Abstract
Sharks are one of the most threatened groups of marine animals worldwide, mostly owing to overfishing and habitat degradation/loss. Although these cartilaginous fish have evolved to fill many ecological niches across a wide range of habitats, they have limited capability to rapidly adapt to human-induced changes in their environments. Contrary to global warming, ocean acidification was not considered as a direct climate-related threat to sharks. Here we show, for the first time, that an early ontogenetic acclimation process of a tropical shark (Chiloscyllium punctatum) to the projected scenarios of ocean acidification (ΔpH = 0.5) and warming (+4°C; 30°C) for 2100 elicited significant impairments on juvenile shark condition and survival. The mortality of shark embryos at the present-day thermal scenarios was 0% both at normocapnic and hypercapnic conditions. Yet routine metabolic rates (RMRs) were significantly affected by temperature, pH and embryonic stage. Immediately after hatching, the Fulton condition of juvenile bamboo sharks was significantly different in individuals that experienced future warming and hypercapnia; 30 days after hatching, survival rapidly declined in individuals experiencing both ocean warming and acidification (up to 44%). The RMR of juvenile sharks was also significantly affected by temperature and pH. The impact of low pH on ventilation rates was significant only under the higher thermal scenario. This study highlights the need of experimental-based risk assessments of sharks to climate change. In other words, it is critical to directly assess risk and vulnerability of sharks to ocean acidification and warming, and such effort can ultimately help managers and policy-makers to take proactive measures targeting most endangered species.
Keywords: sharks, climate change, early ontogeny
1. Introduction
Tropical sea surface temperatures have increased up to 0.5–0.6°C since the mid-nineteenth century owing to the weakening of tropical atmospheric circulation [1,2]. Model projections also suggest that average surface air temperatures will increase between 2°C and 5°C by 2100 owing to anthropogenic forcing [1]. This additional heat will be partially absorbed by the oceans, and future ocean warming is expected to negatively impact the performance and survival of several tropical organisms that already live close to their thermal tolerance limits [3]. Marine tropical biota will be more sensitive to future warming than the subtropical and temperate ones, as they evolved in a relatively stable thermal environment [4,5]. Concomitantly, [CO2]atm has increased from 280 ppm (pre-industrial levels) to values now exceeding 380 ppm [6], and is expected to rise to 730–1020 ppm by the year 2100 [1]. Carbon dioxide reacts with seawater, resulting in a net increase in the concentrations of H+ (lowered pH), H2CO3 and 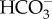 , and a decrease in
, and a decrease in 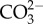 . This process, termed ocean acidification, is projected to decrease the pH of surface waters between 0.14 and 0.5 units, depending on emission scenario, by the end of the twenty-first century [1].
. This process, termed ocean acidification, is projected to decrease the pH of surface waters between 0.14 and 0.5 units, depending on emission scenario, by the end of the twenty-first century [1].
While some species may demonstrate phenotypic plasticity to these future environmental conditions, others are expected to shift their distributions to areas favourable to maintaining physiological optima [7]. Although acclimation (e.g. developmental and transgenerational plasticity) will occur to allow some species to adjust to future ocean conditions [8], standing genetic variability is also an important factor [9]. Yet it is not known whether evolution can occur rapidly enough in certain marine species (e.g. with more complex life histories or long generation times) [9].
Sharks occupy high trophic levels in marine habitats, and play a key role in the structure, function and health of marine ecosystems [10,11]. Most sharks are at a relatively high risk to be negatively impacted by climate change owing to their slow rates of evolution and low phenotypic plasticity. In other words, their tendency towards late age at maturity (and long lifespan) and low fecundity may impair a prompt adaptive response to rapidly changing environmental conditions, especially in tropical regions [12,13]. Despite the ecological relevance of sharks as apex predators, information on physiological impacts of climate change on sharks is still scarce. Although the metabolism of most sharks is known to be determined by ambient temperatures [14], it is puzzling that, to date, no experiments have been conducted to assess the potential impact that ocean warming and acidification may have on these cartilaginous fish. Nonetheless, it has been argued through ‘ecological risk assessments’ for climate change that ocean acidification will not directly affect sharks; these studies hypothesize that sharks may only be indirectly affected through changes in their habitat, marine community structure or prey availability [13].
The aim of this study was to experimentally investigate, for the first time, the impact of ocean warming (+4°C) and ocean acidification (ΔpH = 0.5) on elasmobranchs, namely on the early ontogeny (embryos and recently hatched juveniles) of the tropical bamboo shark Chiloscyllium punctatum. This small, bottom-dwelling and oviparous shark commonly inhabits Indo-West Pacific intertidal areas, from sandy and rocky substrates to sea grass beds and coral reefs [15]. It is worth noting that the Indo-West Pacific region has been warming at a relatively rapid rate compared with several other world regions [2,16]. Moreover, early ontogenetic stages of several marine species are expected to be the most vulnerable to such climatic shifts [17,18], and this higher vulnerability may ultimately become a serious bottleneck for the persistence of those species in the ocean of tomorrow. Under this context, we evaluated the effect of both warming and ocean acidification on the (i) embryonic survival (%), (ii) development time, (iii) embryo's specific growth rate (SGR) (% day−1), (iv) yolk consumption (cm3 day−1), (v) metabolic rates of early, intermediate and pre-hatching embryos, (vi) Fulton condition of newly hatched juveniles, and (vi) survival, metabolic and ventilation rates of juvenile bamboo sharks.
