ABSTRACT
Aminoacyl-tRNA synthetases provide the first step in protein synthesis quality control by discriminating cognate from noncognate amino acid and tRNA substrates. While substrate specificity is enhanced in many instances by cis- and trans-editing pathways, it has been revealed that in organisms such as Streptococcus pneumoniae some aminoacyl-tRNA synthetases display significant tRNA mischarging activity. To investigate the extent of tRNA mischarging in this pathogen, the aminoacylation profiles of class I isoleucyl-tRNA synthetase (IleRS) and class II lysyl-tRNA synthetase (LysRS) were determined. Pneumococcal IleRS mischarged tRNAIle with both Val, as demonstrated in other bacteria, and Leu in a tRNA sequence-dependent manner. IleRS substrate specificity was achieved in an editing-independent manner, indicating that tRNA mischarging would only be significant under growth conditions where Ile is depleted. Pneumococcal LysRS was found to misaminoacylate tRNALys with Ala and to a lesser extent Thr and Ser, with mischarging efficiency modulated by the presence of an unusual U4:G69 wobble pair in the acceptor stems of both pneumococcal tRNALys isoacceptors. Addition of the trans-editing factor MurM, which also functions in peptidoglycan synthesis, reduced Ala-tRNALys production by LysRS, providing evidence for cross talk between the protein synthesis and cell wall biogenesis pathways. Mischarging of tRNALys by AlaRS was also observed, and this would provide additional potential MurM substrates. More broadly, the extensive mischarging activities now described for a number of Streptococcus pneumoniae aminoacyl-tRNA synthetases suggest that adaptive misaminoacylation may contribute significantly to the viability of this pathogen during amino acid starvation.
IMPORTANCE
Streptococcus pneumoniae is a common causative agent of several debilitating and potentially life-threatening infections, such as pneumonia, meningitis, and infectious endocarditis. Such infections are increasingly difficult to treat due to widespread development of penicillin resistance. High-level penicillin resistance is known to depend in part upon MurM, a protein involved in both aminoacyl-tRNA-dependent synthesis of indirect amino acid cross-linkages within cell wall peptidoglycan and in translation quality control. The involvement of MurM in both protein synthesis and antibiotic resistance identify it as a potential target for the development of new and potent antibiotics for pneumococcal infections. The goals of this work were to identify and characterize S. pneumoniae pathways that can synthesize mischarged tRNAs and to relate these activities to expected changes in protein and peptidoglycan biosynthesis during antibiotic and nutritional stress.
INTRODUCTION
Streptococcus pneumoniae is a Gram-positive diplococcus that can be carried asymptomatically in the nasopharynx of healthy individuals. The bacterium is also a significant pathogen and is the common causative agent of many community- and hospital-acquired infections, such as pneumonia and meningitis. In order to successfully colonize the nasopharynx in direct competition with other bacteria, including Haemophilus influenzae, S. pneumoniae routinely produces high levels of the oxidative stressor hydrogen peroxide (1–3). Increased levels of hydrogen peroxide have been directly correlated with enhanced cellular mistranslation rates in other microorganisms (4, 5). When taken together with the finding that pneumococci lack the four typical oxidative stress regulons of other bacteria (RpoS, OxyR, SoxRS, and Mar), it is unclear how this pathogen maintains translational fidelity during its normal life cycle (6, 7).
The aminoacyl-tRNA synthetases (aaRSs) establish and maintain the genetic code by specifically activating their cognate amino acid with ATP to form an aminoacyl-adenylate, which can then be transferred to the cognate tRNA acceptor molecule (8, 9). There are 20 aaRS enzymes in total and they correspond to the 20 standard amino acids present in the cell. Each aaRS is categorized as class I or class II, based on the overall structure and function, except for lysyl-tRNA synthetase (LysRS), which has representatives in both classes (10–14). Common features of class I aaRSs include a HIGH/KMSKS-motif-defined Rossmann nucleotide-binding fold at the active site, binding of the tRNA acceptor stem at the minor groove (with the exception of tyrosyl-tRNA synthetase), and aminoacylation of tRNA at the 2'-hydroxyl group of the terminal adenine (A76) (12, 15–17). In contrast, class II aaRSs are characterized by a triple-motif antiparallel β-sheet fold at the active site, binding of the tRNA acceptor stem at the major groove, and aminoacylation of tRNA at the 3′ hydroxyl group of A76 (with the exception of phenylalanyl-tRNA synthetase) (8).
