Abstract
Nocturnality is widespread among extant mammals and often considered the ancestral behavioural pattern for all mammals. However, mammals are nested within a larger clade, Synapsida, and non-mammalian synapsids comprise a rich phylogenetic, morphological and ecological diversity. Even though non-mammalian synapsids potentially could elucidate the early evolution of diel activity patterns and enrich the understanding of synapsid palaeobiology, data on their diel activity are currently unavailable. Using scleral ring and orbit dimensions, we demonstrate that nocturnal activity was not an innovation unique to mammals but a character that appeared much earlier in synapsid history, possibly several times independently. The 24 Carboniferous to Jurassic non-mammalian synapsid species in our sample featured eye morphologies consistent with all major diel activity patterns, with examples of nocturnality as old as the Late Carboniferous (ca 300 Ma). Carnivores such as Sphenacodon ferox and Dimetrodon milleri, but also the herbivorous cynodont Tritylodon longaevus were likely nocturnal, whereas most of the anomodont herbivores are reconstructed as diurnal. Recognizing the complexity of diel activity patterns in non-mammalian synapsids is an important step towards a more nuanced picture of the evolutionary history of behaviour in the synapsid clade.
Keywords: Synapsida, diel activity pattern, scleral ossicles, phylogenetic flexible discriminant analysis, ancestral state reconstruction
1. Introduction
Diel activity pattern is a behavioural characteristic of vertebrates that is fundamental for temporal and spatial resource partitioning [1]. Four main patterns are recognized [1–3]: (i) diurnal species are strictly active during the day; (ii) nocturnal species are strictly active at night; (iii) cathemeral species are active both day and night; and (iv) crepuscular species are active during twilight periods at dusk and dawn. The majority of extant mammals are nocturnal (45–55% of non-marine species) [4] and many of the remaining species, especially large-bodied ones, are cathemeral [1]. Diurnality is present in a few mammalian clades such as primates, but is much less widespread. Because nocturnality is so pervasive among extant mammals [5], it has long been hypothesized to be the ancestral activity pattern for the clade [6,7].
Conventional wisdom holds that the evolution of nocturnality was intimately tied to the origin of mammals (sensu [8]: a clade defined by the most recent common ancestor of Sinoconodon, morganucodontans and crown mammals) because the early history of mammals includes the evolution of a trait complex that seems consistent with nocturnal activity. Although endothermy is not a prerequisite of nocturnality, it may have provided a selective advantage to early mammals in cooler night environments. Early mammals were capable of bouts of rapid growth [9] and a dense pelage of hair was present in mammals that are phylogenetically not far-removed from the earliest members of the clade [10,11], suggesting that they were at least facultative endotherms (sensu [12]). Dramatically increased relative brain size and complexity in basal mammals compared with close outgroups [13] may reflect the need for improved non-visual sensory processing in low light (scotopic) environments [14]. The morphology and physiology of the visual system of extant mammals may be consistent with a prolonged nocturnal phase in the clade's evolution as well [6,15–19]. However, a recent re-evaluation of photopigment evolution suggests that ancestral mammals were active under twilight (mesopic) conditions [19,20]. Likewise, a proposed mechanism for the hypothesized evolutionary shift to nocturnality in early mammals [21], competition with and predation by diurnal dinosaurs, appears weakly supported. New data suggest that small predatory dinosaurs in particular were active at night [22], so nocturnal activity could not provide a complete refuge from dinosaurs for early mammals. Despite these recent findings, there are multiple lines of evidence supporting the view that at least non-diurnal activity patterns dominated the history of mammals.
Although details on exact timing and evolution of diel activity patterns within mammals are still wanting, an even wider gap of knowledge becomes apparent when considering a broader phylogenetic framework. Mammals are members of a larger amniote clade, Synapsida, and there is a large phylogenetic, morphological and ecological diversity of non-mammalian synapsids, with a fossil record extending back to the Late Carboniferous (Westphalian B; ca 312 Ma) [23]. In the light of this enormous diversity and the excellent quality of many fossils, studies of synapsid functional morphology are expected to yield valuable clues about the evolution of diel activity patterns and temporal resource partitioning among non-mammalian synapsids. Gaining a better understanding of the diel activity patterns of non-mammalian synapsids also may help to improve knowledge of the evolutionary dynamics of this behavioural trait in synapsids in general.
Our study is guided by a new method to indirectly determine the diel activity patterns of fossil amniotes: phylogenetic flexible discriminant analysis [4,22,24] of scleral ring and orbit dimensions, all of which strongly correlate with eyeball shape and optical function [3,25]. Discriminant analysis provides quantitative estimates of ocular image formation in a probabilistic framework that accounts for phylogenetic covariance among species. The morphology and distribution of scleral rings across tetrapods suggest that they are homologous [26], but all mammals (living and extinct) lack scleral rings [26]. However, scleral ossicles occur in nearly all major non-mammalian synapsid clades (figure 1; see the electronic supplementary material for further details). The presence of scleral ossicles in non-mammalian synapsids affords the opportunity to ‘retrodict’ the likely diel activity patterns in members of the mammalian stem lineage, providing a rich resource of fossil information about the early history and palaeobiology of the synapsids.
Figure 1.