2. Material and methods
(a). Egg collection and incubation
Sixty recently spawned embryos of bamboo shark (C. punctatum) were collected by hand by local fishermen between April and July 2013 in the area of Lungsod Ng Cebu (Philippines; around 10°11′N 123°58′E) and transported by Tropical Marine Centre UK. On arrival at the aquaculture facilities of Laboratório Marítimo da Guia (LMG-Cascais, Portugal), all embryos were still irregular in shape and colour, and motionless [19]. The shark embryos were incubated (suspended 5 cm below the water surface with strings to ensure good aeration) within twelve (three replicates per treatment) 50 l incubation systems (n = 5 per system). To understand the development and physiological mechanisms by which shark early ontogenetic stages may (or may not) be able to withstand future ocean changes, the embryos were acclimated at (i) rising pCO2 (ΔpH = 0.5; 0.14% CO2), and (ii) ocean warming expected in 2100 [1], 4°C above the average ambient temperature (26°C) in that Indo-West Pacific region [2,16]. More specifically, the embryos were reared at (i) 26°C pH 8.0 (temperature and pH control), (ii) 26°C pH 7.5 (temperature control and hypercapnic scenario), (iii) 30°C pH 8.0 (warming scenario with pH control), and (iv) 30°C pH 7.5 (warming and hypercapnic scenario). The respective control pCO2 values (see electronic supplementary material, table S1) were found within the intervals obtained by McElhany & Busch's [20] Indo-West Pacific region.
The life support systems were replenished daily with 100 l of new seawater to maintain total alkalinity and dissolved inorganic carbon speciation owing to bacterial activity (i.e. nitrifiers, denitrifiers) and acidification of the treatments (two out of four). The semi-closed systems were filled with 1 µm filtered, UV-irradiated seawater, with the tanks being illuminated from above with white fluorescent lamps under a photoperiod of 14 h light : 10 h dark. Water quality was ensured using wet–dry filters (BioBalls), protein skimmers (Schuran, Jülich, Germany) and 30 W UV-sterilizers (TMC, Chorleywood, UK). Ammonia and nitrite were monitored regularly and kept below detectable levels. pH was adjusted automatically, via solenoid valves, with the Profilux controlling system (Kaiserslautern, Germany) connected to individual pH probes. pH values were monitored every 2 s and lowered by injection of a certified CO2 gas mixture (Air Liquid, Portugal) via air stones or upregulated by aerating the tanks with CO2-filtered (using soda lime, Sigma-Aldrich) air. Seawater carbonate system speciation was calculated weekly from total alkalinity according to Sarazin et al. [21] (spectrophometrically at 595 nm) and pH measurements. All seawater physico-chemical parameters of the different experimental (temperature and pH) set-ups are shown in electronic supplementary material, table S1. pH was quantified via a Metrohm pH meter (826 pH mobile; Metrohm, Filderstadt, Germany) connected to a glass electrode (Schott IoLine, SI analytics, ± 0.001) and calibrated against the seawater buffers Tris–HCl (Tris) and 2-aminopyridine–HCl (Mare, Liège, Belgium) according to Dickson et al. [22]. pH measurements were performed under temperature-controlled conditions using a water bath (Lauda, Germany,±0.1°C). Bicarbonate and pCO2 values were calculated using the CO2SYS software [23], with dissociation constants from Mehrbach et al. [24] as refitted by Dickson & Millero [25].
(b). Survival and development time
Upon arrival at our facilities, the age of each individual shark was estimated according to the embryonic descriptions of Ballard et al. [26] and Harahush et al. [19]. The 60 encapsulated embryos were then individually tagged in the egg capsule (with coloured zip ties), and the opaque external fibrous layer of the case was scraped off to enable visual and photographic analyses. As a result, the developing embryos could be easily surveyed inside their capsules when placed in front of a light source. This procedure was conducted every day to check for survival throughout embryogenesis. Photographs were taken on a weekly basis, and each embryo was analysed through the use of the image-processing software ImageJ to determine the yolk sac length and width, and the embryo's total length (TL). The yolk measurements were then used to calculate the yolk volume using the formulae V = 1/6(πW2 L) (V, volume; W, width; L, length) [27].
The embryo SGR was determined using the formula [(ln embryo TL (T2) – ln embryo TL (T1))/number of days elapsed between T1 and T2] × 100, with T1 being the previous (week) measuring date and T2 being the latter measuring date. Development time was defined as time until hatching.
(c). Fulton condition after hatching
Immediately after hatching, juvenile bamboo sharks were removed from their respective incubation systems, weighed, measured (TL) and individually tagged beneath the dorsal fin (Floy Tag & Mfg). Fulton's condition (measure of individual fish's health) was calculated using the formula K = (weight/TL3) × 100. Afterwards, the juvenile sharks were placed again in the same incubation systems and fed with mysid shrimps ad libitum on a daily basis (during 30 days post-hatching).
(d). Routine metabolic rates, thermal sensitivity and ventilation rates
Oxygen consumption rates, here used as a proxy of routine metabolic rates (RMRs), were determined according to Rosa & Seibel [28,29]. RMR was analysed at four different stages of early development: (i) early embryos (body still somewhat transparent and presence of external filamentous gills; equivalent to stages 25–29 of Ballard et al. [26]); (ii) intermediate embryos (with more or less half the initial yolk volume already consumed and dark brown brands are present over a light pink body; equivalent to stages 30 and 32 of Ballard et al. [26]); (iii) late/pre-hatching embryos (embryo occupies the entire area of the circular pouch, with no visible yolk; equivalent to stages 33–34 in Ballard et al. [26]); and (iv) juveniles (30 days post-hatching). It is worth noting that between 30 and 40 days after deposition, the bottom edge of the egg case of the bamboo shark weakens and the respiratory fissures (or marginal seals) open, allowing the entry of seawater and subsequent circulation within the egg case. Consequently, and contrary to what happens in some invertebrate groups [30], the shark embryonic development does not elicit dramatic changes in the abiotic conditions inside the egg case.
