populations).
(b). Biophysical model and microclimatic variation
We used a biophysical model to predict body temperatures of a butterfly with given morphology under a set of microclimatic conditions (for details, see [28,35–38]; electronic supplementary material S2 and S3). The biophysical model incorporates a simple behavioural thermoregulatory function. At cold temperatures, butterflies orient themselves to maximize solar radiation, whereas at high temperatures, butterflies will orient away from direct solar radiation to minimize exposure to lethal temperatures [39]. The model incorporates this into the solar radiative heating rate by reducing the fractional absorption of direct sunlight by a factor of 0.65 when in avoidance position (i.e. reduction in radiative flux equivalent to 65% of a basking individual) [38]. Thermoregulatory behaviour differs between Chlosyne (dorsal basker) and Colias (lateral basker) [40]. However, both butterflies maximize radiation by basking and orienting towards direct sunlight and minimize radiation by avoiding sunlight and orienting away from the sun.
For microclimatic inputs, we used a HOBO Micro Station Data Loggers (Onset Computers Corporation, MAN-H21-002) to record climatic data every minute. Four sensors were attached to the micro station: a silicon pyranometer smart sensor (S-LIB-M003: resolution 1.25 W m−2), a wind speed smart sensor (S-WSA-M003: resolution 0.38 m s−1, starting threshold 1 m s−1), and two 8-bit temperature smart sensors (S-TMA-M0XX: resolution 0.4°C). For morphological inputs, we collected individuals from each of the sites and measured thorax diameter, body length, forewing length, hindwing absorptivity and fur thickness (electronic supplementary material, S1).
(c). Model validation
To validate the biophysical model, we measured body temperature directly with thermocouples. We inserted a fine-gauge thermocouple wire (Omega TFIR-003-50) attached to a hand-held thermocouple thermometer (Omega HH603A) into the thoraces of anaesthetized or freshly killed females. The female was then placed next to the microclimate station (in open sun) and in a dorsal basking position. We recorded body temperature of the female every minute during the validation procedure and then compared the observed body temperature with the body temperature predicted by the biophysical model. We used a similar procedure involving thermocouples and caged butterflies to roughly characterize thermal performance curves (flight probability versus body temperature) in Arizona and El Salvador (see electronic supplementary material S4 for more details).
(d). Climate change
To determine temperate and tropical population responses to climate change and warming (changes in mean temperature annually), we integrated the biophysical model with a generalized climate change impact model based on variation in thermal performance curves for insect species (fitness versus temperature) across latitude [6,15]. The impact model, as originally derived by Deutsch et al. [6], relates the seasonality of surface air temperature to site-specific insect physiology measures (specifically, warming tolerance; critical thermal maximum minus the habitat temperature and thermal safety margin; optimum temperature minus the habitat temperature) of 38 insect species, and uses that relationship to estimate thermal performance curves for insects globally. Based on projections of changes in air temperature over the next century, the model can then project changes in insect fitness globally based on the thermal performance curves.
Here, we modify the global impact model of Deutsch et al. [6] by incorporating solar radiation and estimating Tb of C. lacinia based on the biophysical model presented such that we have an approximation of the impact model for the species throughout the Americas. We input solar radiation data from the NASA International Satellite Cloud Climatology Project (ISCCP) [41] into the biophysical model as RS. For this model integration exercise, we set ground temperature Tg equal to air temperature Ta and assumed no wind. Following Deutsch et al. [6], we used 1950–2000 Climate Research Unit (CRU) data [42] for present-day surface air temperature data, but instead of calculating seasonality directly, we entered these air temperature data into the biophysical model. We then set the morphological parameters to match the average female El Salvador C. lacinia (see electronic supplementary material S1 and table S1), assumed individuals were in basking posture and solved for Tb as described in the biophysical model above. This produced a climatology (global monthly time series) in C. lacinia body temperature (specifically basking C. lacinia) with which we estimated seasonality in Tb. We extracted Tb seasonality at locations where insect physiology data were available from Deutsch et al. [6] and then regressed the seasonality to the physiological parameters (warming tolerance and thermal safety margin).