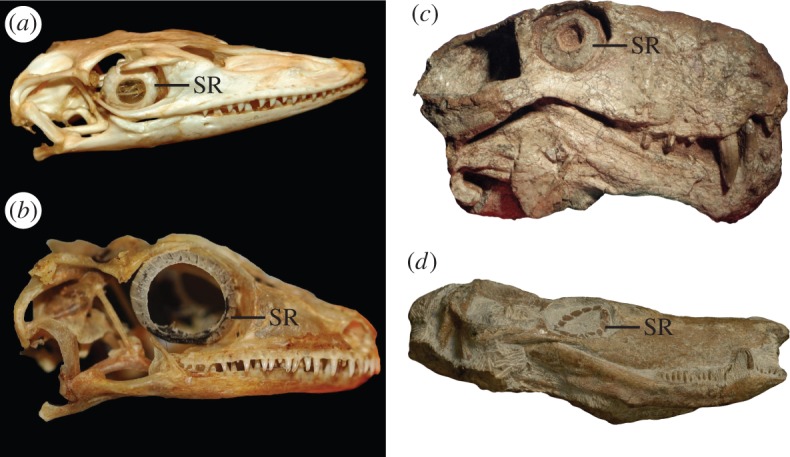
Examples of scleral rings in extant saurians and fossil synapsids. (a) Varanus niloticus (Varanidae; ROM R7303). (b) Rhachodactylus auriculatus (Gekkonidae; ROM R7510). (c) Sauroctonus cf. parringtoni (Gorgonopsia; SAM-PK-K10034). (d) Undescribed Zambian baurioid (Therocephalia; NHCC LB44). See the electronic supplementary material for institutional abbreviations. (Online version in colour.)
2. Results
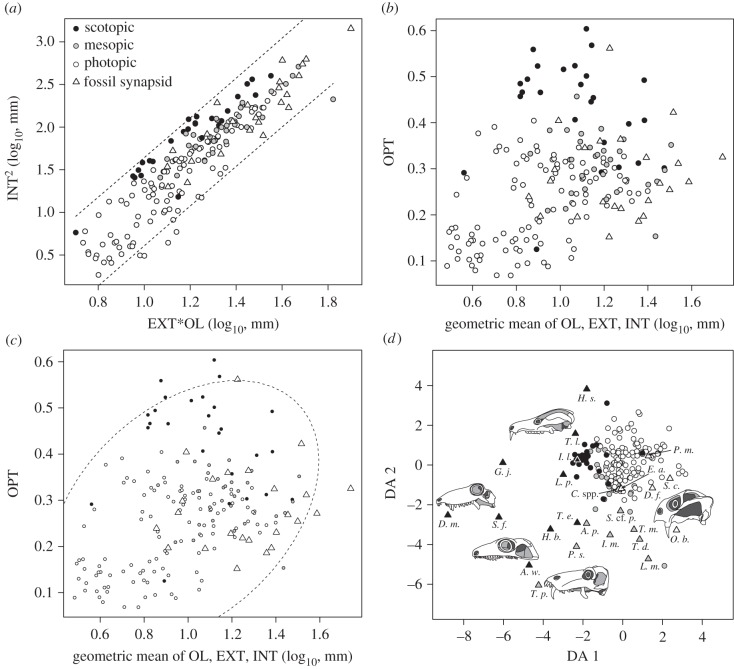
Our results (figures 2–4) show that non-mammalian synapsids featured scleral ring and orbit dimensions (electronic supplementary material) that indicate the presence of the full spectrum of diel activity patterns. First, we used the optical plot (the squared internal scleral ring diameter plotted against the product of external scleral ring diameter and orbit length) of Schmitz & Motani [3] to explore how fossil data compare to extant data (figure 2a). Fossil synapsids tend to have slightly larger overall eye size but are morphologically very similar to extant species. The optical plot further revealed that some fossil synapsids have a large internal scleral ring diameter for given orbit length and external scleral ring diameter, similar to some extant nocturnal species. Next, we introduced a new procedure to characterize eye morphology. We plotted the optical ratio, a proxy for light sensitivity, against the geometric mean of all three eye variables, a proxy for overall eye size (figure 2b). Larger eyes improve both light sensitivity and acuity, and by combining the optical ratio and eye size one may obtain additional information for separating the eyes of species with different diel activity patterns. Indeed, the plot shows that extant diurnal, cathemeral and nocturnal species are well separated. The eyes of diurnal species tend to be small with low optical ratio, whereas the eyes of nocturnal species have high optical ratios. Cathemeral species tend to have intermediate optical ratios but have larger eyes than both diurnal and nocturnal species overall. The eye morphologies of fossil synapsids are very similar to those of extant species and overlap with diurnal, cathemeral and nocturnal species (figure 2c). In order to account for the confounding effects of phylogeny as well as to obtain quantitative predictions of light sensitivity for fossil synapsids, we employed phylogenetic flexible discriminant analysis. The resulting discriminant space is informed by morphology, overall size and phylogenetic covariance and thus cannot be interpreted as a traditional morphospace (figure 2d; electronic supplementary material). Posterior probabilities (figure 3) suggest that nine species were scotopic (active in low light conditions), seven mesopic (intermediate light conditions) and five photopic (bright light conditions). Two species were ambiguously classified as mesopic/scotopic (figure 4). A strong latitudinal effect [28] on ocular image formation resulting from seasonal day length changes is unlikely. None of the species in our sample occurred deep within the polar regions, although species from the Karoo Basin lived at fairly high latitudes (approx. 60° South; electronic supplementary material). Therefore, we assume that the predicted ocular image formation type largely corresponds to diel activity patterns, but acknowledge the possibility that mesopic reconstructions of ocular image formation may be partially influenced by seasonal changes in environmental light levels.
Figure 2.
Scleral ring and orbit morphology of birds, lizards and fossil synapsids. (a) Optical plot sensu Schmitz & Motani [3] showing the similarity of eye morphologies across all groups, including synapsids. (b) The optical ratio [3] plotted against overall eye size showing clear separation between species with different diel activity patterns. (c) Fossil synapsids fall in the range of diurnal, cathemeral and nocturnal birds and lizards. (d) Discriminant space formed by scleral ring and orbit morphology. The two axes were defined with a phylogenetic flexible discriminant analysis of 164 extant saurian species and three categories of ocular image formation (mesopic = cathemeral, photopic = diurnal, scotopic = nocturnal) [23]. Species with large internal ring diameter (INT) compared with external diameter (EXT) tend to have small scores on discriminant axis (DA) 1, and species with large INT for given orbit length (OL) tend to have small values on DA 2. Hence, this discriminant space is in part a graphical representation of the optical ratio (INT2/EXT × OL) [3,22]. Values for fossil synapsids (triangles) occupy a larger area of discriminant space than extant saurians. This pattern is in part driven by their larger eyes, because scores of DA 1 and 2 are correlated with overall eye size [4], but it also reflects phylogenetic differences (electronic supplementary material).