Embryonic and juvenile stages (n = 5 per stage) were placed within a flow-through respirometry set-up (1 l; Loligo Systems, Denmark) connected to the different incubation systems (i.e. sharing the same seawater carbonate characteristics of the different thermal and CO2 treatments). To avoid bacterial contaminations, the seawater from the incubation systems was filtered (0.2 µm) and UV-irradiated before reaching the respirometers. The water was pumped at a constant flow rate (between 5 and 50 ml min−1, for early embryos and juveniles, respectively) through the respirometers with the use of peristaltic pumps (Cole-Parmer, USA). Oxygen concentrations were recorded at the entrance and exit of each respirometer chamber with Clarke-type O2 electrodes connected to a Strathkelvin Instruments 929 Oxygen Interface. Before and after each run, the experimental set-up was calibrated and checked for electrode drift and microbial oxygen consumption. Each experiment was 6 h long: 2 h for acclimation and the following 4 h for oxygen measurement.
Thermal sensitivity (Q10) was determined using the standard equation Q10 = [R(T2)/R(T1)]10/(T2–T1), where R(T2) and R(T1) represent the oxygen consumption rates at temperatures T2 and T1, respectively.
After 30 days post-hatching, each juvenile shark was observed within the incubation systems in order to register their ventilation rates (number of breaths per minute). This procedure was repeated three times per individual (each with 5 min duration). Bamboo sharks were observed in the afternoon before they were fed (to exclude any potential bias caused by feeding on the respiration).
(e). Statistical analyses
Two-way ANOVAs (repeated measurements; using pH and temperatures as variables) were conducted to detect significant differences in development time, yolk consumption, SGRs, juvenile RMR and ventilation rates. The differences in embryos' RMR were investigated with three-way ANOVAs (i.e. with pH, temperature and life stages as variables). Normality and homogeneity of variances were verified by Kolmogorov–Smirnov and Bartlett tests, respectively. Subsequently, post hoc tests (Tukey HSD and unequal N HSD) were performed. All statistical analyses were performed for a significance level of 0.05, using Statistica v. 10.0 software (StatSoft, Tulsa, USA).
3. Results
(a). Embryonic survival and development time
Survival of bamboo shark embryos at the present-day thermal scenario (26°C) was 100% at both normocapnic and hypercapnic conditions (figure 1a). However, under future ocean warming (+4°C), survival significantly decreased (two-way ANOVA, p < 0.05), ranging between 80% and 89% at normocapnic and hypercapnic conditions, respectively. No significant effect of pH on embryonic survival was observed (p > 0.05; see also electronic supplementary material, table S2).
Figure 1.
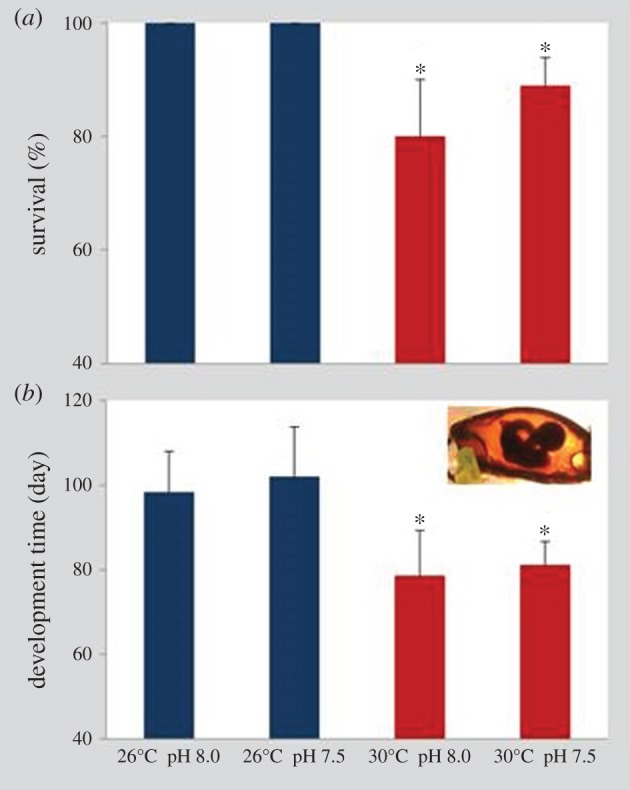
Impact of ocean acidification (ΔpH 0.5) and warming (+4°C) on (a) survival (%) and (b) development time of bamboo shark (Chiloscyllium punctatum) embryos. Values represent mean ± s.d. Asterisks represent significant differences between temperature treatments at the same pH condition. For more statistical details, see electronic supplementary material, table S2. (Online version in colour.)
The duration of the embryonic period was shortened with increasing temperature in both pH treatments (figure 1b, two-way ANOVA, p < 0.05; electronic supplementary material, table S2). At present-day temperature and pH conditions, embryogenesis lasted 98 ± 9 days; warming up to 30°C, under normocapnia, caused embryos to hatch after 79 ± 11 days. Under hypercapnia, embryogenesis at 26°C lasted 102 ± 12 days at 26°C, whereas under the future warming and hypercapnic scenario, it was completed in 81 ± 6 days. The increase in development time at lower pH (7.5) under both thermal treatments was not significant (p > 0.05; see also electronic supplementary material, table S2).
(b). Yolk consumption and specific growth rates
Yolk consumption rates were similar between normocapnia and hypercapnia at both thermal scenarios (figure 2a; two-way ANOVA, p > 0.05). Yet yolk was consumed at a significantly higher rate at 30°C (about 0.16 cm3 day−1) than at 26°C (around 0.12 cm3 day−1; p < 0.05; electronic supplementary material, table S2).