aaRSs provide the first step in quality control of translation. The degeneracy of the genetic code means that, in most cases, there are multiple tRNA isoacceptors specific for the same amino acid present within the cell. Accurate selection of cognate tRNA by the synthetase is typically achieved by a combination of specific identity elements in the tRNA molecule and also the large surface area available for binding and kinetic proofreading (9, 18–20). A more pressing challenge arises from the fact that some amino acids share close similarities in their chemical structures, which can make discrimination from noncognate amino acids particularly problematic. In the event that noncognate amino acids are recognized and activated, an intrinsic aaRS quality control mechanism exists that ensures such errors do not result in mistranslation of the genetic code. These quality control mechanisms clear noncognate amino acids both immediately following ATP-dependent activation (pretransfer editing) and/or following attachment to tRNA (posttransfer editing). For example, accurate discrimination against the isosteric amino acid Val by the class I IleRS involves posttransfer hydrolytic editing of Val-tRNAIle, a reaction in which the D-loop of tRNAIle is particularly important (21–24). The Escherichia coli LysRS, a class II aaRS, has also been shown to have significant tRNA mischarging activity (25). In addition to generating Lys-tRNALys, E. coli LysRS was found to aminoacylate its cognate tRNA with Arg, Thr, Met, Leu, Ala, Ser, and Cys. Furthermore, the weak substrate specificity of the enzyme was exacerbated by a combination of inefficient pretransfer editing mechanisms for some amino acids and an entirely absent posttransfer editing mechanism.
Here we show that pneumococcal IleRS is able to robustly mischarge its cognate tRNAIle with Val and, surprisingly, Leu. However, the overall amino acid specificity of the enzyme is tRNA dependent and may be achieved without the need for editing under conditions when the cellular amino acid pool is balanced. Pneumococcal LysRS preferentially mischarges both isoacceptors of tRNALys robustly with Ala, not Thr, and this likely provides an additional substrate for the Ala/Ser-aminoacyl-tRNA-dependent peptidoglycan biosynthesis enzyme MurM, which we have demonstrated to be a trans-editing factor in previous studies (26, 27). These findings support the hypothesis that broad-specificity tRNA mischarging spans both structural classes of the aminoacyl-tRNA synthetases in S. pneumoniae and provide insight into the mechanisms by which translational quality control has become adapted in this pathogen.
RESULTS
Pneumococcal LysRS mischarges both tRNALys isoacceptors with multiple amino acids.
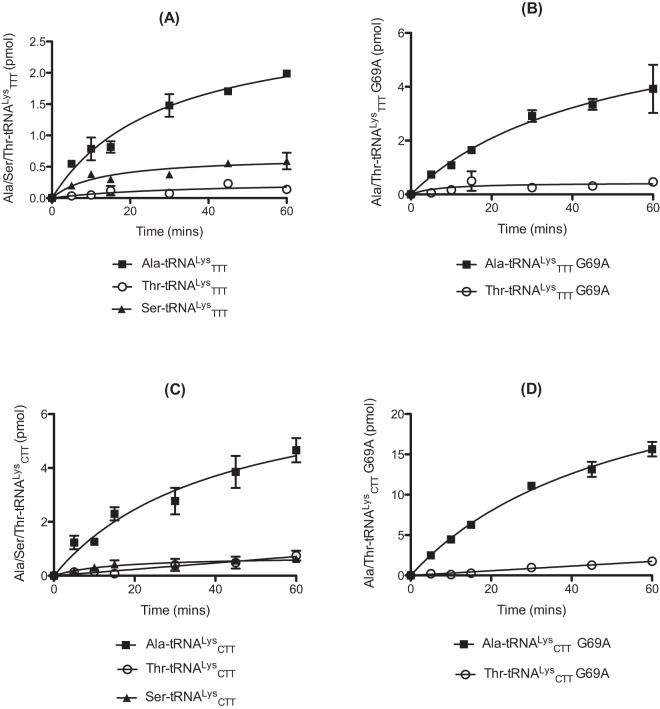
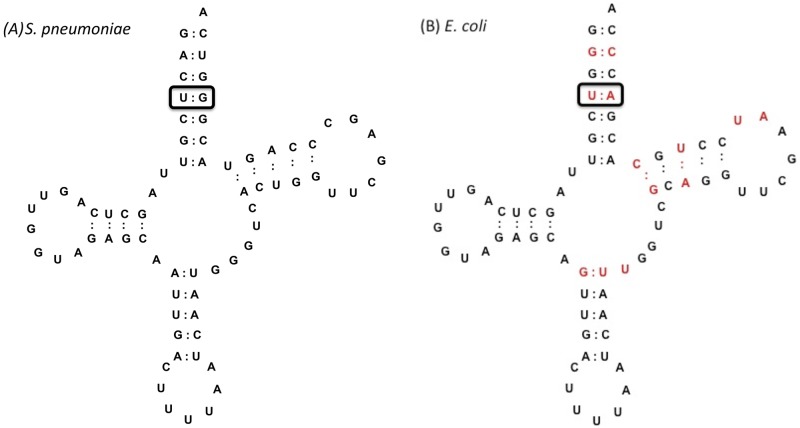
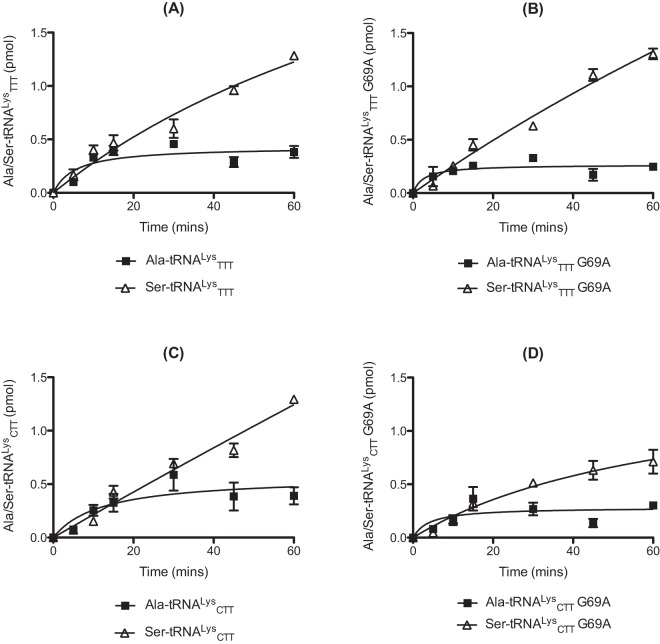
