ABSTRACT
The meningococcal 4CMenB vaccine (Bexsero; Novartis) contains four antigens that can elicit serum bactericidal activity, one of which is factor H (FH)-binding protein (FHbp). FHbp specifically binds human complement FH. When humans are immunized, FHbp is expected to form a complex with FH, which could affect immunogenicity and safety. Wild-type mice (whose FH does not bind to FHbp) and human FH transgenic mice were immunized with three doses of 4CMenB, and their responses were compared. There were no significant differences between the serum bactericidal responses of transgenic and wild-type mice to strains with all of the antigens mismatched for 4CMenB except PorA or NadA. In contrast, against a strain mismatched for all of the antigens except FHbp, the transgenic mice had 15-fold weaker serum bactericidal antibody responses (P = 0.0006). Binding of FH downregulates complement. One explanation for the lower anti-FHbp serum bactericidal activity in the transgenic mice is that their postimmunization serum samples enhanced the binding of FH to FHbp, whereas the serum samples from the wild-type mice inhibited FH binding. Control antiserum from transgenic mice immunized with a low-FH-binding mutant FHbp (R41S) vaccine inhibited FH binding. Two 4CMenB-vaccinated transgenic mice developed serum IgM autoantibodies to human FH. Thus, human FH impairs protective serum anti-FHbp antibody responses, in part by skewing the antibody repertoire to FHbp epitopes outside the FH binding site. FHbp vaccines that bind FH may elicit FH autoantibodies. Mutant FHbp antigens with low FH binding could improve protection and, potentially, vaccine safety in humans.
IMPORTANCE
Two serogroup B meningococcal vaccines contain a novel antigen called factor H (FH)-binding protein (FHbp). FHbp specifically binds human FH, a plasma protein that downregulates complement. One vaccine (4CMenB; Novartis) is licensed in Europe, Canada, and Australia. When humans are immunized, FHbp can complex with FH. We compared the immunogenicity of 4CMenB vaccine in wild-type mice, whose own FH does not bind to FHbp, and human FH transgenic mice. Transgenic mice had respective antibody responses similar to those of wild-type mice to 4CMenB antigens that do not bind FH. However, the protective antibody responses of the transgenic mice to FHbp were impaired, largely because the antibodies did not inhibit but rather enhanced the binding of FH to FHbp. Two transgenic mice developed serum IgM autoantibodies to FH. Mutant FHbp antigens with low FH binding likely will elicit greater protection in humans than FHbp vaccines that bind FH and have a lower risk of FH autoantibodies.
INTRODUCTION
Neisseria meningitidis is an important cause of sepsis and meningitis. Strains with five different capsular structures (serogroups) are responsible for nearly all of the invasive meningococcal infections in North America and Europe (1). Prevention of disease caused by strains with capsular group A, C, W, or Y is possible because of the availability of capsular polysaccharide-based conjugate vaccines. However, the conjugate vaccine approach is not feasible against serogroup B because the serogroup B capsular polysaccharide cross-reacts with host antigens (2) and is poorly immunogenic. Serogroup B strains account for ~30 to 90% of the cases of meningococcal disease in different countries in North America and Europe (1). Therefore, a vaccine that covers serogroup B is important for the control of meningococcal disease. There are many challenges in the development of a broadly protective noncapsular vaccine (reviewed in references 3 and 4). These include identifying noncapsular antigens that do not cross-react with host antigens, that are antigenically conserved and expressed by genetically diverse strains, and that elicit complement-mediated serum bactericidal activity, which is the serologic hallmark of protection against meningococcal disease.
As of 2014, there are two meningococcal serogroup B vaccines in late-stage clinical development in the United States. The Pfizer vaccine targets adolescents (5). The Novartis vaccine (Bexsero) is licensed in Europe, Canada, and Australia and is recommended for infants, adolescents, and adults (4). This vaccine was recently provided to two U.S. universities as an investigational new drug for the control of serogroup B meningococcal outbreaks on campuses (http://medcitynews.com/2014/02/second-college-campus-using-novartis-vaccine-meningitis-b-outbreak/).
Both the Pfizer and Novartis vaccines contain a novel antigen called factor H (FH)-binding protein (FHbp) that can be divided into two antigenically distinct subfamilies, A and B (6). The Pfizer vaccine contains two recombinant FHbp lipoproteins, one from each subfamily. The Novartis vaccine contains a recombinant FHbp antigen from subfamily B (also called variant group 1) (7). This vaccine contains three other components capable of eliciting serum bactericidal antibody responses, recombinant NadA, recombinant Neisseria heparin-binding antigen (NHba) (8), and outer membrane vesicles (OMV), which elicit a bactericidal antibody to PorA (9). This multicomponent vaccine is referred to as the four-component meningococcal B or 4CMenB vaccine (10).
FHbp specifically binds human complement FH (11), which downregulates the alternative pathway (12). In humans immunized with FHbp vaccines, the antigen is expected to form a complex with human FH. The Novartis FHbp antigen is presented as a fusion protein with a second antigen called GNA2091 (13), which is not thought to contribute to bactericidal activity (14). Infants immunized with the 4CMenB vaccine develop serum anti-FHbp bactericidal antibody responses (15, 16). However, it is not known whether the responses to the FHbp fusion protein in 4CMenB are affected by human FH. To investigate this question experimentally, we compared the immunogenicity of the 4CMenB vaccine in human FH transgenic (Tg) mice and wild-type (WT) mice, whose own FH does not bind to FHbp.
RESULTS
4CMenB-vaccinated Tg mice have weaker serum IgG antibody responses to FHbp than WT mice and lower anti-FHbp serum bactericidal activity.
Figure 1 summarizes the serum IgG antibody responses, measured by enzyme-linked immunosorbent assay (ELISA), to two of the components of the 4CMenB vaccine, OMV and FHbp. For the 4CMenB-vaccinated groups, each symbol represents the reciprocal IgG titer of an individual animal. For the negative-control animals immunized with aluminum hydroxide adjuvant, each symbol represents the reciprocal IgG titer of a serum pool from three to six mice. There were no significant differences in the IgG antibody responses of the 4CMenB-vaccinated Tg or WT mice to the OMV (P > 0.24, Fig. 1A and B). In contrast, after dose 2, the IgG antibody responses of Tg mice to FHbp were 8-fold weaker than those of WT mice (reciprocal geometric mean titer [GMT] of 282 versus 2,285, P = 0.003, Fig. 1C) and 2.6-fold weaker after dose 3 (reciprocal GMT of 1,429 versus 3,658, P = 0.008, Fig. 1D).
FIG 1 .