Figure 3.
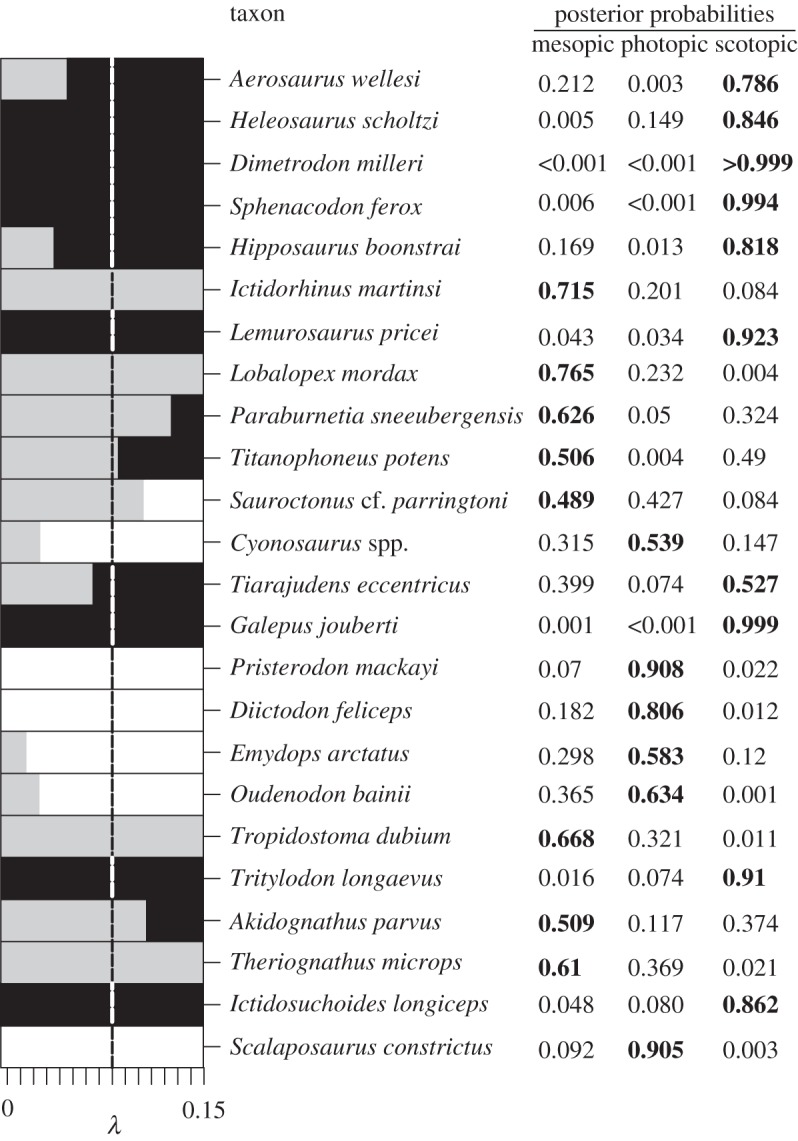
Classifications of fossil synapsids on the basis of phylogenetic flexible discriminant analysis. Posterior probabilities indicate that the full range of diel activity patterns was present in basal synapsids. The bar graph to the left demonstrates the importance of accounting for phylogenetic covariance among species, because varying λ results in different classifications (grey, mesopic; white, photopic; black, scotopic). We performed phylogenetic flexible discriminant analysis at λ = 0.08, which has been found to be optimal in this structure–function system [23], and allowed an error of ±0.02 to account for uncertainty.
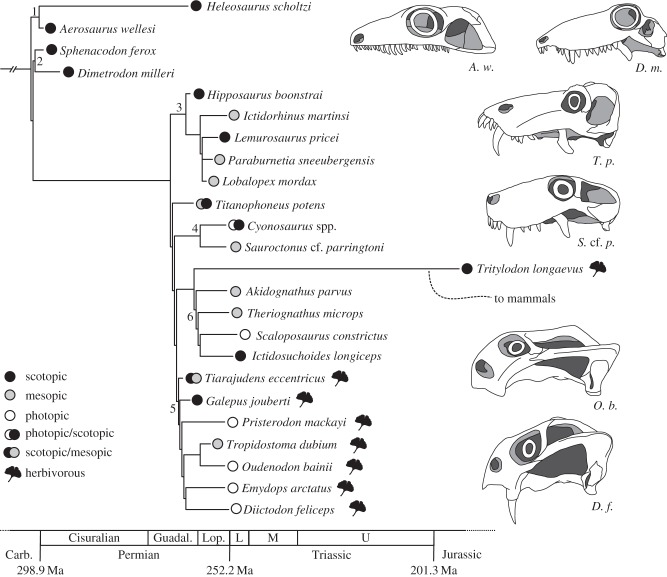
Figure 4.