E. coli LysRS was previously found to catalyze the misaminoacylation of its cognate tRNA with several amino acids, including Ala (25, 28). In our earlier studies focusing on AlaRS, we demonstrated that mischarged Ala- and Ser-tRNA species can potentially enter the peptidoglycan biosynthesis pathway in S. pneumoniae via MurM (27, 29). We now investigated the capacity of pneumococcal LysRS to form mischarged Thr, Ala, and Ser tRNAs, the last two of which are also potential MurM substrates. Of the 3 amino acids tested in this study, mischarging by pneumococcal LysRS was greatest for Ala, regardless of the tRNALys isoacceptor used (Fig. 1A and C). This preference of S. pneumoniae protein for Ala over Thr and Ser differs from E. coli LysRS, which uses Thr most efficiently (25). One possible reason for pneumococcal LysRS having a preference for Ala over Thr may be related to differences in the pneumococcal tRNALys acceptor stem, most notably the presence of a U4:G69 wobble pair in place of the U4:A69 Watson-Crick base pair in E. coli tRNALys (Fig. 2; see also Fig. S1 in the supplemental material). Introduction of a Watson-Crick base pair (G69A) into each pneumococcal tRNALys isoacceptor resulted in an approximately 3-fold increase in lysylation activity by LysRS compared to wild-type tRNAs (Fig. 3). The overall mischarging profile of pneumococcal LysRS remained the same with both the wild-type and the G69A tRNALys transcripts; however, the yield of Ala-tRNALys produced was increased by approximately 2-fold for the TTT G69A transcript and 3-fold for the CTT G69A transcript in comparison to the equivalent wild-type species (Fig. 1B and D; Ser-tRNALys levels were too low to allow accurate determination of the effect of the G69A mutation [data not shown]). This suggests that, in the absence of efficient pre- and/or posttransfer editing mechanisms, the distorted region in the acceptor stem of tRNALys is able to reduce the overall mischarging capacity of pneumococcal LysRS, with an accompanying loss in cognate charging.
FIG 1 .
S. pneumoniae LysRS catalyzed mischarging of wild-type or G69A tRNALys anticodon TTT (A and B, respectively) or CTT (C and D, respectively) with alanine, serine and threonine. Aminoacylation time courses were carried out in the presence of 250 µM [3H]-l-Ala, [3H]-l-Ser or [14C]-l-Thr. Each reaction mixture contained 4 µM active pneumococcal LysRS. Wild-type or G69A S. pneumoniae tRNALys (anticodon CTT or TTT) was used at a concentration of 7 µM. The presented data set is the average of three independent experiments. Error bars show standard errors.
FIG 2 .
Predicted cloverleaf structures of S. pneumoniae (A) and E. coli (B) tRNALys anticodon TTT. Sequence variations from the S. pneumoniae tRNALys sequence are highlighted in red. All tRNA cloverleaf structures are shown without the CCA end.
FIG 3 .
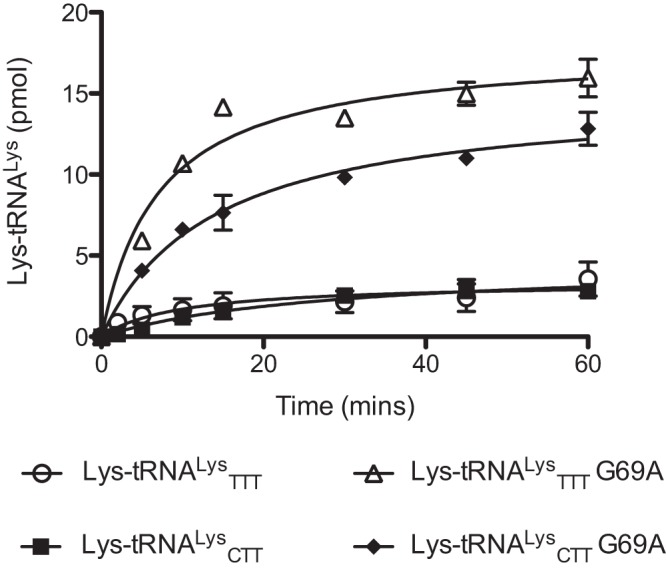
S. pneumoniae LysRS catalyzed Lys-tRNALys formation. The aminoacylation time course in the presence of 40 µM l-[14C]Lys for 3.7 µM active pneumococcal LysRS is shown. Wild-type or G69A pneumococcal tRNALys (anticodon CTT or TTT) was used at a concentration of 7 µM. The presented data set is the average of three independent experiments. Error bars show standard errors.