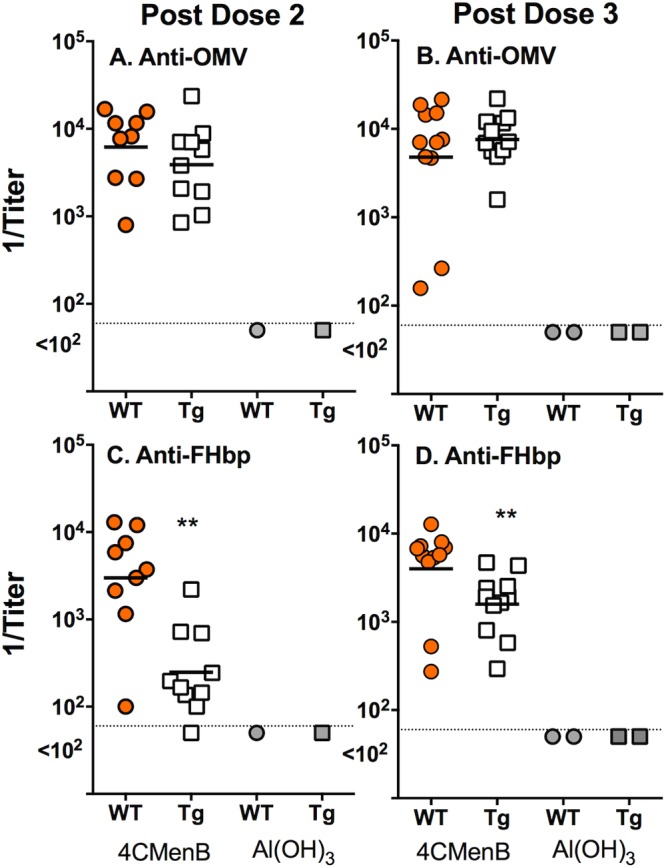
Serum IgG anti-OMV and anti-FHbp antibody responses of Tg and WT mice immunized with the 4CMenB vaccine. For the 4CMenB-vaccinated mice, each symbol represents the serum antibody titer of an individual mouse as measured by ELISA. Orange circles, 4CMenB-vaccinated WT mice. Open squares, 4CMenB-vaccinated Tg mice. Gray circles and squares, serum pools from negative control WT or Tg mice, respectively, immunized with aluminum hydroxide alone. After dose 2, there were sufficient serum samples from 9 of the 11 mice in the WT group and 10 of the 11 mice in the Tg group for the assays. For the aluminum hydroxide adjuvant control group, each symbol represents the titer of a serum pool (three to six mice per pool). (A, B) Anti-OMV antibody titers. There were no significant differences between the respective GMTs of Tg and WT mice after dose 2 (A) or 3 (B) (P > 0.24). (C, D) Anti-FHbp antibody titers. The serum IgG anti-FHbp antibody titers were lower in the 4CMenB-vaccinated Tg mice than in the 4CMenB-vaccinated WT mice (P = 0.003 after dose 2 [C] and P = 0.008 after dose 3 [D]).
To investigate if human FH influenced complement-mediated bactericidal responses, we tested the serum samples against three serogroup B test strains. Each strain expressed only one antigen matching those in the 4CMenB vaccine (see Materials and Methods). Because of small volumes of serum were obtained after dose 2, we measured the bactericidal activity against each of the three strains in pooled serum. Figure 2, left, depicts data from representative experiments measuring the survival of each of the test strains after incubation with a fixed concentration of human complement (20%) and different dilutions of the postdose 2 serum pools from Tg or WT mice. Against strain SK016 (Fig. 2A), which has all of the antigens mismatched to those in the 4CMenB vaccine except PorA P1.4, and strain 5/99 (Fig. 2C), which has all of the antigens mismatched except for NadA, the respective bactericidal activities of the serum pools were not different from each other. In contrast, against strain H44/76 (Fig. 2E), which has all of the antigens mismatched except for FHbp, there was no detectable bactericidal activity in the serum pool from the 4CMenB-vaccinated Tg mice (reciprocal titer for 50% survival, <10). In contrast, the serum pool from the immunized WT mice had high activity (reciprocal titer, ~200). We also measured the bactericidal activities of individual postdose 2 serum samples (n = 10 Tg and 9 WT mice with sufficient serum) against strain H44/76. A few of the Tg mice responded. The reciprocal GMT of the Tg mice was 9, compared to the 177 of the WT mice (P = 0.004) (see Fig. S1 in the supplemental material).
FIG 2 .
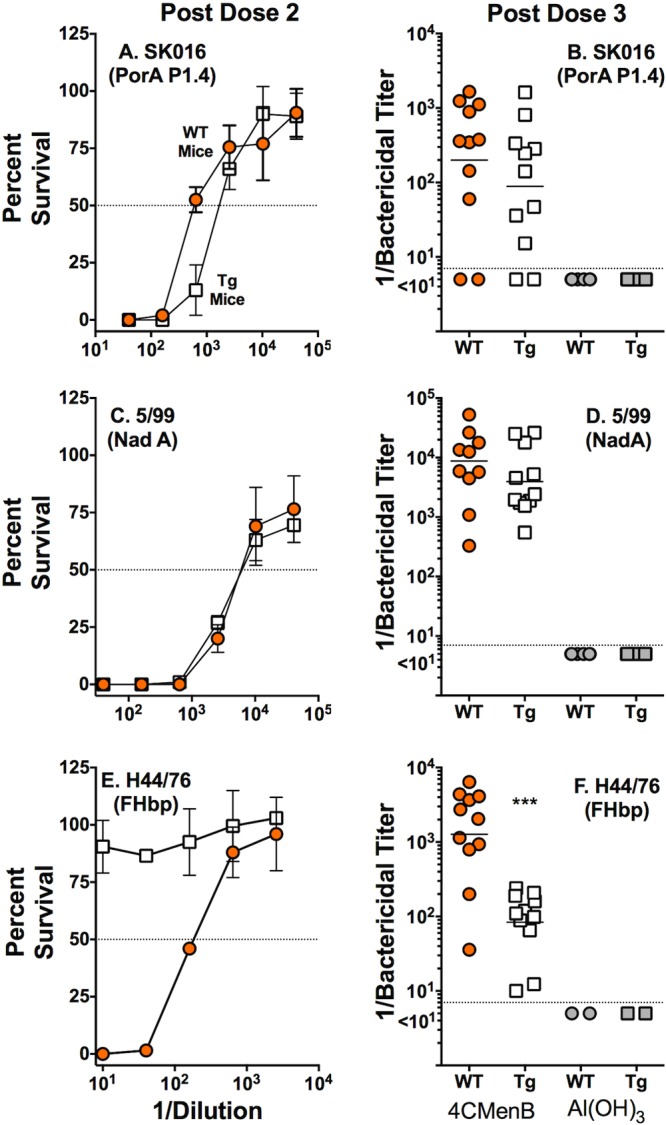
Serum bactericidal antibody responses of Tg and WT mice immunized with the 4CMenB vaccine. Symbols are the same as in Fig. 1. Strain SK016 (A, B) was mismatched for each of the antigens in the 4CMenB vaccine, except PorA P1.4. Strain 5/99 (C, D) was mismatched for each of the antigens except NadA, and strain H44/76 (E, F) was mismatched for each of the antigens except FHbp. (A, C, E) Data from a representative experiment showing the percent survival values of the different test strains after incubation for 1 h at 37°C with 20% human complement and different dilutions of serum pools from 4CMenB-vaccinated Tg or WT mice. Results for each strain were replicated in two or three independent experiments. (B, D, F) Reciprocal serum bactericidal antibody titers of individual Tg or WT mice. There were no significant differences between the respective GMTs (horizontal lines) of 4CMenB-vaccinated Tg or WT mice against strain SK016 (B) (P = 0.19) or strain 5/99 (D) (P = 0.21). Tg mice had lower titers of antibody against H44/76 than WT mice did (F) (P = 0.0006).