Phylogenetic distribution of reconstructed diel activity patterns in non-mammalian synapsids. Our results suggest that fossil synapsids displayed the full range of diel activity patterns. Maximum-likelihood and Bayesian estimates of ancestral states (see the electronic supplementary material for details) indicate synapsids were nocturnal ancestrally, even though further sampling is required to make more formal assessments. Our results also suggest that foraging ecology is linked with diel activity patterns. Small herbivores tend to be diurnal, whereas carnivores are predominantly nocturnal or cathemeral. It is important to account for this complexity when discussing the evolution of diel activity patterns in mammals. Numbers denote the following major synapsid clades: 1 = Varanopidae, 2 = Sphenacodontidae, 3 = Biarmosuchia, 4 = Gorgonopsia, 5 = Anomodontia, 6 = Therocephalia. Titanophoneus potens is a member of Dinocephalia, and Tritylodon longaevus is a member of Cynodontia. Timescale after [27].
3. Discussion
The phylogenetic distribution of diel activity patterns in fossil synapsids indicates a surprisingly deep origin of nocturnality in the clade. All four basal ‘pelycosaur-grade’ species (Aerosaurus wellesi, Dimetrodon milleri, Heleosaurus scholtzi and Sphenacodon ferox) are reconstructed with scotopic ocular image formation (figure 4). The majority of therapsids also are reconstructed as being mesopic or scotopic; the primary exceptions to this pattern are the herbivorous anomodonts, which have scleral ring and orbit dimensions consistent with diurnal activity under photopic conditions (figure 4). Preliminary ancestral state reconstructions using maximum-likelihood and Bayesian approaches (electronic supplementary material) imply that activity under scotopic conditions is the ancestral character state for synapsids. Given that the earliest synapsids occur in the Late Carboniferous (ca 312 Ma) [28] and that scotopic taxa in our analysis such as Sphenacodon and Dimetrodon originate below the Permo-Carboniferous boundary [29], the initial invasion of nocturnal niches by synapsids must have occurred over 300 Myr ago [27], more than 100 Myr before the Late Triassic origin of mammals [8]. Moreover, when combined with recent observations for basal reptiles [30], our results raise the possibility that nocturnal activity patterns were relatively widespread in the early radiation of amniotes.
Although juvenile tetrapods tend to have relatively larger eyes than adults of the same species, it is unlikely that ontogenetic effects have biased our inferences of diel activity patterns among basal synapsids. Only three specimens in our dataset represent very early ontogenetic stages (defined here as having a basal skull length less than 25% of known maximum size for the species) and 21 of the 33 specimens for which we could make numerical estimates are 50% of maximum size or larger (electronic supplementary material). There are also six species in our dataset that are represented by multiple individuals that vary in size. These specimens largely give consistent estimates of optical light sensitivity for each species, and inconsistent cases almost always stem from poor preservation (electronic supplementary material). For example, two of the four specimens of Cyonosaurus we sampled were classified as photopic, one was classified as scotopic and one was classified as mesopic. However, the two best preserved specimens consistently returned a photopic result, and one of these two specimens is the smallest Cyonosaurus specimen in the dataset (approx. 46% of maximum size).
Early mammals feature an array of morphologies that are considered to be consistent with a nocturnal lifestyle, such as impedance-matched hearing capability, a keen olfactory sense and an elevated metabolic rate [31]. These traits are absent or rudimentary in non-mammalian synapsids, but comparisons with extant tetrapods show that they are not required for nocturnal activities. Many amphibians and squamates are nocturnal [32,33], despite having much lower metabolic rates than mammals. Likewise, the Caecilia and Caudata lack tympanic ears [34,35], yet many species in these two clades are nocturnally active [32,36]. Some anurans and squamates also lack tympanic ears [34,37]. Most of these species are diurnal (a few, such as Bombina bombina, are active both day and night), but some have been documented to have alternate means for detecting airborne sound [37–41], underscoring that the lack of a tympanic ear does not eliminate all hearing ability. Conversely, the dorsal sail of the basal synapsid Dimetrodon might be considered as an indicator of diurnal habits, contradicting our reconstruction of D. milleri as nocturnal, because the hypothesized heat exchange function of the sail is usually framed in the context of activity during daylight conditions [42–48]. Several recent studies have questioned the thermoregulatory role of the sail [49–52], however, and alternative functions have been proposed that do not require Dimetrodon to have been diurnal (electronic supplementary material).
Beyond the implications for the evolutionary origins of diel activity patterns in non-mammalian synapsids, our results hint at potential relationships between diel activity and other aspects of ecology. Diurnal activity appears to be correlated with the adoption of herbivorous diets in the synapsids we sampled, with 50% of the included herbivores being classified as photopic versus 6% of the included carnivores (figure 4). These findings are congruent with the patterns observed in Mesozoic archosaurs and extant mammals [22]. The herbivores among non-mammalian synapsids are not as large as their dinosaurian and some mammalian counterparts, so foraging and thermoregulatory constraints dictating a shift to cathemeral activity have diminished influence. Even among herbivores, diel activity patterns may have greater ecological importance than previously considered. For example, the dicynodonts Tropidostoma dubium and Oudenodon bainii display a high degree of morphological similarity, requiring varied data to be reliably differentiated [53], and their stratigraphic ranges overlap in the South African Karoo Basin. In our analysis, T. dubium is reconstructed as mesopic, whereas O. bainii is photopic, offering potential insight into how these species were able to successfully coexist. Diel activity plays an important role in temporal and spatial resource partitioning in modern terrestrial communities [1]; the example of T. dubium and O. bainii suggests that this partitioning was underway by the Late Permian (ca 258 Ma).
