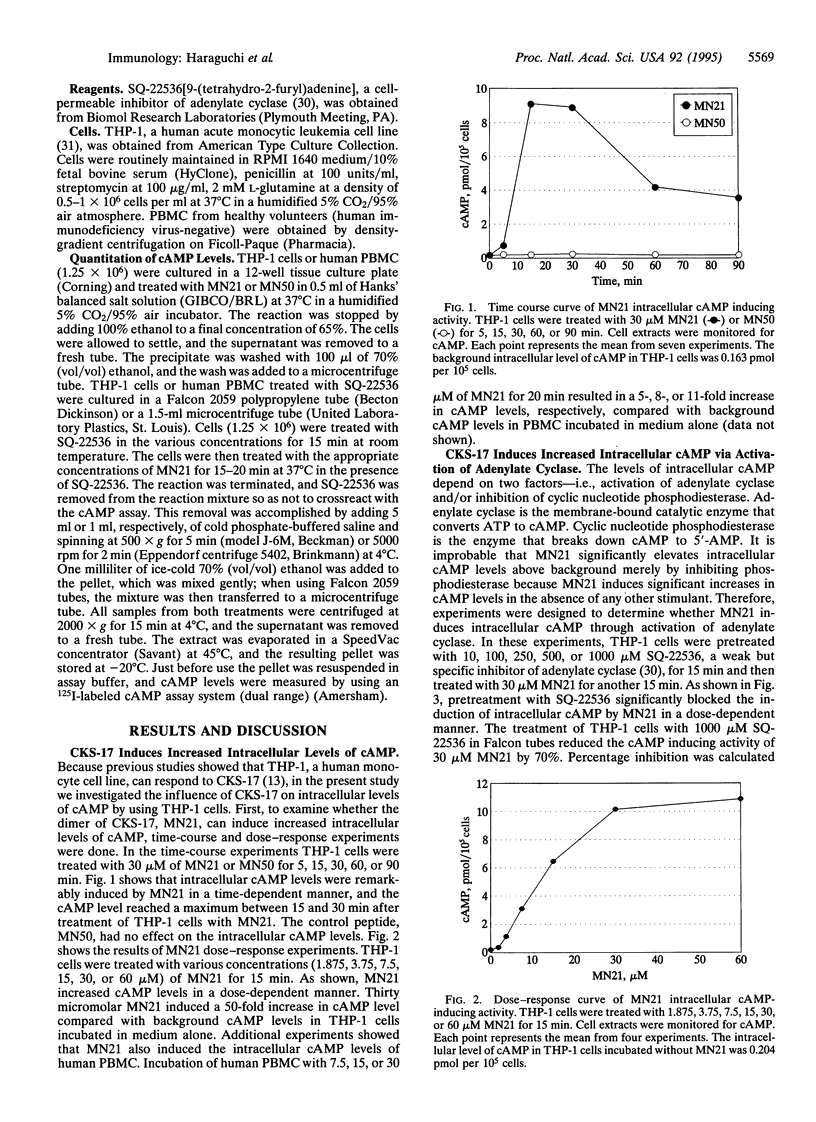
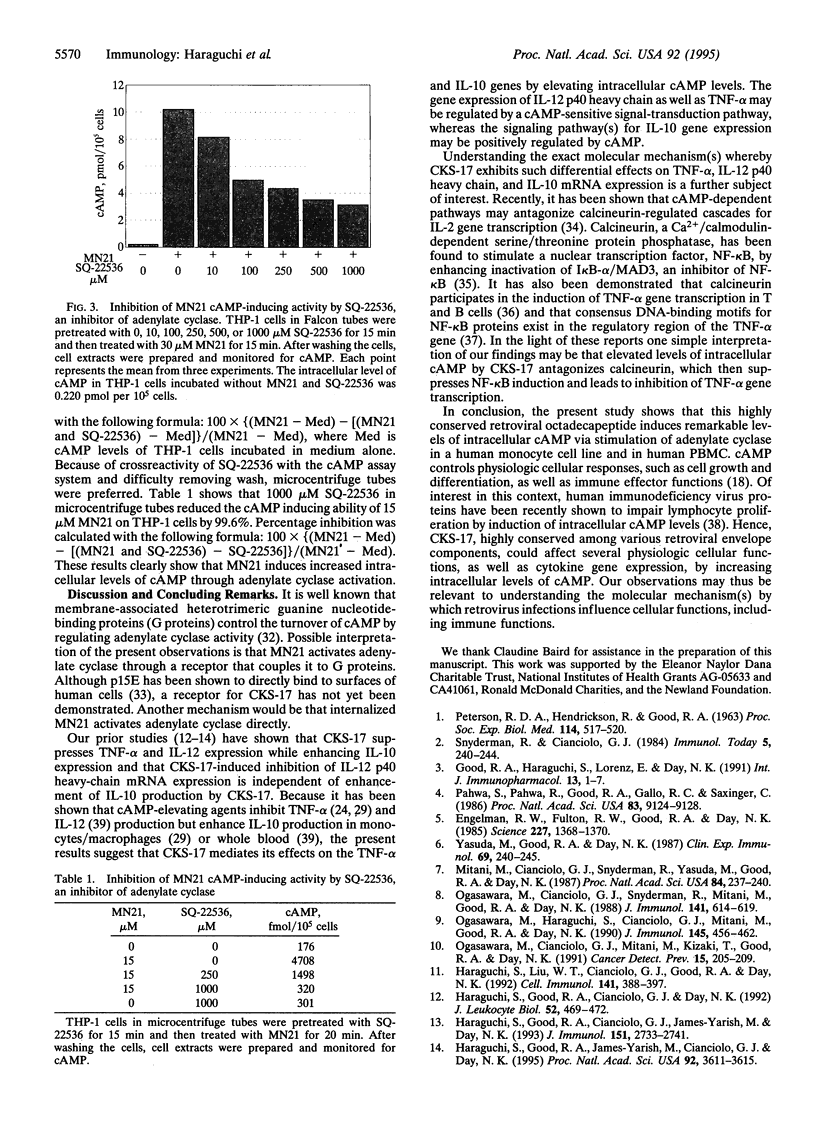
Abstract
A synthetic heptadecapeptide, CKS-17, represents the highly conserved amino acid sequences occurring within the transmembrane envelope protein of many animal and human retroviruses. CKS-17 has been demonstrated to exhibit suppressive properties for numerous immune functions. We have recently shown that CKS-17 acts as an immunomodulatory epitope causing an imbalance of human type 1 and type 2 cytokine production and suppression of cell-mediated immunities. cAMP, an intracellular second messenger, plays an important role in regulation of cytokine biosynthesis--i.e., elevation of intracellular cAMP levels selectively inhibits type 1 cytokine production but has no effect or enhances type 2 cytokine production. Here, we demonstrate that CKS-17 induces dramatic rises in the intracellular cAMP levels of a human monocyte cell line and of human peripheral blood mononuclear cells in a time- and dose-dependent manner. A peptide corresponding to the reverse sequence of CKS-17, used as control, has no effect on intracellular cAMP levels. The cAMP-inducing ability of CKS-17 is significantly blocked by SQ-22536, an inhibitor of adenylate cyclase. These results indicate that CKS-17, a highly conserved component of the transmembrane proteins of immunosuppressive retroviruses, induces increased intracellular levels of cAMP via activation of adenylate cyclase and suggest that this retroviral envelope peptide may differentially modulate type 1 and type 2 cytokine production through elevation of intracellular cAMP levels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastassiou E. D., Paliogianni F., Balow J. P., Yamada H., Boumpas D. T. Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R alpha gene expression at multiple levels. J Immunol. 1992 May 1;148(9):2845–2852. [PubMed] [Google Scholar]
- Betz M., Fox B. S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991 Jan 1;146(1):108–113. [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Kipnis R. J., Snyderman R. Similarity between p15E of murine and feline leukaemia viruses and p21 of HTLV. Nature. 1984 Oct 11;311(5986):515–515. doi: 10.1038/311515a0. [DOI] [PubMed] [Google Scholar]
- Endres S., Fülle H. J., Sinha B., Stoll D., Dinarello C. A., Gerzer R., Weber P. C. Cyclic nucleotides differentially regulate the synthesis of tumour necrosis factor-alpha and interleukin-1 beta by human mononuclear cells. Immunology. 1991 Jan;72(1):56–60. [PMC free article] [PubMed] [Google Scholar]