Based on the statistical relationship between global insect physiology and C. lacinia body temperature seasonality, we then examined the impact of projected changes in body temperature on C. lacinia fitness. We calculated body temperature changes by using an average of two A2 emissions scenario GFDL model projections [15], and inputting changes in air temperature and solar radiation into the biophysical model (see R script in electronic supplementary material S5) as in the statistical evaluation described previously. We used climate simulation data from 2070 to 2100 (air temperature and solar radiation) as the future climate and subtracted out the simulated baseline twentieth-century climate to calculate body temperature change. We then examined the effect of body temperature change on fitness for each point in the grid of interest, which for C. lacinia encompasses most of the Americas. We looked at the effect of morphology by using El Salvador and Arizona morphology (average female characteristics) to measure projected body temperature change, and we looked at the effect of thermoregulation by using an avoidance posture in the biophysical model for future butterflies (2070–2100) and looking at the projected fitness changes.
3. Results
(a). Model validation, morphology and microclimate
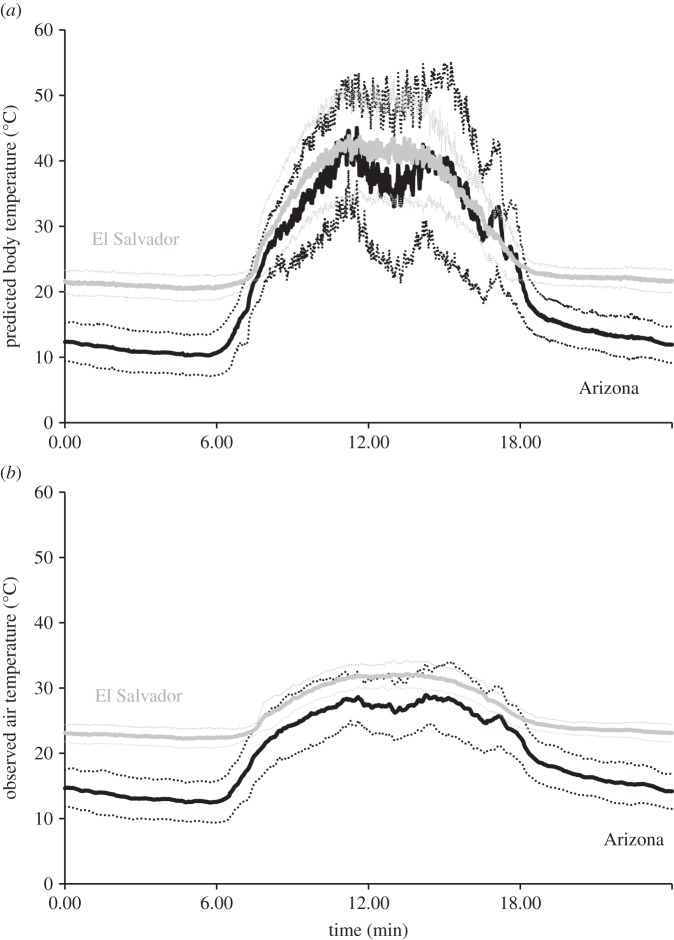
The biophysical model effectively predicted variation in C. lacinia body temperature (electronic supplementary material S4). Based on the biophysical model, while the body temperature of C. lacinia individuals is lower at night and during early morning hours, there is a great deal of overlap between El Salvador and Arizona in the late morning and afternoon hours (figure 1). Peak body temperatures (Tb) for butterflies at both sites during late morning/early afternoon hours are predicted to be about 41°C, although using avoidance posturing butterflies can reduce peak Tb to between 35°C and 39°C. Diurnal variation (standard deviation of hourly means) in Tb (basking body temperature) was 8.4°C (s.d.) for El Salvador and 11.6°C for Arizona. Variation in air temperature (Ta) was 3.7°C for El Salvador and 6.0°C for Arizona.