In summarizing our results, we emphasize the large diversity of scleral ring and orbit morphology in non-mammalian synapsids as well as the variety of inferred diel activity patterns. Our data indicate that non-diurnal activity patterns are far older than the origin of mammals, and even include the possibility of a largely nocturnal phase at the base of Synapsida. Eye morphologies consistent with intermediate light levels were slightly more prevalent among the sampled therapsids, with the exception of the mainly photopic anomodonts. The cynodont Tritylodon longaevus, the closest relative to mammals in our sample, is inferred as mainly active in low-light conditions. Given that we exclusively analysed non-mammalian synapsids, we cannot evaluate the timing of the origin of nocturnality in mammals, but we show that mammals were re-occupying the nocturnal niche and not invading a temporal niche that was fundamentally novel to Synapsida. Our findings demonstrate that eye morphology and likely diel activity patterns vary in a complex way among non-mammalian synapsids. Recognition of this complexity is an important step towards a more nuanced picture of the evolutionary history of diel activity patterns in synapsids. Future research will be needed to determine whether nocturnality was a novel behaviour pattern evolved by basal synapsids, or if they inherited it from an even more distant amniote or tetrapod ancestor.
4. Material and methods
(a). Construction of the time-calibrated phylogeny
The topology of the phylogenetic tree is a composite derived from several works. The backbone topology is primarily based on the study by Sidor & Hopson [54] and Cisneros et al. [55], although the topology also is consistent with Benson's [56] results for pelycosaur-grade synapsids. The topologies for Biarmosuchia and Therocephalia are based on the study by Sidor & Smith [57] and Sigurdson et al. [58], respectively, and the topology for Anomodontia is derived from Kammerer et al. [59]. In order to time-calibrate the tree, we collected information on the full stratigraphic ranges of the included taxa from various literature sources including [29,53,55,57,60–69], ultimately resulting in a non-ultrametric tree (electronic supplementary material). Stratigraphic ranges were converted to numerical time with two main resources, the modelled dates of Montañez et al. [70] for the varanopids and sphenacodontids, and the radiometric dates of Rubidge et al. [71] for the therapsids. In a few cases (e.g. T. longaevus), we used dates presented in [72] to estimate the ages of reported first and last occurrences. In order to convert the stratigraphic ranges and the topology into a fully time-calibrated tree, we followed an approach similar to [22]. In cases where a species was known from a single specimen, we arbitrarily assigned the species a branch length of 1 Myr. We verified that qualitative classification of fossils did not vary when terminal branches were excluded. In cases where stratigraphic ranges imply zero branch lengths, we arbitrarily added 0.5 Myr. After we time-calibrated the synapsid tree, we merged it with the previously established saurian tree by [22], using the bind.tree() function of the ‘ape’ package [73] for the statistical platform R [74]. We set the age of the amniote node to 324.5 Ma [75,76]. The entire nexus file of the complete tree is available in the electronic supplementary material.
(b). Phylogenetic flexible discriminant analysis
To characterize diel activity patterns in non-mammalian synapsids, we measured scleral ring and orbit dimensions with optical relevance in 38 synapsid specimens (electronic supplementary material) representing at least 24 species and belonging to eight major clades (Varanopidae, Sphenacodontidae, Biarmosuchia, Dinocephalia, Gorgonopsia, Anomodontia, Therocephalia and Cynodontia). We inferred ocular image formation for species averages (where applicable) using classification rules established by phylogenetic flexible discriminant analysis of a comparative dataset of 164 extant terrestrial saurians [22,24]. Discriminant analysis is a useful statistical method for predicting a categorical variable (such as ecology or behaviour) on the basis of continuous variables (such as morphological measurements). Group classification rules are established on the basis of a training dataset with known categorical variables, i.e. extant species in palaeobiological applications. The classification rules (‘discriminant functions’) are formed by combinations of those continuous variables that best discriminate between groups. Flexible discriminant analysis uses nonlinear group boundaries, but note that the type of discriminant function varies between different methods. Samples with unknown group membership, such as fossils, are assigned to groups by posterior probabilities calculated for each test sample. Phylogenetic flexible discriminant analysis accounts for phylogenetic covariance when predicting group membership, which should minimize erroneous conclusions. The correctly classified proportion in the training dataset of 164 extant terrestrial saurians is 79.3%, with most of the 34 misclassifications (70.6%) being false inferences of photopic image formation. We performed the analysis on a branch-length transformed tree that maximizes the correlation between form and function [22,24] (Pagel's λ = 0.08). Because the vertex formed by the likelihood distribution is wide, we allowed an error of 0.02 for classification purposes. We informed the discriminant analysis with prior probabilities for diel activity proportions of extant amniotes considered to reflect the full ecological diversity of Late Palaeozoic and Mesozoic ecosystems, with the majority of extant amniotes being photopic (58.5%), followed by scotopic (27.1%) and mesopic species (14.4%). Hence, the classification of non-mammalian synapsids as photopic is favoured a priori, whereas the inference of scotopic and mesopic ocular image formation is penalized. More background information on the method is provided in the electronic supplementary material.
Supplementary Material
Acknowledgements
We thank K. Brink, E. Butler, J. Cisneros, A. Huttenlocker and C. Kammerer for providing measurements of specimens we were unable to examine in person, and C. Kammerer for helpful discussion about the distribution of scleral ossicles in synapsids. R. Motani read earlier versions of the manuscript, and two anonymous reviewers provided helpful feedback. K.D.A. and L.S. designed the research; K.D.A. collected data; K.D.A. and L.S. analysed the data; K.D.A. and L.S. wrote the paper.
Data accessibility
All datasets are available on DRYAD (doi:10.5061/dryad.1v8kj). Fossil synapsid data and the phylogeny are also available in the electronic supplementary material.
Funding statement
Funding for this research was provided by the Field Museum of Natural History Department of Geology.