We measured the postdose 3 bactericidal antibody titers in individual serum samples from 11 4CMenB-vaccinated mice in each group (Fig. 2B, D, and F). There were no significant differences between the respective GMTs of antibodies of the 4CMenB-vaccinated Tg and WT mice against strain SK016 (Fig. 2B) (P = 0.19) or strain 5/99 (Fig. 2D) (P = 0.21). In contrast, against strain H44/76, the reciprocal GMT was 15-fold lower in the Tg than in the WT mice (84 versus 1,273, P = 0.0006, Fig. 2F).
Sera from 4CMenB-vaccinated Tg mice enhance binding of human FH to FHbp.
In previous studies, we found that anti-FHbp monoclonal antibodies (MAbs) that inhibited binding of FH to FHbp had greater complement-mediated bactericidal activity than anti-FHbp MAbs that did not inhibit FH binding (17–19). The reason is that with less FH bound to the bacterial surface, there is less complement downregulation and greater bactericidal activity. To investigate the basis for the discordant bactericidal activity of the anti-FHbp antibodies elicited by the 4CMenB-vaccinated WT and Tg mice, we measured the ability of postdose 3 immunization serum to inhibit the binding of FH to FHbp.
By ELISA, the serum samples from the negative-control aluminum hydroxide-immunized mice showed only background inhibition of binding of FH to FHbp (Fig. 3A). In the 4CMenB-vaccinated groups, the serum samples from the WT mice strongly inhibited the binding of FH to FHbp while the corresponding serum samples from the Tg mice enhanced FH binding (negative inhibition, Fig. 3B). Because of the unexpected enhancement of FH binding, we measured the inhibition of FH binding by control serum samples from additional groups of WT and Tg mice immunized with three doses of a recombinant FHbp vaccine that bound human FH (prepared as previously described) (20). The serum samples from the vaccinated WT mice inhibited FH binding to FHbp, while the corresponding serum samples from the vaccinated Tg mice enhanced FH binding (Fig. 3C). In contrast, control serum samples from groups of Tg and WT mice immunized with a mutant low-FH-binding recombinant FHbp vaccine containing one amino acid substitution (R41S) (20) both inhibited FH binding to FHbp (Fig. 3D). Thus, the enhancement of FH binding to FHbp by the serum samples from the immunized Tg mice was specific for FHbp vaccines that strongly bound human FH.
FIG 3 .
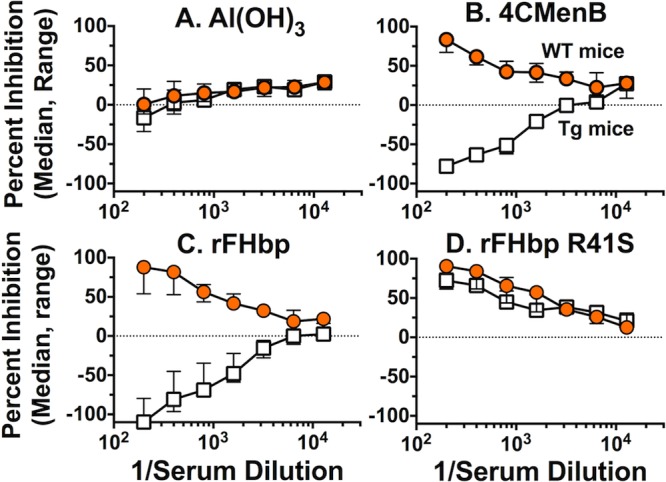
Inhibition of binding of FH to FHbp by serum samples from Tg and WT mice immunized with the 4CMenB vaccine as measured by ELISA. Data represent the median (range) inhibition of FH binding to FHbp in three serum pools each from 4CMenB-vaccinated human FH Tg or WT mice, and two serum pools each from aluminum hydroxide-vaccinated control mice. Orange circles, WT mice; open squares, human FH Tg mice. (A) Serum samples from mice immunized with Al(OH)3 without vaccine antigen. (B) Serum samples from 4CMenB-vaccinated Tg or WT mice. (C) Control serum pools (n = 3 per group) from Tg or WT mice immunized with a recombinant FHbp vaccine that binds human FH. (D) Control serum pools from WT or Tg mice (n = 3 per group) immunized with a recombinant mutant R41S FHbp vaccine with low FH binding.
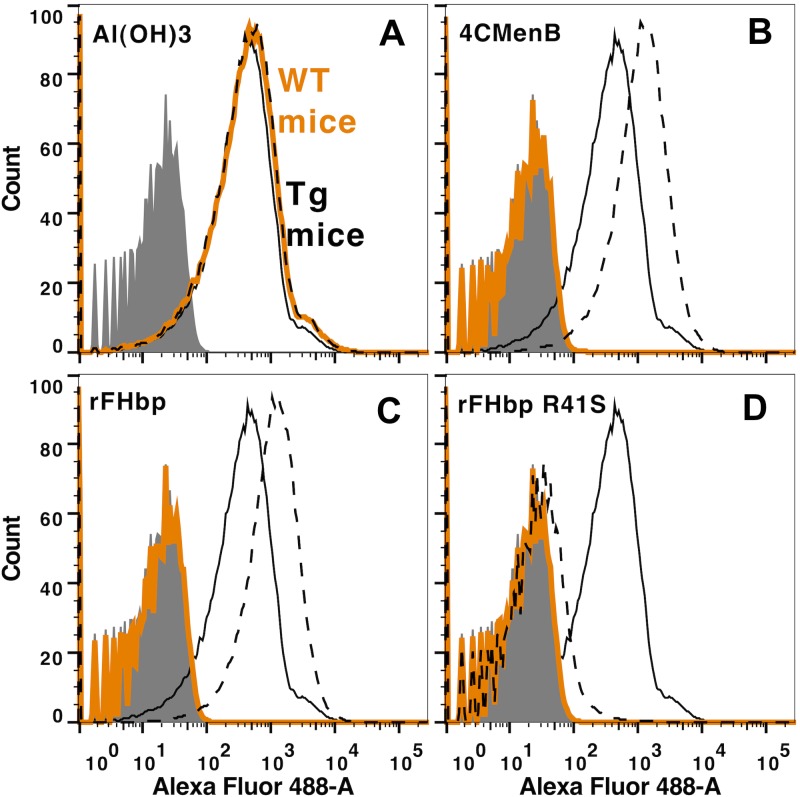
To verify the respective FH inhibitory activities of the different serum pools, we measured the inhibition of FH binding to the surface of live bacteria by flow cytometry (Fig. 4). We used strain H44/76, which is known to be a relatively high expresser of FHbp and a low expresser of NspA, which can also bind human FH (21). The solid black lines in the histograms represent FH binding to bacteria in the absence of serum antibodies. Both the serum samples from Tg mice immunized with the 4CMenB vaccine (Fig. 4B) and the recombinant FHbp vaccine that bound FH (Fig. 4C) enhanced FH binding to the bacterial surface (compare dashed black lines to solid black lines), whereas the corresponding serum samples from the immunized WT mice inhibited FH binding (orange solid lines). The control serum pools from the WT and Tg mice immunized with a low-FH-binding mutant FHbp R41S vaccine both inhibited FH binding (Fig. 4D).