Figure 1.
(a) Diurnal variation of Tb (mean, solid line; standard deviation, dotted lines) based on model for El Salvador and Arizona C. lacinia. Dip in Tb in the mid-afternoon hours in Arizona is a shading effect of a nearby tree. (b) The same for air temperature.
(b). Impacts of climate change
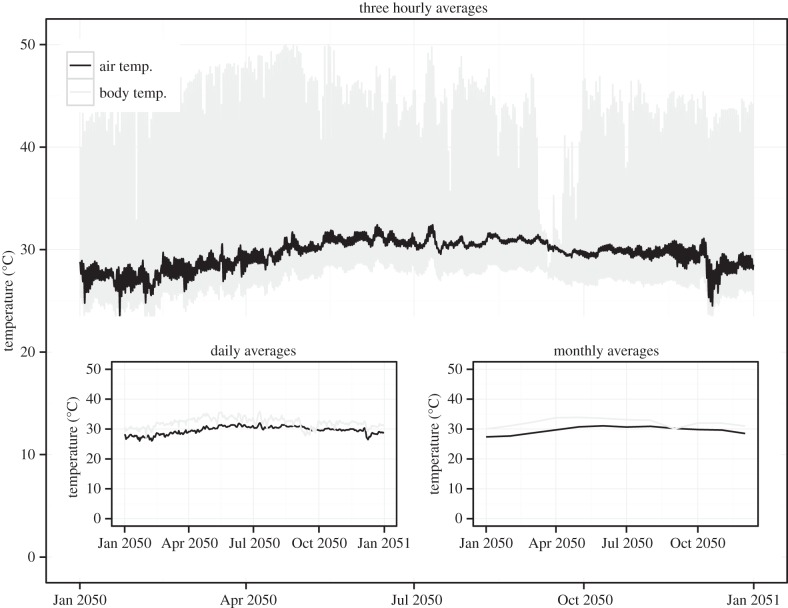
Global modelling (versus microclimatic) of C. lacinia body temperature projected with a biophysical model incorporating solar radiation versus air temperature showed additional high variability in body temperature relative to air temperature, particularly at high temporal resolution (figure 2). The positive correlation between large-scale (1 km) variation in predicted C. lacinia body temperature, insect warming tolerance and thermal safety margins from available studies was significant (R2 = 0.43, p < 0.001 and R2 = 0.43, p < 0.001, respectively), but the relationship is not as strong as warming tolerance for insects and air temperature as analysed by Deutsch et al. [6]. Using this relationship (body temperature variation versus warming tolerance and thermal safety margin), we then estimated thermal performance curves for C. lacinia globally. In the absence of extensive C. lacinia thermal performance data, this is a necessarily imperfect association between multiple insect thermal performances and C. lacinia body temperature. However, the relationship provides a first approximation of how individual and thermoregulatory characteristics might affect the impacts of climate change on insect species.
Figure 2.
Air temperature variation (black) and C. lacinia avoidance body temperature variation (grey) based on solar radiation and air temperature projections (GFDL A2) at three-hourly intervals for the year 2050 (see electronic supplementary material S5 for details). Air and body temperature averaged over days (left inset) and months (right inset) also displayed.