References
- 1.Kronfeld-Schor N, Dayan T. 2003. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Syst. 34, 153–181. ( 10.1146/annurev.ecolsys.34.011802.132435) [DOI] [Google Scholar]
- 2.Tattersall I. 2006. The concept of cathemerality: history and definition. Folia Primatol. 77, 7–14. ( 10.1159/000089692) [DOI] [PubMed] [Google Scholar]
- 3.Schmitz L, Motani R. 2010. Morphological differences between the eyeballs of nocturnal and diurnal amniotes revisited from optical perspectives of visual environments. Vis. Res. 50, 936–946. ( 10.1016/j.visres.2010.03.009) [DOI] [PubMed] [Google Scholar]
- 4.Schmitz L, Motani R. 2011. Response to comment on ‘Nocturnality in dinosaurs inferred from scleral ring and orbit morphology’. Science 334, 1641–1642. ( 10.1126/science.1208489) [DOI] [PubMed] [Google Scholar]
- 5.Jones KE, et al. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648 ( 10.1890/08-1494.1) [DOI] [Google Scholar]
- 6.Walls GL. 1942. The vertebrate eye and its adaptive radiation. Bloomfield Hills, MI: Cranbrook Institute of Science. [Google Scholar]
- 7.Crompton AW, Taylor RT, Jagger JA. 1978. Evolution of homeothermy in mammals. Nature 272, 333–336. ( 10.1038/272333a0) [DOI] [PubMed] [Google Scholar]
- 8.Kielan-Jaworowska Z, Cifelli RL, Luo ZX. 2004. Mammals from the age of dinosaurs: origins, evolution, and structure. New York, NY: Columbia University Press. [Google Scholar]
- 9.Hurum J, Chinsamy-Turan A. 2012. The radiation, bone histology, and biology of early mammals. In Forerunners of mammals: radiation, histology, biology (ed. Chinsamy-Turan A.), pp. 248–270. Bloomington, IL: Indiana University Press. [Google Scholar]
- 10.Meng J, Hu Y, Wang Y, Wang X, Li C. 2006. A Mesozoic gliding mammal from northeastern China. Nature 444, 889–893. ( 10.1038/nature05234) [DOI] [PubMed] [Google Scholar]
- 11.Ji Q, Luo ZX, Yuan X, Tabrum AR. 2006. A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science 311, 1123–1127. ( 10.1126/science.1123026) [DOI] [PubMed] [Google Scholar]
- 12.Grigg GC, Beard LA, Augee ML. 2004. The evolution of endothermy and its diversity in mammals and birds. Physiol. Biochem. Zool. 77, 982–997. ( 10.1086/425188) [DOI] [PubMed] [Google Scholar]
- 13.Rowe TB, Macrini TE, Luo ZX. 2011. Fossil evidence on the origin of the mammalian brain. Science 332, 955–957. ( 10.1126/science.1203117) [DOI] [PubMed] [Google Scholar]
- 14.Jerison HJ. 1973. Evolution of the brain and intelligence. New York, NY: Academic Press. [Google Scholar]
- 15.Menaker M, Moreira LF, Tosini G. 1997. Evolution of circadian organization in vertebrates. Braz. J. Med. Biol. Res. 30, 305–313. ( 10.1590/S0100-879X1997000300003) [DOI] [PubMed] [Google Scholar]
- 16.Jacobs GH, Rowe MP. 2004. Evolution of vertebrate colour vision. Clin. Exp. Optom. 87, 206–216. ( 10.1111/j.1444-0938.2004.tb05050.x) [DOI] [PubMed] [Google Scholar]
- 17.Hall MI, Kamilar JM, Kirk EC. 2012. Eye shape and the nocturnal bottleneck of mammals. Proc. R. Soc. B 279, 4962–4968. ( 10.1098/rspb.2012.2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellingham J, et al. 2006. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 4, e254 ( 10.1371/journal.pbio.0040254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerkema MP, Davies WIL, Foster RG, Menaker M, Hut RA. 2013. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc. R. Soc. B 280, 20130508 ( 10.1098/rspb.2013.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies WIL, Collin SP, Hunt DM. 2012. Molecular ecology and adaptation of visual photopigments in craniates. Mol. Ecol. 21, 3121–3158. ( 10.1111/j.1365-294X.2012.05617.x) [DOI] [PubMed] [Google Scholar]
- 21.Lillegraven JA. 1979. Introduction. In Mesozoic mammals: the first two-thirds of mammalian history (eds Lillegraven JA, Kielan-Jaworowska Z, Clemens WA.), pp. 1–6. Berkeley, CA: University of California Press. [Google Scholar]
- 22.Schmitz L, Motani R. 2011. Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science 322, 705–708. ( 10.1126/science.1200043) [DOI] [PubMed] [Google Scholar]
- 23.Kemp TS. 2012. The origin and radiation of therapsids. In Forerunners of mammals: radiation, histology, biology (ed. Chinsamy-Turan A.), pp. 2–28. Bloomington, IL: Indiana University Press. [Google Scholar]
- 24.Motani R, Schmitz L. 2011. Phylogenetic versus functional signals in the evolution of form-function relationships in terrestrial vision. Evolution 65, 2245–2257. ( 10.1111/j.1558-5646.2011.01271.x) [DOI] [PubMed] [Google Scholar]
- 25.Schmitz L. 2009. Quantitative estimates of visual performance features in fossil birds. J. Morphol. 270, 759–773. ( 10.1002/jmor.10720) [DOI] [PubMed] [Google Scholar]
- 26.Franz-Odendaal TA, Hall BK. 2006. Skeletal elements within teleost eyes and a discussion on homology. J. Morphol. 267, 1326–1337. ( 10.1002/jmor.10479) [DOI] [PubMed] [Google Scholar]