FIG 4 .
Inhibition of binding of FH to the surface of live bacteria by serum samples from Tg and WT mice immunized with the 4CMenB vaccine. Inhibition of FH binding to serogroup B strain H44/76 by 1:40 dilutions of serum pools from immunized Tg or WT mice. Solid black line, human FH (2 µg/ml) without added serum. Dashed black line, FH plus serum from human FH Tg mice. Orange line, FH plus serum from WT mice. Panels A to D show results from the different vaccine groups described in the legend to Fig. 3. The serum samples from WT mice immunized with the 4CMenB or recombinant FHbp vaccine inhibited the binding of FH to the bacteria, while the serum samples from the respective Tg mice enhanced FH binding. The serum samples from the WT and Tg mice immunized with the mutant low-FH-binding FHbp vaccine (R41S) both inhibited FH binding. The results were replicated independently with a second serum pool from each of the vaccine groups.
Sera from 4CMenB-vaccinated Tg mice contain IgM antibodies reactive with human FH.
A theoretical risk of immunizing with a foreign antigen (FHbp) that forms a complex with a host protein (human FH) is the development of autoreactive antibodies to the host protein. We therefore measured the ability of serum antibodies from the 4CMenB-vaccinated mice to bind to human FH by ELISA. There was no significant IgG reactivity to FH in the serum samples from the 4CMenB-vaccinated Tg or WT mice (data not shown). There also was no significant IgM antibody binding in serum samples from the immunized WT mice (data not shown). However, 2 of 11 Tg mice immunized with the 4CMenB vaccine had elevated serum IgM reactivity to human FH when tested at a serum dilution of 1:100 (Fig. 5A). The elevated IgM anti-FH reactivity could be detected at serum dilutions of up to 1:700 (Fig. 5B).
FIG 5 .
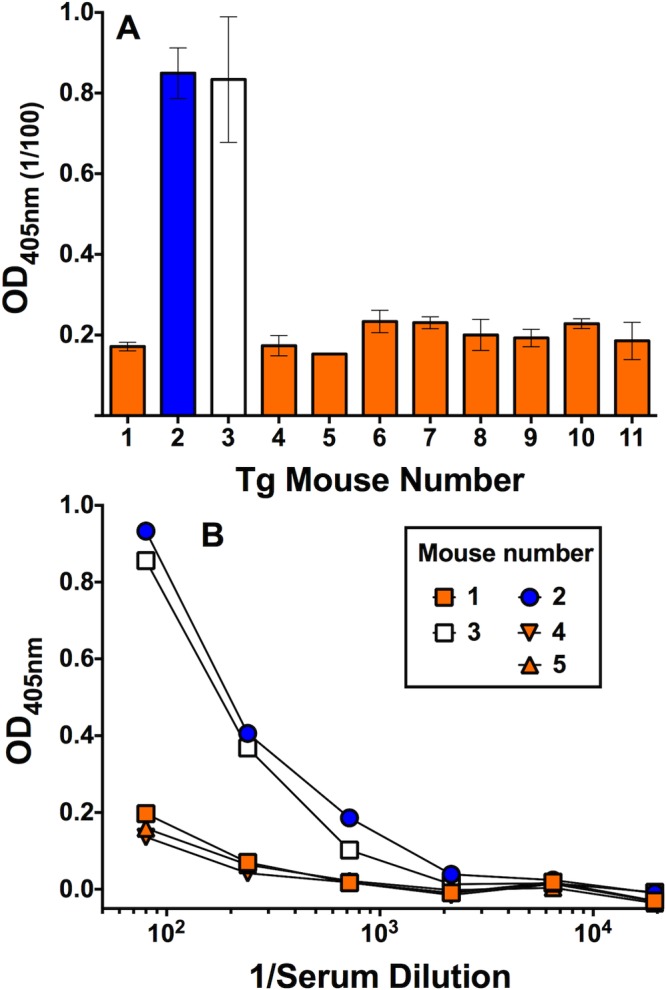
Serum IgM antibody reactivity with human FH as measured by ELISA. (A) IgM reactivity of 1:100 dilutions of serum samples from individual human FH Tg mice immunized with three doses of 4CMenB vaccine. The bars represent median values of duplicate or triplicate measurements, and error bars represent the ranges. (B) IgM reactivity to human FH in representative serum samples tested at different dilutions. The respective results were replicated in four independent experiments.
At a 1:100 dilution, the IgM reactivity with FH in the serum samples from the two 4CMenB-vaccinated Tg mice was inhibited to background levels by the addition of 25 µg/ml of soluble FH (Fig. 6A, compare horizontally hatched orange bars [no inhibitor] with diagonally hatched white bars [FH inhibitor]). FH contains 20 domains (also called short consensus repeats) (22). We used recombinant FH domains 6 and 7 fused to human IgG1 Fc (FH6,7/Fc) and domains 18 to 20 (FH18-20/Fc) as soluble inhibitors to determine the specificity of IgM reactivity. The addition of FH6,7/Fc to serum samples decreased binding to FH by ~60% (white bars); while the addition of FH18-20/Fc had a lesser effect (10 to 20% decrease in binding, blue bars). There was no significant inhibition of serum IgM binding to FH by the negative control IgG1 protein (gray bars).
FIG 6 .
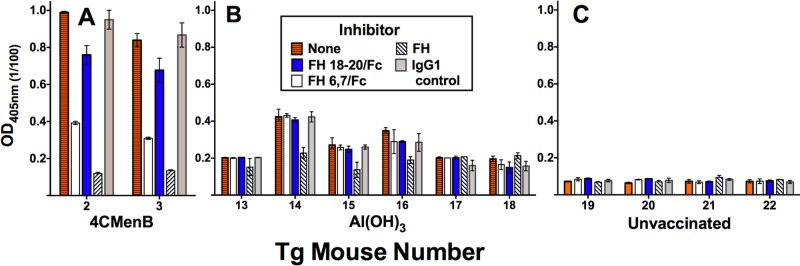
Effects of soluble FH inhibitors on serum IgM reactivity with human FH. Serum samples were tested at a 1:100 dilution. Bars and error bars represent median values and ranges of duplicate or triplicate determinations. Recombinant inhibitors were tested at 25 µg/ml. No inhibitor, orange horizontally hatched bars. Recombinant human FH domains 6 and 7 fused to human IgG1 Fc (FH6,7/Fc), white bars; FH domains 18 to 20 (FH18-20/Fc), blue bars; purified human FH, white diagonally hatched bars. The negative-control inhibitor was 50 µg/ml of a human IgG1 protein (light gray bars). (A) Serum samples from 4CMenB-vaccinated Tg mice 2 and 3 with IgM autoantibodies to FH (see Fig. 5). (B) Serum samples from six mice immunized with aluminum hydroxide without an antigen. (C) Representative serum samples from four of eight control unvaccinated Tg mice.