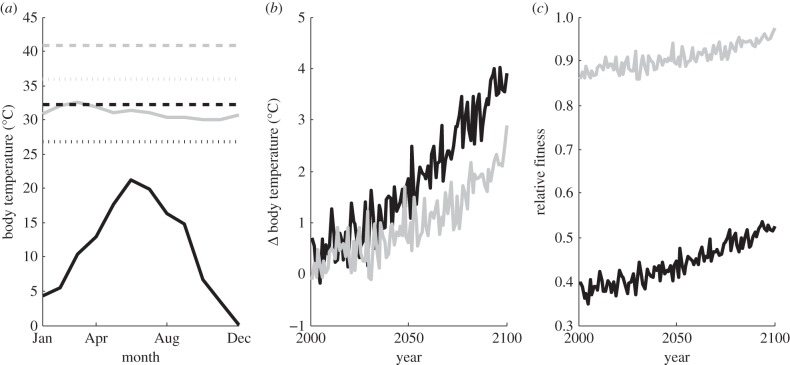
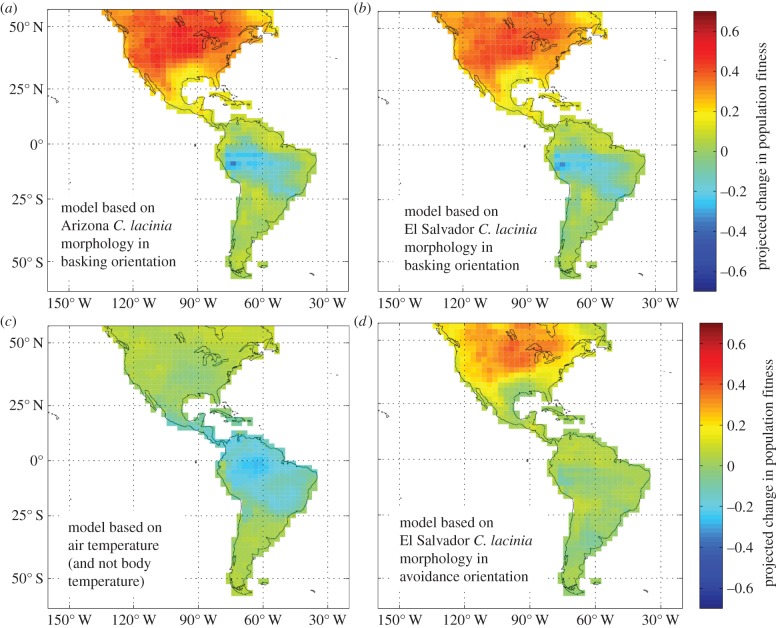
Using the climate projections and warming tolerance relationship, climate change is projected to increase both body temperatures and relative fitness in El Salvador and Arizona C. lacinia populations (figure 3). Across latitude generally, climate change is projected to differentially affect insect populations (figure 4). Regardless of morphology in C. lacinia, which has little effect on the results, some tropical populations are likely to experience negative fitness impacts (especially in South America) due to warming, while temperate populations (and some tropical populations) are expected to exhibit population growth (figure 4). However, reductions in the effects of solar radiation through thermoregulation (in this case, using avoidance posturing) can largely diminish this threat and erase much of the negative fitness impact for tropical insect populations (figure 4).
Figure 3.
(a) Variation in monthly mean C. lacinia body temperature estimates based on observed surface air temperature (CRU) and solar radiation (ISCCP) for Arizona (black) and El Salvador (grey) with theoretically derived critical thermal maximum values or the end of the thermal performance curve where fitness declines to zero (dashed horizontal lines) and thermal optimum estimates (dotted horizontal lines). (b) Projected changes in C. lacinia body temperature based on projected changes in air temperature and solar radiation (GFDL). (c) Projected change in relative fitness given the estimated thermal performance curve and body temperature change.
Figure 4.
Climate change impacts (as measured by intrinsic population growth rates) on C. lacinia based on a relationship between insect physiology (intrinsic population growth rate as a function of temperature) and C. lacinia body temperature seasonality. Results are shown for C. lacinia morphologies typical of (a) temperate Arizona and (b) tropical El Salvador in basking orientation. (c) Fitness impacts based solely on air temperature variation and changes (as in Deutsch et al. [6]) and (d) a tropical (El Salvador) C. lacinia incorporating solar radiation and avoidance posture.