- Engelman R. W., Fulton R. W., Good R. A., Day N. K. Suppression of gamma interferon production by inactivated feline leukemia virus. Science. 1985 Mar 15;227(4692):1368–1370. doi: 10.1126/science.2983424. [DOI] [PubMed] [Google Scholar]
- Frantz B., Nordby E. C., Bren G., Steffan N., Paya C. V., Kincaid R. L., Tocci M. J., O'Keefe S. J., O'Neill E. A. Calcineurin acts in synergy with PMA to inactivate I kappa B/MAD3, an inhibitor of NF-kappa B. EMBO J. 1994 Feb 15;13(4):861–870. doi: 10.1002/j.1460-2075.1994.tb06329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Fitch F. W. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990 Jun 1;144(11):4110–4120. [PubMed] [Google Scholar]
- Goldfeld A. E., Tsai E., Kincaid R., Belshaw P. J., Schrieber S. L., Strominger J. L., Rao A. Calcineurin mediates human tumor necrosis factor alpha gene induction in stimulated T and B cells. J Exp Med. 1994 Aug 1;180(2):763–768. doi: 10.1084/jem.180.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi S., Good R. A., Cianciolo G. J., Day N. K. A synthetic peptide homologous to retroviral envelope protein down-regulates TNF-alpha and IFN-gamma mRNA expression. J Leukoc Biol. 1992 Oct;52(4):469–472. doi: 10.1002/jlb.52.4.469. [DOI] [PubMed] [Google Scholar]
- Haraguchi S., Good R. A., Cianciolo G. J., James-Yarish M., Day N. K. Transcriptional down-regulation of tumor necrosis factor-alpha gene expression by a synthetic peptide homologous to retroviral envelope protein. J Immunol. 1993 Sep 1;151(5):2733–2741. [PubMed] [Google Scholar]
- Haraguchi S., Good R. A., James-Yarish M., Cianciolo G. J., Day N. K. Differential modulation of Th1- and Th2-related cytokine mRNA expression by a synthetic peptide homologous to a conserved domain within retroviral envelope protein. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3611–3615. doi: 10.1073/pnas.92.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi S., Liu W. T., Cianciolo G. J., Good R. A., Day N. K. Suppression of human interferon-gamma production by a 17 amino acid peptide homologous to the transmembrane envelope protein of retroviruses: evidence for a primary role played by monocytes. Cell Immunol. 1992 May;141(2):388–397. doi: 10.1016/0008-8749(92)90157-k. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Desjardins J. V. Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the unidirectional control of platelet function by cyclic AMP. Biochem J. 1978 Oct 15;176(1):83–95. doi: 10.1042/bj1760083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann B., Nishanian P., Nguyen T., Insixiengmay P., Fahey J. L. Human immunodeficiency virus proteins induce the inhibitory cAMP/protein kinase A pathway in normal lymphocytes. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6676–6680. doi: 10.1073/pnas.90.14.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kizaki T., Mitani M., Cianciolo G. J., Ogasawara M., Good R. A., Day N. K. Specific association of retroviral envelope protein, p15E, with human cell surfaces. Immunol Lett. 1991 Apr;28(1):11–18. doi: 10.1016/0165-2478(91)90121-p. [DOI] [PubMed] [Google Scholar]
- Lacour M., Arrighi J. F., Müller K. M., Carlberg C., Saurat J. H., Hauser C. cAMP up-regulates IL-4 and IL-5 production from activated CD4+ T cells while decreasing IL-2 release and NF-AT induction. Int Immunol. 1994 Sep;6(9):1333–1343. doi: 10.1093/intimm/6.9.1333. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Koyano-Nakagawa N., Naito Y., Nishida J., Arai N., Arai K., Yokota T. cAMP activates the IL-5 promoter synergistically with phorbol ester through the signaling pathway involving protein kinase A in mouse thymoma line EL-4. J Immunol. 1993 Dec 1;151(11):6135–6142. [PubMed] [Google Scholar]
- Mitani M., Cianciolo G. J., Snyderman R., Yasuda M., Good R. A., Day N. K. Suppressive effect on polyclonal B-cell activation of a synthetic peptide homologous to a transmembrane component of oncogenic retroviruses. Proc Natl Acad Sci U S A. 1987 Jan;84(1):237–240. doi: 10.1073/pnas.84.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz E., Zubiaga A. M., Merrow M., Sauter N. P., Huber B. T. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990 Jul 1;172(1):95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T. J., Rothenberg E. V. cAMP inhibits induction of interleukin 2 but not of interleukin 4 in T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9353–9357. doi: 10.1073/pnas.87.23.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara M., Cianciolo G. J., Mitani M., Kizaki T., Good R. A., Day N. K. The suppressive effect of a synthetic retroviral peptide on the human IFN gamma production is abrogated by the combined stimulation with IL-1 and IL-2. Cancer Detect Prev. 1991;15(3):205–209. [PubMed] [Google Scholar]