There were insufficient preimmunization serum samples available from the two Tg mice with postimmunization serum IgM FH reactivity to determine whether reactivity was present before vaccination. However, we measured IgM FH reactivity in serum samples from the six control Tg mice immunized with the aluminum hydroxide adjuvant alone and eight control unvaccinated Tg mice. At a 1:100 dilution, the serum samples from all eight unvaccinated mice had very low IgM FH binding (OD of 0.1 or less; representative data for four unvaccinated mice, Fig. 6C). The serum samples from the six Tg mice immunized with the aluminum hydroxide adjuvant alone had slightly higher IgM binding (OD of 0.2 to 0.4, Fig. 6B). In three of these serum samples (from mice 14 to 16), the low-level serum IgM binding was partially inhibited by the addition of purified human FH but not by the recombinant FH domain inhibitors. These data suggest that the majority of the low-level serum IgM FH binding in mice immunized with the adjuvant only is not specific. Further, the portion of FH binding that may be specific is directed at FH domains that are different from those in the serum samples from the two 4CMenB-vaccinated mice with IgM anti-FH reactivity.
DISCUSSION
The search for noncapsular serogroup B vaccine candidates is necessitated by observations that antibodies to the serogroup B capsular polysaccharide cross-react with glycosylated mammalian proteins such as the neural cell adhesion molecule (2) and the desire to avoid safety concerns from using a vaccine antigen with autoreactivity. While none of the new meningococcal protein vaccine candidates are known to elicit antibodies that cross-react with host antigens, after clinical trials had begun, FHbp (previously called GNA 1870 [7] or LP2086 [23]) was discovered to bind to the complement inhibitor FH (24) in a human-specific manner (11).
In this study, we used a previously characterized human FH Tg BALB/c mouse line (20) to investigate the effect of human FH on the immunogenicity of the FHbp fusion protein contained in the 4CMenB vaccine. Our most important findings were that, compared with WT mice, whose own FH does not bind to FHbp, the Tg mice had weaker serum IgG anti-FHbp antibody responses and weaker serum bactericidal antibody responses against a serogroup B strain with all of the antigens mismatched to the 4CMenB vaccine except FHbp.
The mechanism responsible for the lower FHbp immunogenicity in the Tg mice is not known. Conceivably, presenting FHbp in a complex with human FH adversely affects antigen presentation. Another possibility is that the vaccine activates complement, which results in C3 fragment deposition on the antigens. In other systems, C3 fragments bound to an antigen can have adjuvant activity (25–27). In Tg mice, the presence of human FH in a complex with the FHbp vaccine antigen would downregulate the deposition of C3 fragments and result in lower C3 adjuvant activity and weaker antibody responses. This hypothesis merits further investigation.
The effect of human FH on decreasing serum anti-FHbp bactericidal antibody responses in the 4CMenB-vaccinated Tg mice appeared to be disproportionately greater than the decrease in IgG anti-FHbp antibody responses (after dose 3, 15-fold lower bactericidal antibody titers than in WT mice, with a corresponding 2.6-fold decrease in IgG titers). FHbp is relatively sparsely exposed on many meningococcal strains (18). In previous studies, a critical determinant of anti-FHbp bactericidal activity was the ability of low-level C3b deposited by activation of the classical pathway to be amplified by the alternative pathway (17), which was enhanced if the anti-FHbp antibodies inhibited the binding of FH to FHbp (17). In the present study, we found that while the serum anti-FHbp antibodies elicited by 4CMenB vaccination of WT mice inhibited the binding of FH to FHbp, the corresponding antibodies elicited in the Tg mice enhanced FH binding. These data suggested that the anti-FHbp antibody repertoire of the Tg mice was skewed toward FHbp epitopes outside the FH combining site. The basis for the enhanced FH binding by the postimmunization serum samples from the Tg mice is not known. Conceivably, binding of antibodies to certain FHbp epitopes can result in conformational changes in FHbp that rendered the molecule more accessible for FH binding. For example, in a previous study, the binding of an anti-FHbp MAb (MAb 502) to FHbp enhanced FH binding (28). On the basis of nuclear magnetic resonance data, Scarselli et al. found that the binding of this MAb to FHbp increased the accessibility of certain FHbp residues, such as Ala206 and Val207 (29), that have been implicated in FH binding (30, 31).
In the present study, two human FH Tg mice immunized with the 4CMenB vaccine developed serum IgM antibodies reactive with human FH. The majority of the IgM reactivity was inhibited by the FH domain 6,7/Fc fragment, which is the portion of FH that binds specifically to meningococcal FHbp (30). While it might be counterintuitive that the presence of FHbp bound with FH induces antibodies against the FH domains that bind to FHbp, a likely explanation is that the anti-FH antibodies are directed at amino acid residues in domains 6 and 7 that are not in contact with FHbp. The serum IgM reactivity of the two mice was also partially inhibited by FH domains 18 to 20. While FH domains 18 to 20 have been reported to interact with FH ligands in other bacteria, such as Streptococcus pyogenes (32) or Haemophilus influenzae (33), these domains do not bind directly to N. meningitidis (34). However, when FH is bound, full-length FH may have a folded-back structure where the N and C termini are adjacent (35). Thus, multiple sites in FH may be in close proximity to FHbp and create neoepitopes that are not entirely predictable on the basis of known structures.
FH is the main alternative complement pathway inhibitory protein in human plasma, and serum IgG autoantibodies to FH can result in functional FH deficiency (36, 37). IgG autoantibodies to FH are found at an increased frequency in patients with atypical hemolytic-uremic syndrome (38–40), which is a rare disease characterized by microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure. Serum FH autoantibodies are also found at an increased frequency in patients with certain rheumatoid diseases (41). In the present study, the anti-FH antibodies in serum samples from 4CMenB-vaccinated human FH Tg mice were limited to IgM, which may be transient and not have clinical importance. However, a class switch from IgM to IgG can rarely occur and would have greater disease potential. It is important to emphasize that our data are from a Tg mouse model that may be more susceptible to developing autoantibodies to human FH than immunized humans are. Extensive safety studies with humans have not identified evidence of functional FH deficiency or atypical hemolytic-uremic syndrome associated with FHbp-containing vaccines. The importance of our 4CMenB findings in the Tg mice is that the data underscore a theoretical concern that vaccination of humans with FHbp antigens that bind to human FH may induce anti-FH autoantibodies. Therefore, the presence of such antibodies should be investigated in serum samples from vaccinated humans.
In previous studies, mutant FHbp vaccines with low binding to human FH circumvented the limitations of decreased immunogenicity of FHbp vaccines that bind human FH (20, 42–44). These data, together with the possibility that FHbp vaccines that bind human FH may elicit anti-FH autoantibodies, suggest that mutant FHbp antigens with low FH binding offer the prospect of developing safer and more effective FHbp-based meningococcal vaccines.