Generation of Ala-tRNALys by pneumococcal LysRS may provide an additional substrate for peptidoglycan cross-linking by MurM.
The relative instability of wild-type Ala-tRNALys in solution (see Fig. S2 in the supplemental material) compared to previously studied mischarged pneumococcal tRNAs (27) resulted in an inability to isolate sufficient quantities of this product for use in direct deacylation assays. Therefore, the effect of MurM addition on the ability of pneumococcal LysRS to produce mischarged Ala-tRNALys was investigated. MurM reduced the mischarging capacity of pneumococcal LysRS in the presence of Ala regardless of the isoacceptor of tRNALys present in the reaction mixture (Fig. 4). Our earlier studies with Ser-tRNAAla showed that reduction in the yield of this product by AlaRS upon addition of MurM was correlated with the trans-editing activity of the latter protein (27). In addition, the use of mischarged Ser-tRNAAla by MurM in peptidoglycan biosynthesis has already been demonstrated (29); therefore, it is likely that production of Ala-tRNALys by LysRS provides another substrate that can be diverted from protein synthesis into this pathway.
FIG 4 .
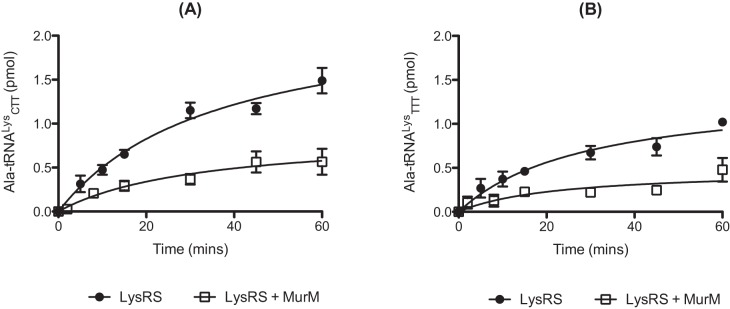
MurM decreases production of alanylated tRNALys anticodons CTT (A) and TTT (B) by LysRS. Misaminoacylation time courses for generation of [3H]Ala-tRNALys by 2 µM active pneumococcal LysRS in the presence of 0.6 µM MurM are shown. The concentration of wild-type pneumococcal tRNALys used was 7 µM. The concentration of [3H]Ala used was 250 µM. Data sets are the averages of three independent experiments. Error bars indicate standard errors.
The presence of the U4:G69 wobble pair in the acceptor stems of both tRNALys isoacceptors raised the question of whether these species could be substrates for the pneumococcal AlaRS enzyme, as previously demonstrated for tRNAPhe (27). Full-length pneumococcal AlaRS preferentially mischarged both wild-type tRNALys transcripts with Ser over cognate Ala (Fig. 5A and C). The mutated G69A transcripts were also aminoacylated by full-length AlaRS, although slightly less efficiently in the case of the anticodon CTT transcript (Fig. 5B and D).
FIG 5 .
Comparative S. pneumoniae full-length AlaRS-catalyzed mischarging of wild-type or G69A tRNALys anticodon TTT (A and B, respectively) or CTT (C and D, respectively) with alanine and serine. Aminoacylation time courses were carried out in the presence of 250 µM l-[3H]Ala or l-[3H]Ser. Each reaction mixture contained 0.5 µM active full-length pneumococcal AlaRS. Wild-type or G69A S. pneumoniae tRNALys (anticodon CTT or TTT) was used at a concentration of 7 µM. The presented data set is the average of three independent experiments. Error bars show standard errors.
Pneumococcal IleRS robustly mischarges its cognate tRNA with Leu and Val.
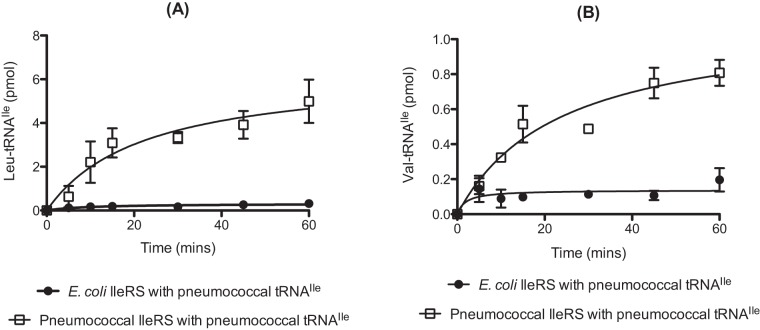
Taken together with results of previous studies, the above findings now show extensive mischarging by S. pneumoniae class II-type aaRSs. To investigate if similar activities are found for class I-type aaRSs, the substrate specificity of IleRS during amino acid activation and aminoacylation was investigated. In the case of E. coli IleRS, the primary potential noncognate substrate is Val, which is effectively dealt with by a combination of pretransfer editing of Val-AMP and posttransfer editing of Val-tRNAlle (21, 23, 30). Consequently, E. coli IleRS does not accumulate Val-tRNAlle to levels expected to affect the overall error rate of translation. The abilities of pneumococcal and E. coli IleRS to mischarge cognate tRNA with both l-Leu and l-Val were compared. Pneumococcal IleRS was able to mischarge tRNAIle with Leu to approximately 5-fold-higher levels than the E. coli enzyme in vitro (Fig. 6A). Val-tRNAIle was also synthesized to higher levels by pneumococcal IleRS than by E. coli IleRS, although the difference was less significant than that observed with Leu (Fig. 6B).