- PETERSON R. D., HENDRICKSON R., GOOD R. A. REDUCED ANTIBODY FORMING CAPACITY DURING THE INCUBATION PERIOD OF PASSAGE A LEUKEMIA IN C3H MICE. Proc Soc Exp Biol Med. 1963 Nov;114:517–520. doi: 10.3181/00379727-114-28720. [DOI] [PubMed] [Google Scholar]
- Pahwa S., Pahwa R., Good R. A., Gallo R. C., Saxinger C. Stimulatory and inhibitory influences of human immunodeficiency virus on normal B lymphocytes. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9124–9128. doi: 10.1073/pnas.83.23.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliogianni F., Kincaid R. L., Boumpas D. T. Prostaglandin E2 and other cyclic AMP elevating agents inhibit interleukin 2 gene transcription by counteracting calcineurin-dependent pathways. J Exp Med. 1993 Nov 1;178(5):1813–1817. doi: 10.1084/jem.178.5.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandené L., Vandenbussche P., Crusiaux A., Alègre M. L., Abramowicz D., Dupont E., Content J., Goldman M. Differential effects of pentoxifylline on the production of tumour necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) by monocytes and T cells. Immunology. 1992 May;76(1):30–34. [PMC free article] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Strassmann G., Patil-Koota V., Finkelman F., Fong M., Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994 Dec 1;180(6):2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashiba S., Shapira L., Amar S., Van Dyke T. E. Cloning and characterization of human TNF alpha promoter region. Gene. 1993 Sep 15;131(2):307–308. doi: 10.1016/0378-1119(93)90314-s. [DOI] [PubMed] [Google Scholar]
- Thanhäuser A., Reiling N., Böhle A., Toellner K. M., Duchrow M., Scheel D., Schlüter C., Ernst M., Flad H. D., Ulmer A. J. Pentoxifylline: a potent inhibitor of IL-2 and IFN-gamma biosynthesis and BCG-induced cytotoxicity. Immunology. 1993 Sep;80(1):151–156. [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Yasuda M., Good R. A., Day N. K. Influence of inactivated feline retrovirus on feline alpha interferon and immunoglobulin production. Clin Exp Immunol. 1987 Aug;69(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- van der Pouw Kraan T. C., Boeije L. C., Smeenk R. J., Wijdenes J., Aarden L. A. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995 Feb 1;181(2):775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]