MATERIALS AND METHODS
Mice.
The protocols were approved by the Institutional Animal Care and Use Committees at Children’s Hospital Oakland Research Institute (CHORI). The human FH Tg BALB/c mouse line has been previously described (20). The transgene contains a cytomegalovirus (CMV) enhancer, a chicken β-actin promoter, and cDNA encoding the full-length human FH protein. The CMV enhancer and chicken β-actin promoter achieved high levels of human FH expression that approximate human serum FH concentrations (20). At approximately 5 weeks of age, the animals were bled via the facial vein to measure concentrations of human FH in serum with an FHbp capture ELISA, which was performed as previously described (20). In our previous study, we found an inverse correlation between human FH concentrations in serum and serum bactericidal antibody responses to a recombinant FHbp vaccine that bound human FH (20). Therefore, only Tg mice with human FH concentrations in serum of ≥240 µg/ml were included in the present study. These concentrations are similar to those found in human serum. WT BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) and housed at the CHORI facility for 3 weeks before the immunogenicity protocol was begun.
Vaccines.
4CMenB vaccine was purchased in the United Kingdom, where the vaccine is licensed. A human dose (0.5 ml) contains 50 µg each of three recombinant proteins. These proteins are combined with 25 µg of OMV from N. meningitidis group B strain NZ98/254 and adsorbed with aluminum hydroxide (0.5 mg Al3+ per human dose).
Mouse immunogenicity.
Beginning at 7 to 12 weeks of age, WT and Tg mice (n = 12 per group) were immunized with three intraperitoneal doses of 4CMenB at 3-week intervals. The dose used was one-fifth of the human dose. During the study, two animals (1 WT and 1 Tg) died from causes that did not appear to be related to vaccination, leaving 11 animals in each group. Control WT and Tg mice (n = 6 per group) were immunized with aluminum hydroxide without a vaccine antigen at a dose of 0.1 mg Al3+ (to match that in one-fifth of the human dose of 4CMenB). Blood samples were obtained from the facial vein 2 weeks after the second dose, and via cardiac puncture 2 weeks after the third dose, at which time the animals were euthanized. Serum samples were stored frozen at −70°C until use. All serum samples were heated at 56°C for 30 min to inactivate internal complement.
Serum IgG antibody responses.
The ELISA for measurement of titers of IgG antibody to FHbp in serum, which has been previously described (44), used recombinant FHbp (ID 1) as the target antigen. For measurement of serum anti-OMV titers, we prepared detergent-extracted OMV from a mutant of N. meningitidis strain NZ98/254 in which the gene encoding FHbp had been inactivated (45). The OMV procedure has been described previously in detail (46). In brief, bacteria were grown for 5 h in Frantz medium. The cells were resuspended in 0.1 M Tris-HCl containing 10 mM EDTA and 0.5% sodium deoxycholate to extract OMV. The OMV was isolated by ultracentrifugation for 2 h at 100,000 × g, resuspended in 3% sucrose, and stored frozen at −20°C until use. The anti-OMV ELISA was performed as previously described for the measurement of IgG antibodies to FHbp, except that for the anti-OMV antibody titers, the wells of the microtiter plate were incubated with 5 µg/ml of the OMV diluted in phosphate-buffered saline for 48 h at 4°C instead of 2 µg/ml of FHbp overnight at 4°C.
Serum bactericidal antibody activity.
The serum bactericidal antibody activity assay was performed as previously described (17), except that bacteria were grown to mid-exponential phase in Frantz medium supplemented with 4 mM d,l-lactate (Sigma catalog no. L-1250) and 2 mM CMP–N-acetylneuraminic acid (Carbosynth catalog no. MC04931). The complement for the bactericidal assay was IgG-depleted human serum prepared as previously described (20). The bactericidal antibody titer was the dilution of serum that resulted in 50% fewer CFU/ml than the number of CFU/ml in negative-control wells after 60 min of incubation.
N. meningitidis test strains. We used three serogroup B strains, each mismatched for three of the four antigens in the 4CMenB vaccine. Two of the strains, H44/76 and 5/99, have been previously characterized and used to measure FHbp- and NadA-specific 4CMenB bactericidal activity, respectively (15, 16). The third strain, SK016 (sequence type 103 complex), has PorA P1.7-2,4, which matches the OMV of 4CMenB. This strain lacks the gene for NadA, has FHbp ID 25 in subfamily A, and is resistant to complement-mediated bactericidal activity of serum samples from mice immunized with recombinant NHba. We confirmed that all three strains were killed by human complement only with the specific mouse antiserum or anti-PorA MAb that matched the vaccine antigen.
Human FH.
Purified human FH was purchased from Complement Technology, Inc. (Tyler, TX). According to the manufacturer, the preparation is 97% pure on the basis of SDS-PAGE. Trace contaminants may include human IgG, IgA, IgM, factor I, factor B, factor P, C3, C4, and albumin.
Inhibition of binding of human FH.
The ability of serum anti-FHbp antibodies to inhibit the binding of human FH to FHbp was measured by ELISA as previously described (20). In brief, 2 µg/ml of recombinant FHbp was added to wells of a microtiter plate and incubated overnight at 4°C. After aspiration and blocking, serial dilutions of the test serum samples were added to the plate together with a fixed concentration (1.5 µg/ml) of purified human FH. The plates were incubated at room temperature for 2 h. After washing, bound human FH was detected with a sheep anti-human FH antibody (ab8842; Abcam), followed by donkey anti-sheep IgG (whole molecule) conjugated to alkaline phosphatase (Sigma catalog no. A5187). The percent inhibition of FH binding in the presence of serum antibody was calculated by comparison with human FH bound in the absence of mouse serum.
The ability of serum anti-FHbp antibodies to inhibit the binding of human FH to the bacterial surface was also measured on live bacteria of strain H44/76 by flow cytometry (17). After the bacteria were washed, bound FH was detected as described above for the ELISA, except that we used a donkey anti-sheep IgG (H+L) antibody (Invitrogen catalog no. A11015) conjugated with Alexa Fluor 488.
Serum antibody reactivity with human FH.
Binding of serum IgG and IgM antibodies to human FH was measured by ELISA. The assay was performed as described above for serum titers of IgG antibody to FHbp, except that wells of a microtiter plate were coated with human FH (5 µg/ml incubated overnight at 4°C). After aspiration and blocking, diluted test serum samples were added. Bound IgG was detected with goat anti-mouse IgG (whole molecule) conjugated with alkaline phosphatase (Sigma) and bound IgM was detected with goat anti-mouse IgM (μ chain specific) conjugated with alkaline phosphatase (Sigma). In some experiments, we investigated whether the addition of soluble recombinant human FH domains 6 and 7 or 18 to 20 fused to human IgG1 Fc (FH6,7/Fc or FH18-20/Fc, respectively) inhibited serum anti-FH reactivity. The recombinant fragments (provided by Jutamas Shaughnessy, University of Massachusetts School of Medicine, Worcester) were similar to those previously described (47), except that the mouse IgG2a Fc had been replaced with human IgG1 Fc. As a negative-control inhibitor, we used a human IgG1 kappa myeloma protein (AG502; Millipore).