FIG 6 .
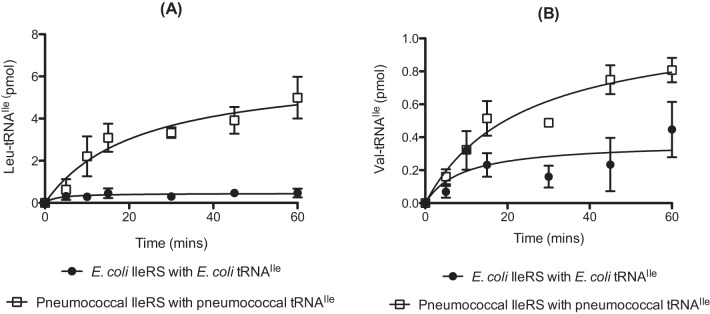
Comparative S. pneumoniae and E. coli IleRS-catalyzed mischarging of cognate tRNAIle with leucine (A) or valine (B). Aminoacylation time courses were evaluated in the presence of 200 µM l-[14C]Leu (A) or 200 µM l-[14C]Val (B) for 1 µM active pneumococcal or E. coli IleRS. Wild-type pneumococcal or E. coli tRNAIle (anticodon GAT) was used at a concentration of 10 µM. The presented data set is the average of three independent experiments. Error bars show standard errors.
To investigate if differences in mischarging between pneumococcal and E. coli IleRS result from variations in tRNA sequence (see Fig. S3 in the supplemental material), noncognate aminoacylation experiments were performed. Replacement of E. coli tRNAIle with the S. pneumoniae tRNAIle did not significantly increase yields of Leu- or Val-tRNAIle produced by the E. coli IleRS enzyme (Fig. 7A and B, respectively). Further examination of the possible role of tRNAIle in amino acid specificity was investigated by using a G16C mutation in pneumococcal tRNAIle, as this residue has been implicated in the editing of Val-tRNAIle by IleRS (24, 31). The aminoacylation capacity of pneumococcal IleRS was reduced by almost 50% for tRNAIle G16C compared to wild-type tRNA with both Ile (Fig. 8A) and Leu (Fig. 8B). However, no difference was seen for Val mischarging between the wild-type and the G16C transcript (Fig. 8C).
FIG 7 .
Comparative S. pneumoniae and E. coli IleRS-catalyzed mischarging of pneumococcal tRNAIle with leucine or valine. Aminoacylation time courses were evaluated in the presence of 200 µM l-[14C]Leu (A) or 200 µM l-[14C]Val (B) for 1 µM active pneumococcal of E. coli IleRS. Wild-type pneumococcal tRNAIle (anticodon GAT) was used at a concentration of 10 µM. The presented data set is the average of three independent experiments. Error bars show standard errors.
FIG 8 .
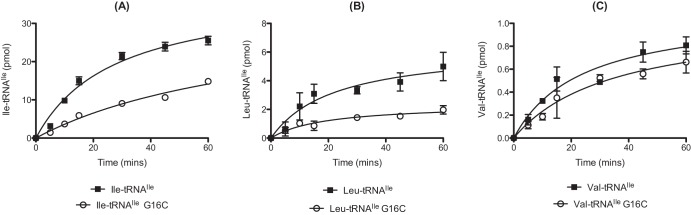
Comparative S. pneumoniae IleRS-catalyzed mischarging of wild-type and G16C tRNAIle with isoleucine (A), leucine (B), or valine (C). For the cognate amino acid, aminoacylation time courses were carried out in the presence of 22 µM l-[14C]Ile and 500 nM active pneumococcal IleRS (A). For the noncognate amino acids, aminoacylation time courses were carried out in the presence of 200 µM l-[14C]Leu or l-[14C]Val for 1 µM active pneumococcal IleRS. Wild-type or G16C pneumococcal tRNAIle (anticodon GAT) was used at a concentration of 10 µM. The presented data set is the average of three independent experiments. Error bars show standard errors.
IleRS has weak posttransfer editing activity against Leu-tRNAIle.