4. Discussion
Based on the morphological, biophysical and climate change model results, tropical ectotherm populations may be more vulnerable to climate change than temperate populations, but impacts will depend heavily on thermoregulatory behaviour, habitat and regional contexts. These results generally corroborate previous studies based on air temperature changes that have demonstrated significant climate change threats to tropical organisms [6,15]. On the other hand, the results also show that heat avoidance behavioural responses can alter climate change impact projections and the negative consequences for tropical organisms can be diminished. Sunday et al. [20] suggested in a recent analysis of multiple ectotherm taxa (using a lizard biophysics model) that thermoregulatory behaviour will be a necessity for organisms to avoid warming impacts in the future. Yet there are fitness costs to behavioural thermoregulation (e.g. a butterfly cannot fly or reproduce when in heat avoidance posture). That tropical ectotherms are likely to be threatened by future climate change therefore remains a troubling prospect given these results together.
Though we have long known that organismal body temperature does not vary directly and linearly with changes in air temperature [27], the importance of including body temperature at large spatial scales for global climate change impact models is relatively new [22,28,43]. Here, we established that air temperature variation was not a good surrogate for body temperature variation in C. lacinia. Solar influences on body temperatures are particularly likely to have critical effects on organismal physiology [44,45]. Other regional climatic factors not included in this model such as surface temperatures (ground, plants, etc.), wind and precipitation are furthermore likely to affect impacts of climate [27,46,47], emphasizing the need for further research in biophysical components of global change ecology models [29].
Similarly, using biophysical and solar radiative forcings allows for more detailed behavioural analyses critical for understanding of climate change impacts [48]. Our results suggest that many tropical insects (and other organisms) of open habitats may be able to escape many of the direct consequences of warming by avoiding sun behaviourally through shade-seeking, orienting away from the sun or otherwise minimizing solar radiation. However, adult butterflies also have high behavioural flexibility relative to other life stages (eggs, larvae and pupae). Warming impacts may therefore not be avoidable for much of an organism's life cycle [23,45], though individuals can further respond to stress through diapause and plasticity, potentially [49]. The incorporation of habitat and landscape features can further provide for more complex behavioural responses to warming impacts [21,22].
Thermal variation is exhibited at multiple spatial and temporal scales. Temporally, recent studies have shown the importance of examining diurnal, seasonal and inter-annual time scales for understanding warming impacts across latitude [2,6,50,51]. In addition to altering performance curves [49], temporal variation can be critical for species in buffering climate change impacts, especially for species with limited behavioural thermoregulation capacity [52]. As shown in this study (figures 1 and 2), the variation in air temperature at different scales (minutes, hours, years) may not be entirely sufficient to characterize the thermal environment given the high variation in body temperature at these scales. Spatial thermal heterogeneity can similarly complicate warming predictions [15,26,43,53]. High-resolution spatial and temporal data will probably therefore be key in understanding larger-scale global change patterns [54].
Biophysical and mechanistic studies have significant predictive advantages in ecological climate warming models but also have the limitations that they are data-intensive and often case-specific [55]. Worse yet, even the most detailed biophysical models have limitations, such as lacking dynamic thermal profiles as a function of height [56]. Each of these concerns applies in this study because we include microclimate, thermal performance and morphological data for one species across latitude (C. lacinia), and yet even these data are incomplete (e.g. biophysics of juvenile stages and latitudinal variation in activity times in the field are not considered in this model). However, despite these limitations, the results presented here suggest that models that ignore biophysical and physiological factors do so at the risk of misrepresenting realistic thermal variation in the environment.
Starting at small temporal (e.g. minute) and spatial (e.g. metre) scales, we were able to identify the climatic parameters that dictate organismal experience of body temperature. Based on those results, we integrated the small-scale observations with a macroclimatic model that examined climate change effects at large temporal (century) and spatial (kilometre) scales. Future research will do well to manage the multiple scales at which climatic variation and adaptation are operating to achieve the goal of effectively assessing climate change vulnerability of species globally, and tropical species in particular.