Figure 2.
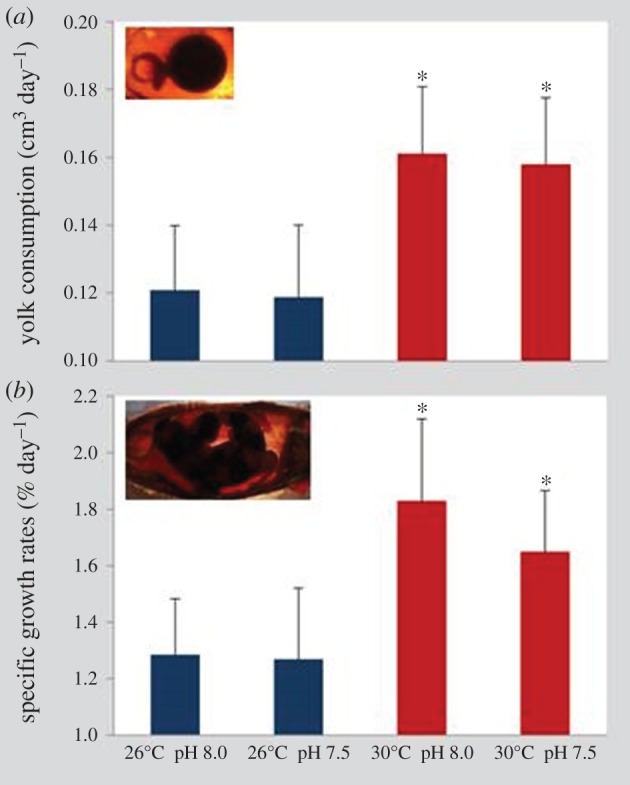
Impact of ocean acidification (ΔpH 0.5) and warming (+4°C) on (a) yolk consumption (cm3 day−1) and (b) specific growth rate (% day−1) of developing embryos of bamboo shark (Chiloscyllium punctatum). Values represent mean ± s.d. Asterisks represent significant differences between temperature treatments at the same pH condition. For more statistical details, see electronic supplementary material, table S2. (Online version in colour.)
SGRs showed similar trends in treatment-specific differences; that is, while pH did not elicit any significant change (figure 2b; two-way ANOVA, p > 0.05), the warming scenario significantly increased embryonic growth (about 1.8% day−1; p < 0.05; electronic supplementary material, table S2) when compared with the present-day thermal scenario (around 1.3% day−1).
(c). Metabolic rates and thermal sensitivity of shark embryos
RMRs were significantly affected by temperature, pH and embryonic stage (early, intermediate and late/pre-hatching embryos; figure 3; three-way ANOVA, p < 0.05; electronic supplementary material, table S3). At all three embryonic stages, hypercapnia led to a significant decrease in RMR under the warming scenario (p < 0.05). RMR values ranged between 6.33 (in early stages under 26°C and pH 7.5) and 127.01 µmol O2 h−1 per embryo (pre-hatching stage under 30°C and pH 8.0). Consequently, embryonic Q10 values (i.e. thermal sensitivity of metabolism between 26°C and 30°C) ranged above 3 under normocapnia across all embryonic stages, and close to 1.5 in intermediate and late stages acclimated to low pH (grey line in figure 4).
Figure 3.
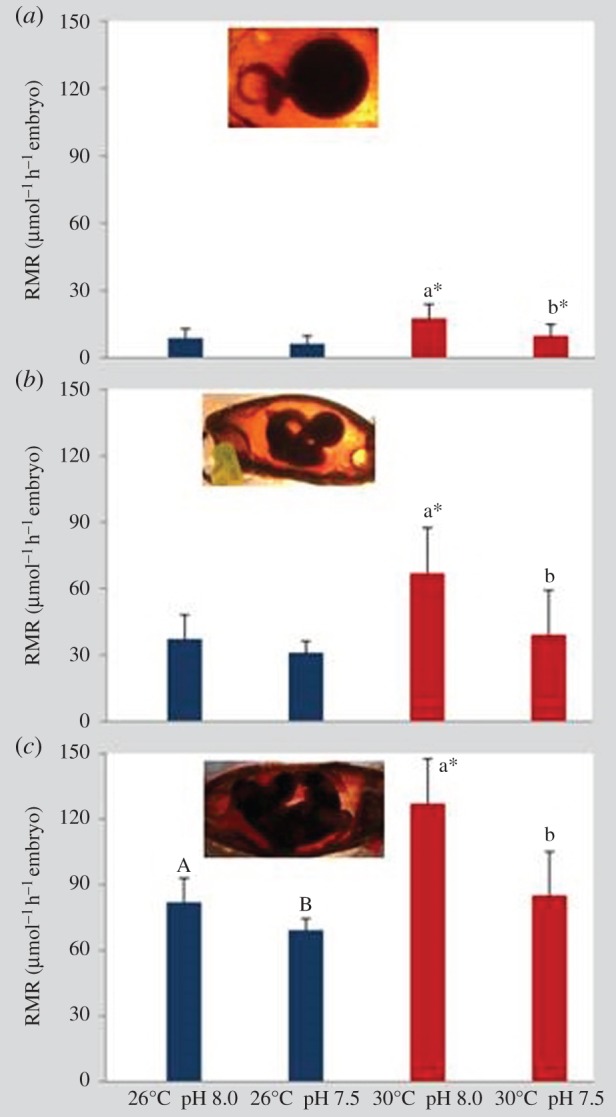
Impact of ocean acidification (ΔpH 0.5) and warming (+4°C) on routine metabolic rate (µmol O2 h−1 embryo) of (a) early stage embryos, (b) intermediate stage embryos and (c) pre-hatching embryos of bamboo shark (Chiloscyllium punctatum). n = 5 per stage. Values represent mean ± s.d. Different upper- and lower-case letters represent statistical differences between pH treatments at 26°C and 30°C, respectively (p < 0.05). Asterisks represent significant differences between temperature treatments at the same pH condition. For more statistical details, see electronic supplementary material, table S3. (Online version in colour.)