- 27.Gradstein FM, Ogg J, Schmitz MA, Ogg GA. 2012. Geologic time scale 2012. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 28.Pearce E, Dunbar R. 2012. Latitudinal variation in light levels drives human visual system size. Biol. Lett. 8, 90–93. ( 10.1098/rsbl.2011.0570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris SK, Lucas SG, Berman DS, Henrici AC. 2004. Vertebrate fossil assemblage from the Upper Pennsylvanian Red Tanks Member of the Bursum Formation, Lucero Uplift, Central New Mexico. New Mex. Mus. Nat. Hist. Sci. Bull. 25, 267–284. [Google Scholar]
- 30.Müller J, Tsuji LA. 2007. Impedance-matching hearing in Paleozoic reptiles: evidence of advanced sensory perception at an early stage of amniote evolution. PLoS ONE 2, e889 ( 10.1371/journal.pone.0000889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemp TS. 2005. The origin and evolution of mammals. Oxford, UK: Oxford University Press. [Google Scholar]
- 32.Wells KD. 2010. The ecology and behavior of amphibians. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 33.Vitt LJ, Pianka ER. 2007. Feeding ecology in the natural world. Lizard ecology (eds Reilly SM, McBrayer LD, Miles DB.), pp. 141–172. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 34.Wever EG. 1985. The amphibian ear. Princeton, NJ: Princeton University Press. [Google Scholar]
- 35.Mason MJ. 2007. Pathways for sound transmission to the inner ear in amphibians. In Hearing and sound communication in amphibians. Springer Handbook of Auditory Research (eds Narins PM, Feng AS, Fay RR, Popper AN.), pp. 147–183. New York, NY: Springer. [Google Scholar]
- 36.Wake MH. 1985. The comparative morphology and evolution of the eyes of caecilians (Amphibia, Gymnophiona). Zoomorphology 105, 277–295. ( 10.1007/BF00312059) [DOI] [Google Scholar]
- 37.Wever EG. 1978. The reptile ear. Princeton, NJ: Princeton University Press. [Google Scholar]
- 38.Hetherington TE, Linquist ED. 2010. Lung-based hearing in an ‘earless’ amphibian. J. Comp. Physiol. A 184, 395–401. ( 10.1007/s003590050338) [DOI] [Google Scholar]
- 39.Lindquist ED, Hetherington TE, Volman SF. 1998. Biomechanical and neurophysiological studies on audition in eared and earless harlequin frogs (Atelopus). J. Comp. Physiol. A 183, 265–271. ( 10.1007/s003590050254) [DOI] [PubMed] [Google Scholar]
- 40.Boistel R, Aubin T, Cloetens P, Langer M, Gillet B, Josset P, Pollet N, Herrel A. 2011. Whispering to the deaf: communication by a frog without external vocal sac or tympanic membrane in noisy environments. PLoS ONE 6, e22080 ( 10.1371/journal.pone.0022080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hetherington TE. 2001. Laser vibrometric studies of sound-induced motion of the body walls and lungs of salamanders and lizards: implications for lung-based hearing. J. Comp. Physiol. A 187, 499–507. ( 10.1007/s003590100220) [DOI] [PubMed] [Google Scholar]
- 42.Romer AS. 1948. Relative growth in pelycosaurian reptiles. In Robert Broom commemorative volume (ed. Du Toit AL.), pp. 45–55. Cape Town, South Africa: Royal Society of South Africa. [Google Scholar]
- 43.Robard S. 1949. On the dorsal sail of Dimetrodon. Copeia 1949, 224 ( 10.2307/1438996) [DOI] [Google Scholar]
- 44.Bramwell CD, Fellgett PB. 1973. Thermal regulation in sail lizards. Nature 242, 203–205. ( 10.1038/242203a0) [DOI] [Google Scholar]
- 45.Haack SC. 1986. A thermal model of the sailback pelycosaur. Paleobiology 12, 450–458. [Google Scholar]
- 46.Tracy CR, Turner JS, Huey RB. 1986. A biophysical analysis of possible thermoregulatory adaptations in sailed pelycosaurs. In The ecology and biology of mammal-like reptiles (eds Hotton N, MacLean PD, Roth JJ, Roth EC.), pp. 195–206. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 47.Florides GA, Wrobel LC, Kalogirou SA, Tassou SA. 1999. A thermal model for reptiles and pelycosaurs. J. Therm. Biol. 24, 1–13. ( 10.1016/S0306-4565(98)00032-1) [DOI] [PubMed] [Google Scholar]
- 48.Florides GA, Kalogirou SA, Tassou SA, Wrobel LC. 2001. Natural environment and thermal behaviour of Dimetrodon limbatus. J. Therm. Biol. 25, 15–20. ( 10.1016/S0306-4565(00)00019-X) [DOI] [PubMed] [Google Scholar]
- 49.Huttenlocker AK, Rega E, Sumida SS. 2010. Comparative anatomy and osteohistology of hyperelongate neural spines in the sphenacodontids Sphenacodon and Dimetrodon (Amniota: Synapsida). J. Morphol. 271, 1407–1421. ( 10.1002/jmor.10876) [DOI] [PubMed] [Google Scholar]
- 50.Huttenlocker AK, Mazierski D, Reisz RR. 2011. Comparative osteohistology of hyperelongate neural spines in the Edaphosauridae (Amniota: Synapsida). Palaeontology 54, 573–590. ( 10.1111/j.1475-4983.2011.01047.x) [DOI] [Google Scholar]
- 51.Tomkins JL, LeBas NR, Witton MP, Martill DM, Humphries S. 2010. Positive allometry and the prehistory of sexual selection. Am. Nat. 176, 141–148. ( 10.1086/653001) [DOI] [PubMed] [Google Scholar]
- 52.Rega EA, Noriega K, Sumida SS, Huttenlocker A, Lee A, Kennedy B. 2012. Healed fractures in the neural spines of an associated skeleton of Dimetrodon: implications for dorsal sail morphology and function. Fieldiana Life Earth Sci. 5, 104–111. ( 10.3158/2158-5520-5.1.104) [DOI] [Google Scholar]