Supplementary Material
Acknowledgements
Ward Watt provided essential guidance in the early stages of this project. Reynaldo Hernandez worked tirelessly in the field and we are grateful for the support from our Salvadoran collaborators; especially, Celia Castellanos, Oliver Komar, Karla Lara and Francisco Serrano. Sally Bonebrake's donation of a red 1995 Honda Passport to T.C.B. and subsequent repairs subsidized by Ken Bonebrake facilitated C. lacinia collection efforts.
Funding statement
Funding through the National Science Foundation (OISE-0832204) and the American Museum of Natural History (Student Support Award) made this research possible.
References
- 1.Janzen D. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 203–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 2.Ghalambor C, Huey R, Martin P, Tewksbury J, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 3.Corlett RT. 2012. Climate change in the tropics: the end of the world as we know it? Biol. Conserv. 151, 22–25. ( 10.1016/j.biocon.2011.11.027) [DOI] [Google Scholar]
- 4.Bonebrake TC. 2013. Conservation implications of adaptation to tropical climates from a historical perspective. J. Biogeogr. 40, 409–415. ( 10.1111/jbi.12011) [DOI] [Google Scholar]
- 5.IPCC. 2013. Climate change 2013: the physical science basis Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor C, Haak DC, Martin P. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillon M, Wang G, Huey R. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706. ( 10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 8.Sinervo B, et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899. ( 10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 9.Somero G. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine winners and losers. J. Exp. Biol. 213, 912–920. ( 10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 10.Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G. 2008. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 6, 2621–2626. ( 10.1371/journal.pbio.0060325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chown SL. 2012. Trait-based approaches to conservation physiology: forecasting environmental change risks from the bottom up. Phil. Trans. R. Soc. B 367, 1615–1627. ( 10.1098/rstb.2011.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCain C. 2009. Vertebrate range sizes indicate that mountains may be higher in the tropics. Ecol. Lett. 12, 550–560. ( 10.1111/j.1461-0248.2009.01308.x) [DOI] [PubMed] [Google Scholar]
- 15.Bonebrake TC, Deutsch C. 2012. Climate heterogeneity modulates impacts of warming on tropical insects. Ecology 93, 449–455. ( 10.1890/11-1187.1) [DOI] [PubMed] [Google Scholar]
- 16.Helmuth B, et al. 2006. Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol. Monogr. 76, 461–479. ( 10.1890/0012-9615(2006)076[0461:MPOTSI]2.0.CO;2) [DOI] [Google Scholar]
- 17.Ashcroft M, Chisholm L, French K. 2009. Climate change at the landscape scale: predicting fine-grained spatial heterogeneity in warming and potential refugia for vegetation. Glob. Change Biol. 15, 657–667. ( 10.1111/j.1365-2486.2008.01762.x) [DOI] [Google Scholar]
- 18.Bonebrake TC, Boggs CL, McNally JM, Ranganathan J, Ehrlich PR. 2010. Oviposition behavior and offspring performance in herbivorous insects: consequences of climatic and habitat heterogeneity. Oikos 119, 927–934. ( 10.1111/j.1600-0706.2009.17759.x) [DOI] [Google Scholar]
- 19.Chapperon C, Seuront L. 2011. Space-time variability in environmental thermal properties and snail thermoregulatory behaviour. Funct. Ecol. 25, 1040–1050. ( 10.1111/j.1365-2435.2011.01859.x) [DOI] [Google Scholar]