Figure 4.
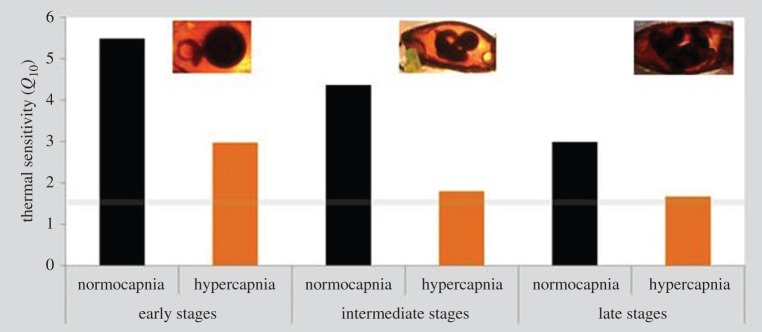
Impact of ocean acidification (ΔpH 0.5) and warming (+4°C) on the thermal sensitivity (Q10; between 26°C and 30°C) of early, intermediate and late embryonic stages of bamboo shark (Chiloscyllium punctatum). Grey line represents a threshold below which the levels are indicative of active metabolic depression. (Online version in colour.)
(d). Fulton condition of recently hatched sharks and survival
Immediately after hatching (day 0), the Fulton condition (K) of juvenile bamboo sharks was significantly different in individuals that experienced future warming and hypercapnia (figure 5a; two-way ANOVA, p < 0.05; electronic supplementary material, table S4). Nonetheless, while pH did not affect K under present-day thermal conditions (K-values between 0.37 and 0.39), K changed under the future warming condition (K = 0.34). Thirty days after hatching, survival rapidly declined in individuals experiencing both ocean warming and acidification (figure 5b; two-way ANOVA, p < 0.05; electronic supplementary material, table S4). At present-day temperature, survival decreased significantly from 100% under normocapnia to 61% under hypercapnia. At future warming conditions, survival decreased from 71% under normocapnia to 44% under hypercapnia.
Figure 5.
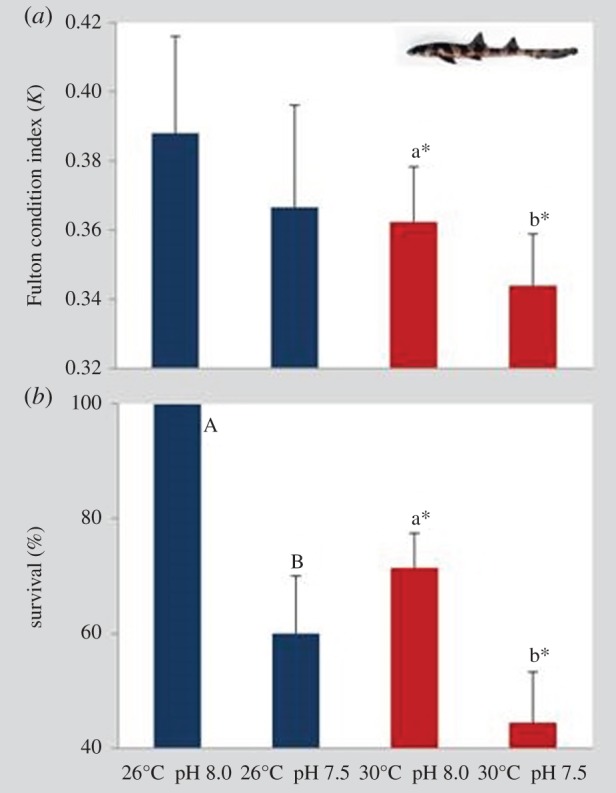
Impact of ocean acidification (ΔpH 0.5) and warming (+4°C) on (a) Fulton condition index (K) on newly hatched (day 0) juveniles and (b) survival (%) of 30 days post-hatching juveniles of bamboo shark (Chiloscyllium punctatum). Values represent mean ± s.d. Different upper- and lower-case letters represent statistical differences between pH treatments at 26°C and 30°C, respectively (p < 0.05). Asterisks represent significant differences between temperature treatments at the same pH condition. For more statistical details, see electronic supplementary material, table S4. (Online version in colour.)
(e). Metabolic and ventilation rates and Q10-values of juvenile sharks
The RMR of juvenile sharks (30 days after hatching) was significantly affected by temperature and pH (figure 6a; two-way ANOVA, p < 0.05). Moreover, there was a significant interaction between these two environmental variables (p < 0.05; electronic supplementary material, table S4). Temperature elicited a significant increase in RMR both at normocapnic (Q10-value of 5.37) and hypercapnic (Q10-value of 2.98) conditions (p < 0.05). Although pH did not cause any relevant change at 26°C (normocapnia: 82.6 µmol ± 12.3 O2 h−1 ind−1; hypercapnia: 74.6 µmol ± 3.9 O2 h−1 ind−1), it significantly decreased RMR under the future warming scenario (from 161 ± 2.6 O2 h−1 ind−1 to 115 ± 17.2 O2 h−1; p < 0.05). Ventilation rates of juvenile sharks showed a similar trend, with the number of breaths per minute increasing significantly with warming and normocapnia (figure 6b; two-way ANOVA, p < 0.05; electronic supplementary material, table S4). Yet such an increasing pattern with warming did not happen under hypercapnia. Additionally, the impact of ocean acidification on ventilation rates was significant only under the higher thermal scenario (p < 0.05).