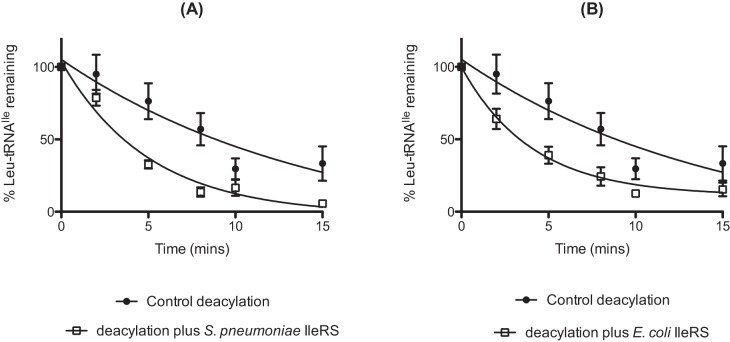
The robust level of mischarging seen with pneumococcal IleRS and Leu allowed aminoacylation kinetic parameters to be determined for tRNAIle (Table 1). The comparatively low kcat values derived in this study are likely due to the absence of the N-6-threonylcarbamoyl modification of adenine-37 in the anticodon loop of in vitro-transcribed tRNAIle, as previously described in other systems (22, 31, 32). Pneumococcal IleRS has a KM for Leu that is ~8,000-fold higher and a kcat almost 3-fold higher than that for cognate Ile. Consequently, the catalytic efficiency of pneumococcal IleRS is approximately 2,850 times greater for Ile than Leu, giving a specificity constant comparable to overall error rates in protein synthesis which are generally estimated to be in the range of 1 in 3,000 to 10,000 (33). Specificity constants of less than 1 in 3,000 are typically associated with the lack of a requirement for editing among aminoacyl-tRNA synthetases under conditions that favor maintenance of a balanced intracellular amino acid pool (34). Therefore, the editing capacity of pneumococcal IleRS against Leu-tRNAIle was tested (Fig. 9). Both pneumococcal and E. coli IleRS were demonstrated to have relatively weak posttransfer editing activities, consistent with the specificity constant obtained from our kinetic studies.
TABLE 1 .
Kinetic parameters for aminoacylation of tRNAIle with Ile and Leu by pneumococcal IleRSa
| Amino acid | Km (μM) | Vmax (μM/min/mg) | kcat (min−1) | kcat/Km | Specificity constant |
|---|---|---|---|---|---|
| Ile | 0.4 ± 0.1 | 0.0005 ± 0.0002 | 0.06 ± 0.02 | 0.14 | 1:2,850 |
| Leu | 3,200 ± 290 | 0.0015 ± 0.0002 | 0.16 ± 0.02 | 0.000049 |
Means and standard errors are shown. Vmax and kcat values were determined using protein concentration estimations obtained via the Bradford assay.
FIG 9 .
Deacylation of mischarged pneumococcal Leu-tRNAIle by S. pneumoniae and E. coli IleRS. Deacylation time courses were carried out as described above by incubation of 50 pM [14C]Leu-tRNAIle with 0.5 µM pneumococcal (A) or E. coli (B) IleRS. For the control reaction mixture, an equal volume of protein storage buffer was added. Error bars indicate standard errors.
DISCUSSION
Pneumococcal IleRS retains fidelity without editing under conditions where the amino acid pool is balanced.
Pneumococcal IleRS was found to mischarge tRNAIle with Leu significantly more efficiently than the E. coli enzyme misaminoacylated tRNAIle with Val. Despite this significant mischarging activity, pneumococcal IleRS maintains a substrate specificity for Ile over Leu that is consistent with reported error rates of translation. This is achieved in the absence of efficient editing by virtue of a 2,850-fold difference in catalytic efficiencies that ultimately favors turnover of cognate Ile over noncognate Leu (Table 1). Nevertheless, conditions causing an imbalance of the cellular amino acid pool might be expected to result in increased mistranslation rates at Ile codons within this bacterium. In S. pneumoniae it is possible that both oxidative stress, caused by high-level hydrogen peroxide production, and the infection process itself may cause amino acid pool imbalances. Production of hydrogen peroxide by pneumococcus results in an alpha-hemolytic appearance on blood agar due to partial lysis of erythrocytes, and the pneumolysin toxin produced during host infection can cause full lysis of blood cells and other cells (35, 36). Therefore, serum is an expected source of amino acids for the bacterium during human infection. Plasma levels of Ile, Val, and Leu in humans are known to decrease during infection, with Leu remaining the most abundant postinoculation with S. pneumoniae in some instances (37–39). It has also been demonstrated for the intraerythrocytic protozoan malaria parasite Plasmodium falciparum that Ile becomes the limiting amino acid in the human host during infection. This is because Ile is absent from adult hemoglobin, which is the main source of nutrients for the parasite (40, 41). The relative abundance of Leu and the absence of an efficient editing mechanism may have allowed pneumococcal IleRS to evolve so that it remains functional, regardless of the severity and duration of host infection, even under conditions of limited availability of the cognate amino acid Ile.
Pneumococcal tRNALys has evolved to function in both protein and peptidoglycan biosynthesis.