- 20.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Álvarez Pérez HJ, Garland T., Jr 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948. ( 10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan ML, Huynh RK, Precious RA, Calsbeek RG. 2013. The impact of climate change measured at relevant spatial scales: new hope for tropical lizards. Glob. Change Biol. 19, 3093–3102. ( 10.1111/gcb.12253) [DOI] [PubMed] [Google Scholar]
- 23.Kearney M, Shine R, Porter W. 2009. The potential for behavioral thermoregulation to buffer cold-blooded animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840. ( 10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huey RB, Tewksbury JJ. 2009. Can behavior douse the fire of climate warming? Proc. Natl Acad. Sci. USA 106, 3647–3648. ( 10.1073/pnas.0900934106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowlton JL, Graham CH. 2010. Using behavioral landscape ecology to predict species responses to land-use and climate change. Biol. Conserv. 143, 1342–1354. ( 10.1016/j.biocon.2010.03.011) [DOI] [Google Scholar]
- 26.Sears MW, Raskin E, Angilletta MJ., Jr 2011. The world is not flat: defining relevant thermal landscapes in the context of climate change. Integr. Comp. Biol. 51, 666–675. ( 10.1093/icb/icr111) [DOI] [PubMed] [Google Scholar]
- 27.Urban MC, Tewksbury JJ, Sheldon KS. 2012. On a collision course: competition and dispersal differences create no-analogue communities and cause extinctions during climate change. Proc. R. Soc. B 279, 2072–2080. ( 10.1098/rspb.2011.2367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gates DM. 1980. Biophysical ecology. New York, NY: Springer. [Google Scholar]
- 29.Helmuth B, Kingsolver JG, Carrington E. 2005. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu. Rev. Physiol. 67, 177–201. ( 10.1146/annurev.physiol.67.040403.105027) [DOI] [PubMed] [Google Scholar]
- 30.Buckley LB, Miller EF, Kingsolver JG. 2013. Ectotherm thermal stress and specialization across altitude and latitude. Integr. Comp. Biol. 53, 571–581. ( 10.1093/icb/ict026) [DOI] [PubMed] [Google Scholar]
- 31.Clusella-Trullas S, Blackburn TM, Chown SL. 2011. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am. Nat. 177, 738–751. ( 10.1086/660021) [DOI] [PubMed] [Google Scholar]
- 32.Ehrlich PR, Hanski IK. 2004. On the wings of checkerspots. Oxford, UK: Oxford University Press. [Google Scholar]
- 33.Neck RW. 1977. Food plant ecology of the butterfly Chlosyne lacinia (Nymphalidae) III. Adult resources. J. Res. Lepidoptera 16, 147–154. [Google Scholar]
- 34.Bonebrake TC, Watt W, Perez A, Boggs C. 2011. One variable species or multiple cryptic? Mitochondrial phylogeny of Central and North American Chlosyne lacinia (Lepidoptera: Nymphalidae). Eur. J. Entomol. 108, 529–535. ( 10.14411/eje.2011.068) [DOI] [Google Scholar]
- 35.Kingsolver JG, Moffat RJ. 1982. Thermoregulation and the determinants of heat transfer in Colias butterflies. Oecologia 53, 27–33. ( 10.1007/BF00377132) [DOI] [PubMed] [Google Scholar]
- 36.Kingsolver JG. 1983. Thermoregulation and flight in Colias butterflies: elevational patterns and mechanistic limitations. Ecology 64, 534–545. ( 10.2307/1939973) [DOI] [Google Scholar]
- 37.Helmuth BST. 1998. Intertidal mussel microclimates: predicting the body temperature of a sessile invertebrate. Ecol. Monogr. 68, 51–74. ( 10.1890/0012-9615(1998)068[0051:IMMPTB]2.0.CO;2) [DOI] [Google Scholar]
- 38.Stamberger JA. 2006. Adaptation to temporal scales of heterogeneity in the thermal environment. Dissertation, Stanford University, Stanford, CA. [Google Scholar]
- 39.Kingsolver JG, Watt WB. 1983. Thermoregulatory strategies in Colias butterflies: thermal stress and the limits to adaptation in temporally varying environments. Am. Nat. 121, 32–55. ( 10.1086/284038) [DOI] [Google Scholar]