Figure 6.
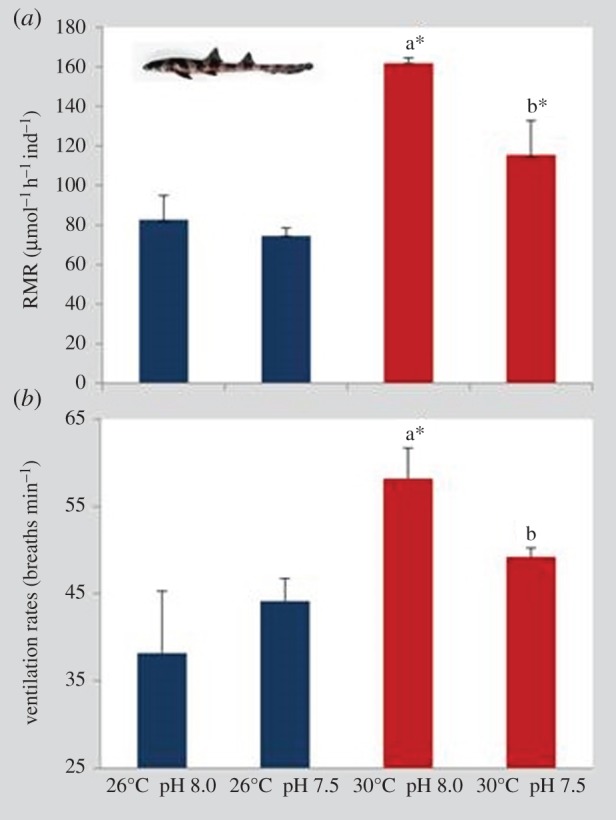
Impact of ocean acidification (ΔpH 0.5) and warming (+4°C) on (a) metabolic rates (µmol O2 h−1 ind−1) and (b) ventilation rates (number of breaths min−1) of 30 days post-hatching juveniles of bamboo shark (Chiloscyllium punctatum). Values represent mean ± s.d. Different lower-case letters represent statistical differences between pH treatments at 30°C (p < 0.05). Asterisks represent significant differences between temperature treatments at the same pH condition. For more statistical details, see electronic supplementary material, table S4. (Online version in colour.)
4. Discussion
One of the most important mechanisms that will allow marine organisms to cope with future environmental changes is acclimation [31]. Here, we showed that, after an acclimation process that comprised an extensive embryonic period and 30 days post-hatching, the hypercapnic and warming scenario was already outside the range of tolerance of this tropical shark. This finding was clearly supported by the high mortality (>50%; figure 5b) and decreased fitness recorded in the juvenile phase (figure 5a). Under future ocean conditions, this relatively inactive bottom-dwelling shark displayed a more lethargic behaviour (data not shown), and decreased metabolic and ventilatory capabilities, which might have cascading effects on its growth and subsequent reproduction. Such potential impacts may be stronger in more active shark species displaying higher metabolic costs associated with energetically demanding life strategies (e.g. pelagic species with ram ventilation). Temperature-induced respiratory acidosis and hypercapnia cause short-term acid–base imbalance, but as in most fish, pH compensation in sharks is done rapidly and efficiently across the gills through the direct transfer of acid–base equivalents between the animal and the external environment [32,33]. As shark ventilation may be influenced by acid–base status [33,34], the decreased (although not significantly) ventilation observed under ocean warming and acidification conditions (figure 6b) likely indicates acid–base imbalance (metabolic and respiratory acidosis). If compensation of acid–base imbalance was not achieved, the exposure to reduced pH (and elevated pCO2) would explain the reduction of shark metabolism (figure 6a) [35–37]. One should keep in mind that metabolic depression, or even suppression, is considered an adaptive strategy to cope with short-term hypercapnia (and hypoxia), but it is not advantageous under the chronically high CO2 conditions predicted for the ocean of tomorrow [28,30].
Early-life stages are known to display narrow environmental tolerance windows owing to developmental constraints and insufficient capacity of central organs [7,38]. Consequently, at high temperature borders, oxygen (O2) supply in shark embryos may have become limited via ventilatory and circulatory constraints, and its combined effect with CO2 should have decreased their thermal tolerance [7]. Based on this concept, the scope for aerobic performance in the bamboo shark should decline above the optimal temperature range, with consequences for locomotion, growth and reproduction. In this study, ocean warming had more pronounced impacts on shark early-life stages than acidification. During embryogenesis, with the exception of RMR (figure 3c), none of the measured parameters (survival, development time, yolk consumption and SGR) was significantly affected by hypercapnia (see electronic supplementary material, table S2). However, RMR under the warming scenario was significantly affected by pH (figure 3; electronic supplementary material, table S3). Hypercapnia was indeed a crucial factor on embryos' RMR as energy expenditure rates were highly temperature-dependent under normocapnia, with Q10-values ranging between 3 and 6. Yet, under high CO2 conditions, such rates became less temperature-dependent (Q10-values around 1.5). Reduced pH caused the embryos to become more ‘hypometabolic’ (figure 4), which is expected to be accompanied by a reduction in protein synthesis and, consequently, decreased SGR (figure 2b) [39,40]. A greater degree of physiological impairment in shark embryos under hypercapnia had deleterious repercussions on juvenile fitness (figure 5a) and survival (figure 5b). Although not proven here [41], our findings may also suggest the idea that subtle events in shark's early-life (embryo) stages can promote ‘carry-over effects’ to later periods of its life cycle.