Our findings demonstrate that like E. coli, pneumococcal LysRS has relaxed amino acid substrate specificity, although in this case Ala rather than Thr is the preferred substrate for tRNALys mischarging. This difference in noncognate amino acid specificity is accompanied by significant changes in the structure of both tRNALys isoacceptors, namely, the presence of an unusual wobble pair (U4:G69) reminiscent of the G3:U70 pair critical for recognition of tRNAAla by AlaRS (18, 42–45). The presence of this wobble region in the acceptor stem of pneumococcal tRNALys controls the efficiency of tRNA aminoacylation by LysRS and to a lesser extent that by AlaRS. Replacement of the wobble region with a correct Watson-Crick base pair (mutant tRNALys G69A) resulted in improved aminoacylation by LysRS, suggesting that the wobble pair has been specifically selected for and retained during evolution, although the underlying selection pressure remains unclear. A similar improvement in isoacceptor aminoacylation capacity was demonstrated for phenylalanylation of tRNAPhe U4G by pneumococcal PheRS in comparison to that by the wild-type species (27). In addition, our studies have demonstrated that the presence of the aminoacyl-tRNA-dependent peptidoglycan cross-linking enzyme, MurM, lowers the yield of Ala-tRNALys produced by pneumococcal LysRS. This supports our earlier finding that MurM can also act as a trans-editing factor by effectively directing both mischarged Ala and Ser species away from protein synthesis and into peptidoglycan biosynthesis (27).
The identification of an unusual wobble region in the acceptor stems of both tRNAPhe and tRNALys suggests that pneumococcus may have evolved to have a specific subset of its tRNA species accessible to both pathways. This is in contrast to the mechanism by which Staphylococcus aureus is known to ensure adequate provision of Gly-tRNAGly for cell wall cross-linking and protein synthesis. In S. aureus, peptidoglycan is indirectly cross-linked by virtue of a pentaglycine bridge that is formed by the activity of the glycyl-tRNA-dependent FemXAB proteins (46). It has been established that there are four fully annotated tRNAGly isoacceptors encoded in the genome of this bacterium plus a fifth pseudogene that encodes an unusual Gly isoacceptor. All five isoacceptors are efficiently aminoacylated by glycyl-tRNA synthetase; however, three of them contain sequence-specific identity elements that are consistent with weak EF-Tu binding and are, therefore, likely to be specifically shuttled into the peptidoglycan biosynthesis pathway (47). In pneumococcus, peptidoglycan is indirectly cross-linked by the addition of Ala-Ala or Ser-Ala dipeptide bridges. The MurM and MurN proteins specifically catalyze dipeptide bridge formation by using Ala and/or Ser tRNA species originally thought to be provided selectively by alanyl- and seryl-tRNA synthetase, respectively (48–50). However, no unique tRNAAla or tRNASer isoacceptors have been identified in this bacterium. As a result, the mechanism by which pneumococcus ensures adequate provision of substrates for both protein and peptidoglycan biosynthesis has remained elusive. Our studies demonstrate that the mechanism used by pneumococcus may reside in the unique evolutionary modification of a specific subset of tRNAs within the cell which subsequently alters the substrate specificity of the aminoacyl-tRNA synthetases.
Broad-specificity tRNA mischarging occurs across both classes of tRNA synthetase in S. pneumoniae.
The translation quality control systems of S. pneumoniae are somewhat unique in terms of both the mischarging and editing profiles of the corresponding aaRSs and the absence of trans-editing factors such as AlaXp and Ybak, commonly found in other bacteria (27). For the class I enzyme IleRS, elevated mischarging activity offers a potential mechanism for adaptive translation during cognate amino acid limitation (51). For the class II enzymes AlaRS, LysRS, and PheRS, the ability to generate and/or protect a broad range of Ala and Ser mischarged tRNA offers a versatile mechanism to provide substrates for peptidoglycan biosynthesis. Further studies are now warranted to explore in vivo the apparently widespread role of aaRS-catalyzed misaminoacylation in S. pneumoniae. In addition to aaRS-specific adaptations, pneumococcus is also known to have a unique EF-Tu protein that differs in sequence from that of other bacteria at four positions: P129K, M140L, T230S, and E234D (52). All of these adaptations may have, in part, been driven by evolutionary pressure for the bacterium to adapt to its unusual lifestyle. It is well documented that pneumococcus routinely produces and, therefore, exposes itself to high levels of hydrogen peroxide during its natural life cycle as a means of competing with other bacterial species for colonization of the nasopharynx (3). Further characterization of aaRS-specific and other types of adaptations that pneumococcus has made to maintain quality control of translation while ensuring adequate provision of aminoacylated tRNA substrates for both peptidoglycan and protein synthesis may enable identification of new drugs targets in the future.
MATERIALS AND METHODS
Strains, plasmids, and general protein expression and purification.