- 40.Kingsolver JG. 1985. Butterfly thermoregulation: organismic mechanisms and population consequences. J. Res. Lepidoptera 24, 1–20. [Google Scholar]
- 41.New M, Lister D, Hulme M, Makin I. 2002. A high-resolution data set of surface climate over global land areas. Clim. Res. 21, 1–25. ( 10.3354/cr021001) [DOI] [Google Scholar]
- 42.Zhang Y, Rossow WB, Lacis AA, Oinas V, Mishchenko MI. 2004. Calculation of radiative fluxes from the surface to top of atmosphere based on ISCCP and other global data sets: refinements of the radiative transfer model and the input data. J. Geophys. Res. 109, D19105 ( 10.1029/2003JD004457) [DOI] [Google Scholar]
- 43.Gilman SE, Wethey DS, Helmuth B. 2006. Variation in the sensitivity of organismal body temperature to climate change over local and geographic scales. Proc. Natl Acad. Sci. USA 103, 9560–9565. ( 10.1073/pnas.0510992103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall DJ, McQuaid CD, Williams GA. 2010. Non-climatic thermal adaptation: implications for species’ responses to climate warming. Biol. Lett. 6, 669–673. ( 10.1098/rsbl.2010.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kingsolver JG, Woods HA, Buckley LB, Potter KA, MacLean HJ, Higgins JK. 2011. Complex life cycles and the responses of insects to climate change. Integr. Comp. Biol. 51, 719–732. ( 10.1093/icb/icr015) [DOI] [PubMed] [Google Scholar]
- 46.Bonebrake TC, Mastrandrea MD. 2010. Tolerance adaptation and precipitation changes complicate latitudinal patterns of climate change impacts. Proc. Natl Acad. Sci. USA 107, 12 581–12 586. ( 10.1073/pnas.0911841107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clusella-Trullas S, Chown S. 2011. Comment on ‘erosion of lizard diversity by climate change and altered thermal niches’. Science 332, 537 ( 10.1126/science.1195193) [DOI] [PubMed] [Google Scholar]
- 48.Chapperon C, Seuront L. 2011. Behavioral thermoregulation in a tropical gastropod: links to climate change scenarios. Glob. Change Biol. 17, 1740–1749. ( 10.1111/j.1365-2486.2010.02356.x) [DOI] [Google Scholar]
- 49.Hoffmann AA. 2010. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880. ( 10.1242/jeb.037630) [DOI] [PubMed] [Google Scholar]
- 50.Paaijmans KP, Heinig RL, Seliga RA, Blanford JI, Blanford S, Murdock CC, Thomas MB. 2013. Temperature variation makes ectotherms more sensitive to climate change. Glob. Change Biol. 19, 2373–2380. ( 10.1111/gcb.12240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kingsolver JG, Diamond SE, Buckley LB. 2013. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct. Ecol. 27, 1415–1423. ( 10.1111/1365-2435.12145) [DOI] [Google Scholar]
- 52.Kearney MR, Matzelle A, Helmuth B. 2012. Biomechanics meets the ecological niche: the importance of temporal data resolution. J. Exp. Biol. 215, 922–933. ( 10.1242/jeb.059634) [DOI] [PubMed] [Google Scholar]
- 53.Buckley LB, Tewksbury JJ, Deutsch CA. 2013. Can terrestrial ectotherms escape the heat of climate change by moving? Proc. R. Soc. B 280, 20131149 ( 10.1098/rspb.2013.1149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasseur DA, DeLong JP, Gilbert B, Greig HS, Harley CDG, McCann KS, Savage V, Tunney TD, O'Connor MI. 2014. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B 281, 20132612 ( 10.1098/rspb.2013.2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckley LB, Urban MC, Angilletta MJ, Crozier LG, Rissler LJ, Sears MW. 2010. Can mechanism inform species’ distribution models? Ecol. Lett. 13, 1041–1054. ( 10.1111/j.1461-0248.2010.01506.x) [DOI] [PubMed] [Google Scholar]
- 56.Buckley LB, Kingsolver JG. 2012. The demographic impacts of shifts in climate means and extremes on alpine butterflies. Funct. Ecol. 26, 969–977. ( 10.1111/j.1365-2435.2012.01969.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.