Although the vulnerability of tropical sharks to climate change is species-specific, factors such as temperature, sea level rise and freshwater input are assumed to elicit the greatest effects upon estuarine, coastal inshore and reef species [13]. Such effects are likely to be expressed through changes in species abundance and distribution (e.g. poleward movements and migrations to deeper waters) [42]. Until now, ocean acidification was not considered as a primary climate-related threat to elasmobranchs; only indirectly through habitat and community changes (e.g. reef sharks) [12,13]. Experimental approaches such as those shown in the present study are critical to directly assess risk and vulnerability of sharks to ocean acidification and warming, and can ultimately help managers and policy-makers to take proactive measures targeting most endangered species.
Supplementary Material
Acknowledgements
The authors thank Bernardo Quintella for helping in the shark tagging, and Myron Peck, Mark Felix, Guy Claireaux and Megan Thompson for helpful suggestions and critically reviewing the manuscript. The Portuguese Foundation for Science and Technology (FCT) supported this study through a Programa Investigador FCT 2013 development grant, and project grants PTDC/MAR/0908066/2008 and PTDC/AAG-GLO/3342/2012 to R.R.
References
- 1.Meehl GA, Stocker TF, Collins WD, others. 2007. Global climate projections. In Climate change 2007: the physical science basis (eds Solomon S, Qin D, Manning M.), pp. 686–688. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Vecchi GA, Wittenberg AT, Held IM, Leetmaa A, Harrison MJ. 2006. Weakening of tropical Pacific atmospheric circulation due to anthropogenic forcing. Nature 441, 73–76. ( 10.1038/nature04744) [DOI] [PubMed] [Google Scholar]
- 3.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 4.Tewksbury JJ, Huey RB, Deutsch CA. 2008. Putting the heat on tropical animals. Science 320, 1296–1297. ( 10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 5.Nilsson GE, Crawley N, Lunde IG, Munday PL. 2009. Elevated temperature reduces the respiratory scope of coral reef fishes. Glob. Change Biol. 15, 1405–1412. ( 10.1111/j.1365-2486.2008.01767.x) [DOI] [Google Scholar]
- 6.Petit JR, et al. 1999. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 399, 429–436. ( 10.1038/20859) [DOI] [Google Scholar]
- 7.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690–691. ( 10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 8.Donelson JM, Munday PL, McCormick MI, Nilsson GE. 2011. Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Glob. Change Biol. 17, 1712–1719. ( 10.1111/j.1365-2486.2010.02339.x) [DOI] [Google Scholar]
- 9.Sunday JM, Calosi P, Dupont S, Munday PL, Stillman JH, Reusch TBH. 2014. Evolution in an acidifying ocean. Trends Ecol. Evol. 29, 117–125. ( 10.1016/j.tree.2013.11.001) [DOI] [PubMed] [Google Scholar]
- 10.Baum JK, Myers RA, Kehler DG, Worm B, Harley SJ, Doherty PA. 2003. Collapse and conservation of shark populations in the Northwest Atlantic. Science 299, 389–392. ( 10.1126/science.1079777) [DOI] [PubMed] [Google Scholar]
- 11.Baum JK, Worm B. 2009. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714. ( 10.1111/j.1365-2656.2009.01531.x) [DOI] [PubMed] [Google Scholar]
- 12.Field IC, Meekan MG, Buckworth RC, Bradshaw CJA. 2009. Susceptibility of sharks, rays and chimaeras to global extinction. Adv. Mar. Biol. 56, 275–363. ( 10.1016/S0065-2881(09)56004-X) [DOI] [PubMed] [Google Scholar]
- 13.Chin A, Kyne PM, Walker TI, McAuley RB. 2010. An integrated risk assessment for climate change: analysing the vulnerability of sharks and rays on Australia's Great Barrier reef. Glob. Change Biol. 16, 1936–1953. ( 10.1111/j.1365-2486.2009.02128.x) [DOI] [Google Scholar]
- 14.Carlson JK, Goldman KJ, Lowe CG. 2004. Metabolism, energetic demand, and endothermy. In Biology of sharks and their relatives (eds Carrier JC, Musick JA, Heithaus MR.), pp. 203–224. CRC Marine Biology Series; Boca Raton, FL: CRC Press. [Google Scholar]
- 15.Compagno LJV. 2001. Sharks of the world: an annotated and illustrated catalogue of shark species known to date. Vol. 2: bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). Rome, Italy: FAO. [Google Scholar]
- 16.Luo J-J, Sasaki W, Masumoto Y. 2012. Indian Ocean warming modulates Pacific climate change. Proc. Natl Acad. Sci. USA 109, 18 701–18 706. ( 10.1073/pnas.1210239109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pimentel M, Faleiro F, Dionísio G, Repolho R, Pousão-Ferreira P, Machado J, Rosa R. 2014. Defective skeletogenesis and oversized otoliths in fish early stages in a changing ocean. J. Exp. Biol. 217, 2062–2070. ( 10.1242/jeb.092635) [DOI] [PubMed] [Google Scholar]
- 18.Rosa R, et al. 2014. Differential impacts of ocean acidification and warming on winter and summer progeny of a coastal squid (Loligo vulgaris). J. Exp. Biol. 217, 518–525. ( 10.1242/jeb.096081) [DOI] [PubMed] [Google Scholar]
- 19.Harahush BK, Fischer ABP, Collin SP. 2007. Captive breeding and embryonic development of Chiloscyllium punctatum Muller & Henle, 1838 (Elasmobranchii: Hemiscyllidae). J. Fish Biol. 71, 1007–1022. ( 10.1111/j.1095-8649.2007.01569.x) [DOI] [Google Scholar]
- 20.McElhany P, Busch DS. 2013. Appropriate pCO2 treatments in ocean acidification experiments. Mar. Biol. 160, 1807–1812. ( 10.1007/s00227-012-2052-0) [DOI] [Google Scholar]
- 21.Sarazin G, Michard G, Prevot F. 1999. A rapid and accurate spectroscopic method for alkalinity measurements in seawater samples. Wat. Res. 33, 290–294. ( 10.1016/S0043-1354(98)00168-7) [DOI] [Google Scholar]
- 22.Dickson A, Sabine C, Christian J. 2007. Guide to best practices for ocean CO2 measurements. PICES Spec. Pub. 3, 191. [Google Scholar]
- 23.Lewis E, Wallace DWR. 1998. CO2SYS-Program developed for the CO2 system calculations. Carbon Dioxide Inf Anal Center Report ORNL/CDIAC-105. See http://cdiac.ornl.gov/oceans/co2rprt.html.