Statistical analyses.
For calculation of geometric mean titers, IgG and bactericidal antibody titers were transformed (log10). We used a nonparametric (Mann-Whitney) test to compare the respective antibody titers of two independent groups of mice. A two-tailed P value of ≤0.05 was considered statistically significant.
SUPPLEMENTAL MATERIAL
Serum bactericidal activities against serogroup B strain H44/76 measured in postimmunization dose 2 serum samples from WT and Tg mice immunized with 4CMenB vaccine. The test strain was mismatched for each of the antigens in 4CMenB except FHbp. Each symbol represents the reciprocal serum bactericidal antibody titer of an individual Tg or WT mouse. Orange circles and open squares, 4CMenB-vaccinated WT and Tg mice, respectively. Gray circles and squares, WT and Tg mice immunized with aluminum hydroxide, respectively. Horizontal lines represent reciprocal GMTs. Dotted line, serum samples with reciprocal titers of <10. Tg mice had lower titers than WT mice (reciprocal GMTs of 9 and 177, respectively; P = 0.004 by Mann-Whitney test). Download
ACKNOWLEDGMENTS
This work was supported by grants AI046464 and AI082263 from the National Institute of Allergy and Infectious Diseases, NIH. The work at CHORI was performed in a facility funded by Research Facilities Improvement Program grant C06 RR 016226 from the National Center for Research Resources, NIH.
We are grateful to Sanjay Ram, University of Massachusetts Medical School, Worcester, MA, for providing critical comments and to Jutamas Shaughnessy (University of Massachusetts Medical School) for providing the recombinant chimeric FH domain inhibitors.
Footnotes
Citation Costa I, Pajon R, Granoff DM. 2014. Human factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enhance FH binding. mBio 5(5):e01625-14. doi:10.1128/mBio.01625-14.
REFERENCES
- 1. Harrison LH, Trotter CL, Ramsay ME. 2009. Global epidemiology of meningococcal disease. Vaccine 27:B51–B63. 10.1016/j.vaccine.2008.10.030 [DOI] [PubMed] [Google Scholar]
- 2. Finne J, Leinonen M, Mäkelä PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357 [DOI] [PubMed] [Google Scholar]
- 3. Granoff DM. 2010. Review of meningococcal group B vaccines. Clin. Infect. Dis. 50(Suppl 2):S54–S65. 10.1086/648966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin NG, Snape MD. 2013. A multicomponent serogroup B meningococcal vaccine is licensed for use in Europe: what do we know, and what are we yet to learn? Expert Rev. Vaccines 12:837–858. 10.1586/14760584.2013.814862 [DOI] [PubMed] [Google Scholar]
- 5. Mcneil LK, Zagursky RJ, Lin SL, Murphy E, Zlotnick GW, Hoiseth SK, Jansen KU, Anderson AS. 2013. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol. Mol. Biol. Rev. 77:234–252. 10.1128/MMBR.00056-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, Ambrose K, Borrow R, Findlow J, Taha MK, Deghmane AE, Kriz P, Musilek M, Kalmusova J, Caugant DA, Alvestad T, Mayer LW, Sacchi CT, Wang X, Martin D, Von Gottberg A, Du Plessis M, Klugman KP, Anderson AS, Jansen KU, Zlotnick GW, Hoiseth SK. 2009. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J. Infect. Dis. 200:379–389. 10.1086/600141 [DOI] [PubMed] [Google Scholar]
- 7. Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799. 10.1084/jem.20021911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serruto D, Spadafina T, Ciucchi L, Lewis LA, Ram S, Tontini M, Santini L, Biolchi A, Seib KL, Giuliani MM, Donnelly JJ, Berti F, Savino S, Scarselli M, Costantino P, Kroll JS, O’Dwyer C, Qiu J, Plaut AG, Moxon R, Rappuoli R, Pizza M, Aricò B. 2010. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc. Natl. Acad. Sci. U. S. A. 107:3770–3775. 10.1073/pnas.0915162107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelly C, Arnold R, Galloway Y, O’Hallahan J. 2007. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Am. J. Epidemiol. 166:817–823. 10.1093/aje/kwm147 [DOI] [PubMed] [Google Scholar]
- 10. Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. 2012. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30(Suppl 2):B87–B97. 10.1016/j.vaccine.2012.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Granoff DM, Welsch JA, Ram S. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect. Immun. 77:764–769. 10.1128/IAI.01191-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneider MC, Exley RM, Ram S, Sim RB, Tang CM. 2007. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 15:233–240. 10.1016/j.tim.2007.03.005 [DOI] [PubMed] [Google Scholar]
- 13. Giuliani MM, Adu-Bobie J, Comanducci M, Aricò B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, Cartocci E, Ciucchi L, Di Marcello F, Ferlicca F, Galli B, Luzzi E, Masignani V, Serruto D, Veggi D, Contorni M, Morandi M, Bartalesi A, Cinotti V, Mannucci D, Titta F, Ovidi E, Welsch JA, Granoff D, Rappuoli R, Pizza M. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834–10839. 10.1073/pnas.0603940103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bos MP, Grijpstra J, Tommassen-Van Boxtel R, Tommassen J. 2014. Involvement of Neisseria meningitidis lipoprotein GNA2091 in the assembly of a subset of outer membrane proteins. J. Biol. Chem. 289:15602–15610. 10.1074/jbc.M113.539510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, Oster P, Miller E, Pollard AJ. 2010. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin. Infect. Dis. 51:1127–1137. 10.1086/656741 [DOI] [PubMed] [Google Scholar]
- 16. Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E, Toneatto D, Pollard AJ. 2010. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr. Infect. Dis. J. 29:e71–e79. 10.1097/INF.0b013e3181b0602e [DOI] [PubMed] [Google Scholar]
- 17. Giuntini S, Reason DC, Granoff DM. 2011. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect. Immun. 79:3751–3759. 10.1128/IAI.05182-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Welsch JA, Ram S, Koeberling O, Granoff DM. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J. Infect. Dis. 197:1053–1061. 10.1086/528994 [DOI] [PubMed] [Google Scholar]
- 19. Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM. 2008. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate, factor H-binding protein. Infect. Immun. 76:4232–4240. 10.1128/IAI.00367-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186:3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. 2010. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 6(7):e1001027. 10.1371/journal.ppat.1001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langford-Smith A, Keenan TD, Clark SJ, Bishop PN, Day AJ. 2014. The role of complement in age-related macular degeneration: heparan sulphate, a ZIP code for complement factor H? J. Innate Immun. 6:407–416. 10.1159/000356513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, Xie X, Zagursky R, Zhang Y, Zlotnick GW. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 72:2088–2100. 10.1128/IAI.72.4.2088-2100.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Madico G, Welsch JA, Lewis LA, Mcnaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501–510. 10.4049/jimmunol.177.1.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Test ST, Mitsuyoshi J, Connolly CC, Lucas AH. 2001. Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide. Infect. Immun. 69:3031–3040. 10.1128/IAI.69.5.3031-3040.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hostetter MK. 1986. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J. Infect. Dis. 153:682–693. 10.1093/infdis/153.4.682 [DOI] [PubMed] [Google Scholar]