- 53.Botha J, Angielczyk KD. 2007. An integrative approach to distinguishing the Late Permian dicynodont species Oudenodon bainii and Tropidostoma microtrema (Therapsida: Anomodontia). Palaeontology 50, 1175–1209. ( 10.1111/j.1475-4983.2007.00697.x) [DOI] [Google Scholar]
- 54.Sidor CA, Hopson JA. 1998. Ghost lineages and ‘mammalness’: assessing the temporal pattern of character acquisition in the Synapsida. Paleobiology 24, 254–273. [Google Scholar]
- 55.Cisneros JC, Abdala F, Rubidge BS, Dentzien-Dias PC, de Oliveira Bueno A. 2011. Dental occlusion in a 260-million-year-old therapsid with saber canines from the Permian of Brazil. Science 331, 1603–1605. ( 10.1126/science.1200305) [DOI] [PubMed] [Google Scholar]
- 56.Benson RBJ. 2012. Interrelationships of basal synapsids: cranial and postcranial morphological partitions suggest different topologies. J. Syst. Palaeontol. 10, 601–624. ( 10.1080/14772019.2011.631042) [DOI] [Google Scholar]
- 57.Sidor CA, Smith RMH. 2007. A second burnetiamorph therapsid from the Permian Teekloof Formation of South Africa and its associated fauna. J. Vertebr. Paleontol. 27, 420–430. ( 10.1671/0272-4634(2007)27[420:ASBTFT]2.0.CO;2) [DOI] [Google Scholar]
- 58.Sigurdson T, Huttenlocker AK, Modesto SP, Rowe TB. 2012. Reassessment of the morphology and paleobiology of the therocephalian Tetracynodon darti (Therapsida), and the phylogenetic relationships of Baurioidea. J. Vertebr. Paleontol. 32, 1113–1134. ( 10.1080/02724634.2012.688693) [DOI] [Google Scholar]
- 59.Kammerer CF, Angielczyk KD, Fröbisch J. 2011. A comprehensive taxonomic revision of Dicynodon (Therapsida, Anomodontia), and its implications for dicynodont phylogeny, biogeography, and biostratigraphy. Soc. Vert. Paleontol. Mem. 11, 1–158. ( 10.1080/02724634.2011.627074) [DOI] [Google Scholar]
- 60.Reisz RR. 1986. Pelycosauria. Handbuch der Paläoherpetologie 17A. Stuttgart, Germany: Gustav Fischer Verlag. [Google Scholar]
- 61.Hopson JA, Barghusen HR. 1986. An analysis of therapsid relationships. In The ecology and biology of mammal-like reptiles (eds Hotton N, MacLean PD, Roth JJ, Roth EC.), pp. 83–106. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 62.King GM. 1988. Anomodontia. Handbuch der Paläoherpetologie 17C. Stuttgart, Germany: Gustav Fischer Verlag. [Google Scholar]
- 63.Rubidge BS. 1995. Biostratigraphy of the Beaufort Group (Karoo Supergroup). S. Afr. Committee Stratigr. Biostratigr. Ser. 1, 1–46. [Google Scholar]
- 64.Angielczyk KD, Fröbisch J, Smith RMH. 2005. On the stratigraphic range of the dicynodont taxon Emydops (Therapsida, Anomodontia) in the Karoo Basin, South Africa. Palaeontol. Afr. 41, 23–33. [Google Scholar]
- 65.Knoll F. 2005. The tetrapod fauna of the Upper Elliot and Clarens formations in the main Karoo Basin (South Africa and Lesotho). Bull. Soc. Geol. France 176, 81–91. ( 10.2113/176.1.81) [DOI] [Google Scholar]
- 66.Fröbisch J. 2008. Global taxonomic diversity of anomodonts (Tetrapoda, Therapsida) and the terrestrial rock record across the Permian–Triassic boundary. PLoS ONE 3, e3733 ( 10.1371/journal.pone.0003733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huttenlocker A. 2009. An investigation into the cladistic relationships and monophyly of therocephalian therapsids (Amniota: Synapsida). Zool. J. Linn. Soc. Lond. 157, 865–891. ( 10.1111/j.1096-3642.2009.00538.x) [DOI] [Google Scholar]
- 68.Campione NE, Reisz RR. 2010. Varanops brevirostris (Eupelycosauria: Varanopidae) from the Lower Permian of Texas, with discussion of varanopid morphology and interrelationships. J. Vertebr. Paleontol. 30, 724–746. ( 10.1080/02724631003762914) [DOI] [Google Scholar]
- 69.Lucas SG, et al. 2010. Vertebrate paleontology, biostratigraphy, and biochronology of the Pennsylvanian-Permian Cutler Group, Cañon del Cobre, northern New Mexico. New Mex. Mus. Nat. Hist. Sci. Bull. 49, 115–123. [Google Scholar]
- 70.Montañez IP, et al. 2007. CO2-forced climate and vegetation instability during Late Paleozoic deglaciation. Science 315, 87–91. ( 10.1126/science.1134207) [DOI] [PubMed] [Google Scholar]
- 71.Rubidge BS, Erwin DH, Ramezani J, Bowring SA, de Klerk WJ. 2013. High-precision temporal calibration of Late Permian vertebrate biostratigraphy: U-Pb zircon constraints from the Karoo Supergroup, South Africa. Geology 41, 363–366. ( 10.1130/G33622.1) [DOI] [Google Scholar]
- 72.Gradstein FJ, Ogg JG, Smith A. 2004. A geologic time scale. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 73.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 74.R Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 75.Hedges SB, Dudley J, Kumar S. 2006. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics 22, 2971–2972. ( 10.1093/bioinformatics/btl505) [DOI] [PubMed] [Google Scholar]
- 76.Kumar S, Hedges SB. 2011. TimeTree2: species divergence times on the iPhone. Bioinformatics 27, 2023–2024. ( 10.1093/bioinformatics/btr315) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets are available on DRYAD (doi:10.5061/dryad.1v8kj). Fossil synapsid data and the phylogeny are also available in the electronic supplementary material.