- 24.Mehrbach C, Culberson C, Hawley J, Pytkowicz R. 1973. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907. ( 10.4319/lo.1973.18.6.0897) [DOI] [Google Scholar]
- 25.Dickson A, Millero F. 1987. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 34, 1733–1743. ( 10.1016/0198-0149(87)90021-5) [DOI] [Google Scholar]
- 26.Ballard WW, Mellinger J, Lechenault H. 1993. A series of normal stages for the development of Scyliorhinus canicula, the lesser spotted dogfish (Chondrichthyes: Scyliorhinidae). J. Exp. Zool. 267, 318–336. ( 10.1002/jez.1402670309) [DOI] [Google Scholar]
- 27.Rosa R, Calado R, Narciso L, Nunes ML. 2007. Embryogenesis of decapod crustaceans with different life history traits, feeding ecologies and habitats: a fatty acid approach. Mar. Biol. 151, 935–947. ( 10.1007/s00227-006-0535-6) [DOI] [Google Scholar]
- 28.Rosa R, Seibel BA. 2008. Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc. Natl Acad. Sci. USA 105, 20 776–20 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosa R, Seibel BA. 2010. Metabolic physiology of the Humboldt squid, Dosidicus gigas: implications for vertical migration in a pronounced oxygen minimum zone. Prog. Oceanogr. 86, 72–80. ( 10.1016/j.pocean.2010.04.004) [DOI] [Google Scholar]
- 30.Rosa R, et al. 2013. Lower hypoxia thresholds of cuttlefish early life stages living in a warm acidified ocean. Proc. R. Soc. B 280, 20131695 ( 10.1098/rspb.2013.1695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donelson JM, Munday PL. 2012. Thermal sensitivity does not determine acclimation capacity for a tropical reef fish. J. Anim. Ecol. 81, 1126–1131. ( 10.1111/j.1365-2656.2012.01982.x) [DOI] [PubMed] [Google Scholar]
- 32.Perry SF, Gilmour KM. 2006. Acid–base balance and CO2 excretion in fish: unanswered questions and emerging models. Respir. Physiol. Neurobiol. 154, 199–215. ( 10.1016/j.resp.2006.04.010) [DOI] [PubMed] [Google Scholar]
- 33.Heisler N, Toews DP, Holeton GF. 1988. Regulation of ventilation and acid–base status in the elasmobranch Scyliorhinus stellaris during hyperoxia-induced hypercapnia. Respir. Physiol. 71, 227–246. ( 10.1016/0034-5687(88)90018-7) [DOI] [PubMed] [Google Scholar]
- 34.Heisler N. 1988. Acid–base regulation. In Acid–base regulation physiology of elasmobranch fishes (ed. Shuttleworth TJ.), pp. 215–251. Berlin, Germany: Springer. [Google Scholar]
- 35.Guppy M, Withers P. 1999. Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol. Rev. 74, 1–40. ( 10.1017/S0006323198005258) [DOI] [PubMed] [Google Scholar]
- 36.Pörtner HO, Langenbuch M, Reipschlager A. 2004. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718. ( 10.1007/s10872-004-5763-0) [DOI] [Google Scholar]
- 37.Pörtner HO, Reipschlager A, Heisler N. 1998. Acid-base regulation, metabolism and energetics in Sipunculus nudus as a function of ambient carbon dioxide level. J. Exp. Biol. 201, 43–55. [DOI] [PubMed] [Google Scholar]
- 38.Pörtner HO, Mark FC, Bock C. 2004. Oxygen limited thermal tolerance in fish? Answers obtained by nuclear magnetic resonance techniques. Respir. Physiol. Neurobiol. 141, 243–260. ( 10.1016/j.resp.2004.03.011) [DOI] [PubMed] [Google Scholar]
- 39.Hochachka PW, Somero GN. 2002. Biochemical adaptation: mechanisms and process in physiological evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 40.Storey KB, Storey JM. 2004. Oxygen limitation and metabolic rate depression. In Functional metabolism regulation and adaptation (ed. Storey KB.), pp. 415–442. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 41.Hettinger A, Sanford E, Hill TM, Russell AD, Sato KN, Hoey J, Forsch M, Page HN, Gaylord B. 2012. Persistent carry-over effects of planktonic exposure to ocean acidification in the Olympia oyster. Ecology 93, 2758–2768. ( 10.1890/12-0567.1) [DOI] [PubMed] [Google Scholar]
- 42.Perry AL, Low PJ, Ellis JR, Reynolds JD. 2005. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915. ( 10.1126/science.1111322) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