- 27. Kerekes K, Cooper PD, Prechl J, Józsi M, Bajtay Z, Erdei A. 2001. Adjuvant effect of gamma-inulin is mediated by C3 fragments deposited on antigen-presenting cells. J. Leukoc. Biol. 69:69–74 PubMed; [PubMed] [Google Scholar]
- 28. Giuntini S, Beernink PT, Reason DC, Granoff DM. 2012. Monoclonal antibodies to meningococcal factor H binding protein with overlapping epitopes and discordant functional activity. PLoS One 7(3):e34272. 10.1371/journal.pone.0034272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scarselli M, Aricò B, Brunelli B, Savino S, Di Marcello F, Palumbo E, Veggi D, Ciucchi L, Cartocci E, Bottomley MJ, Malito E, Lo Surdo P, Comanducci M, Giuliani MM, Cantini F, Dragonetti S, Colaprico A, Doro F, Giannetti P, Pallaoro M, Brogioni B, Tontini M, Hilleringmann M, Nardi-Dei V, Banci L, Pizza M, Rappuoli R. 2011. Rational design of a meningococcal antigen inducing broad protective immunity. Sci. Transl. Med. 3:91ra62. 10.1126/scitranslmed.3002234 [DOI] [PubMed] [Google Scholar]
- 30. Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. 2009. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature 458:890–893. 10.1038/nature07769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pajon R, Beernink PT, Granoff DM. 2012. Design of meningococcal factor H binding protein mutant vaccines that do not bind human complement factor H. Infect. Immun. 80:2667–2677. 10.1128/IAI.00103-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reuter M, Caswell CC, Lukomski S, Zipfel PF. 2010. Binding of the human complement regulators CFHR1 and factor H by streptococcal collagen-like protein 1 (Scl1) via their conserved C termini allows control of the complement cascade at multiple levels. J. Biol. Chem. 285:38473–38485. 10.1074/jbc.M110.143727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleury C, Su YC, Hallström T, Sandblad L, Zipfel PF, Riesbeck K. 2014. Identification of a Haemophilus influenzae factor H-binding lipoprotein involved in serum resistance. J. Immunol. 192:5913–5923. 10.4049/jimmunol.1303449 [DOI] [PubMed] [Google Scholar]
- 34. Shaughnessy J, Lewis LA, Jarva H, Ram S. 2009. Functional comparison of the binding of factor H short consensus repeat 6 (SCR 6) to factor H binding protein from Neisseria meningitidis and the binding of factor H SCR 18 to 20 to Neisseria gonorrhoeae porin. Infect. Immun. 77:2094–2103. 10.1128/IAI.01561-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kopp A, Hebecker M, Svobodová E, Józsi M. 2012. Factor H: a complement regulator in health and disease, and a mediator of cellular interactions. Biomolecules 2:46–75. 10.3390/biom2010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kopp A, Strobel S, Tortajada A, Rodríguez de Córdoba S, Sánchez-Corral P, Prohászka Z, López-Trascasa M, Józsi M. 2012. Atypical hemolytic uremic syndrome-associated variants and autoantibodies impair binding of factor H and factor H-related protein 1 to PENTRAXIN 3. J. Immunol. 189:1858–1867. 10.4049/jimmunol.1200357 [DOI] [PubMed] [Google Scholar]
- 37. Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Frémeaux-Bacchi V. 2005. Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J. Am Soc. Nephrol. 16:555–563. 10.1681/ASN.2004050380 [DOI] [PubMed] [Google Scholar]
- 38. Moore I, Strain L, Pappworth I, Kavanagh D, Barlow PN, Herbert AP, Schmidt CQ, Staniforth SJ, Holmes LV, Ward R, Morgan L, Goodship TH, Marchbank KJ. 2010. Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood 115:379–387. 10.1182/blood-2009-05-221549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Józsi M, Licht C, Strobel S, Zipfel SL, Richter H, Heinen S, Zipfel PF, Skerka C. 2008. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 111:1512–1514. 10.1182/blood-2007-09-109876 [DOI] [PubMed] [Google Scholar]
- 40. Zipfel PF, Edey M, Heinen S, Józsi M, Richter H, Misselwitz J, Hoppe B, Routledge D, Strain L, Hughes AE, Goodship JA, Licht C, Goodship TH, Skerka C. 2007. Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet. 3(3):e41. 10.1371/journal.pgen.0030041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foltyn Zadura A, Zipfel PF, Bokarewa MI, Sturfelt G, Jönsen A, Nilsson SC, Hillarp A, Saxne T, Trouw LA, Blom AM. 2012. Factor H autoantibodies and deletion of complement factor H-related protein-1 in rheumatic diseases in comparison to atypical hemolytic uremic syndrome. Arthritis Res. Ther. 14:R185. 10.1186/ar4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rossi R, Granoff DM, Beernink PT. 2013. Meningococcal factor H-binding protein vaccines with decreased binding to human complement factor H have enhanced immunogenicity in human factor H transgenic mice. Vaccine 31:5451–5457. 10.1016/j.vaccine.2013.08.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. 2012. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog. 8(5):e1002688. 10.1371/journal.ppat.1002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Der Veen S, Johnson S, Jongerius I, Malik T, Genovese A, Santini L, Staunton D, Ufret-Vincenty RL, Pickering MC, Lea SM, Tang CM. 2014. Nonfunctional variant 3 factor H binding proteins as meningococcal vaccine candidates. Infect. Immun 82:1157–1163. 10.1128/IAI.01183-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Konar M, Granoff DM, Beernink PT. 2013. Importance of inhibition of binding of complement factor H for serum bactericidal antibody responses to meningococcal factor H-binding protein vaccines. J. Infect. Dis. 208:627–636. 10.1093/infdis/jit239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frasch CE, Van Alphen L, Holst J, Poolman JT, Rosenqvist E. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p. 81–107 In Pollard AJ, Maiden MC. (ed), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 47. Lewis LA, Vu DM, Vasudhev S, Shaughnessy J, Granoff DM, Ram S. 2013. Factor H-dependent alternative pathway inhibition mediated by porin B contributes to virulence of Neisseria meningitidis. mBio 4(5):e00339-13. 10.1128/mBio.00339-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum bactericidal activities against serogroup B strain H44/76 measured in postimmunization dose 2 serum samples from WT and Tg mice immunized with 4CMenB vaccine. The test strain was mismatched for each of the antigens in 4CMenB except FHbp. Each symbol represents the reciprocal serum bactericidal antibody titer of an individual Tg or WT mouse. Orange circles and open squares, 4CMenB-vaccinated WT and Tg mice, respectively. Gray circles and squares, WT and Tg mice immunized with aluminum hydroxide, respectively. Horizontal lines represent reciprocal GMTs. Dotted line, serum samples with reciprocal titers of <10. Tg mice had lower titers than WT mice (reciprocal GMTs of 9 and 177, respectively; P = 0.004 by Mann-Whitney test). Download