S. pneumoniae strain D39 chromosomal DNAs for use as a template in the cloning of genes encoding IleRS, LysRS, EF-Tu, AlaRS, and MurM were a gift from B. Lazazzera (University of California, Los Angeles). E. coli ileS was cloned by amplification of the gene from strain BL21(DE3) by colony PCR. The gene encoding S. pneumoniae ileS was cloned into pQE-31 (Qiagen) by virtue of the BamHI and HindIII restriction sites. The subsequent expression construct allowed for the production of a recombinant protein extended at the N terminus by a six-histidine tag. The genes encoding E. coli IleRS, S. pneumoniae AlaRS, and S. pneumoniae MurM were cloned into pET21b (Novagen) by virtue of the NdeI and XhoI restriction sites, allowing for the production of recombinant protein extended at the C terminus by a six-histidine tag. All cloned expression constructs were checked for accuracy against the appropriate protein sequences found in the comprehensive microbial resource database (J. Craig Venter Institute) by Sanger DNA sequencing (Plant Microbial Genomics Facility, The Ohio State University) with the appropriate primers. Both IleRS proteins were overexpressed in E. coli strain B834(DE3) by the addition of a final concentration of 1 mM isopropyl-β-d-1-thigalactopyranoside at an optical density at 600 nm (OD600) of 0.4 followed by a reduction in growth temperature from 37°C to 25°C for 3 to 5 h. EF-Tu and MurM were overexpressed in the same way; however, the expression strain was changed to E. coli BL21(DE3). Proteins were purified on BD Talon cobalt resin using equilibration/wash buffer (50 mM sodium phosphate [pH 7.2], 500 mM sodium chloride, and 20% glycerol) containing 250 mM imidazole. MurM was solubilized prior to purification as described elsewhere (29). S. pneumoniae tRNAIle, E. coli tRNAIle, and S. pneumoniae tRNALys (anticodons CTT and TTT) were produced by in vitro T7 RNA polymerase runoff transcription as described previously (53, 54).
Determination of protein concentration by active site titration or Bradford assay.
To determine active protein concentrations for IleRS, AlaRS, and LysRS, 5 µl of undiluted protein or protein diluted 1:10 or 1:20 was incubated in three separate reaction mixtures for 10 min at 37°C in the presence of 0.1 M Na-HEPES (pH 7.2), 30 mM KCl, 10 mM MgCl2, 2 mM ATP, 2 µmol min−1 ml−1 inorganic pyrophosphatase (Roche), and 40 µM cognate amino acid ([14C]Lys, [3H]Ala, or [14C]Ile from PerkinElmer or Moravek Biochemicals). A control reaction was also carried out where the reaction volume of protein was replaced by protein storage buffer. Samples were processed by vacuum filtration onto Whatman Protran BA85 filter paper circles. After sample spotting, each filter paper was washed three times with buffer comprised of 50 mM Na-HEPES (pH 7.2), 15 mM KCl, 5 mM MgCl2 prior to drying and quantification by liquid scintillation counting (55). For determination of protein concentration by the Bradford assay, Bradford reagent was obtained from Bio-Rad and used as per the manufacturer’s instructions. A standard curve with known concentrations of bovine serum albumin (resuspended in IleRS storage buffer) was obtained to improve accuracy of estimations.
Aminoacylation.
Aminoacylation time courses were carried out over a time period of 1 h at 37°C in the presence of 0.1 M Na-HEPES (pH 7.2), 30 mM KCl, 10 mM MgCl2, 2 mM ATP, 2 µmol min−1 ml−1 inorganic pyrophosphatase (Roche), 10 µM tRNAIle or tRNALys transcript, 20 to 40 µM cognate amino acid ([14C]Lys or [14C]Ile from PerkinElmer or Moravek Biochemicals, respectively) or 200 µM noncognate amino acid ([3H]Ser, [14C]Thr, [3H]Ala, [14C]Leu, or [14C]Val) at 150 to 500 cpm/pmol and 0.5 to 4.0 µM active IleRS, AlaRS, or LysRS (as determined by active site titration). Where appropriate, reactions were repeated in the presence of 500 nM S. pneumoniae MurM. Ten-microliter samples were taken for each time point and spotted onto 3-mm Whatman filter paper discs, which were immediately dropped into 5% trichloroacetic acid (TCA). Discs were subjected to two further washes with 5% TCA and ethanol prior to drying and scintillation counting.
Kinetics of isoleucylation and leucylation of tRNAIle by pneumococcal IleRS.
To determine the steady-state kinetic parameters for pneumococcal IleRS with either l-Ile or l-Leu, aminoacylation time courses were carried out at 37°C for both the lowest (1.5 µM Ile and 30 µM Leu) and the highest (50 µM Ile and 1 mM Leu) amino acid concentrations in the presence of 0.1 M Na-HEPES (pH 7.2), 30 mM KCl, 10 mM MgCl2, 2 mM ATP, 2 µmol min−1 ml−1 inorganic pyrophosphatase (Roche), 10 µM pneumococcal tRNAIle transcript, and 500 nM or 1 µM active IleRS for Ile and Leu, respectively (as determined by active site titration). For Ile, the linear region was determined to be within the first 5 min; therefore, 10-µl samples were spotted onto 3-mm Whatman filter paper and dropped into 5% TCA at four time points (1, 2, 3, and 5 min) at each of the amino acid concentrations (1.5, 3, 5, 8, 10, 15, 20, 30, 40, and 50 µM) for determination of gradients and key kinetic parameters from triplicate data sets, using the Hanes-Woolf method. For Leu, the linear region was determined to be within the first 30 min; therefore, 10-µl samples were spotted onto 3-mm Whatman filter paper and dropped into 5% TCA at four time points (10, 15, 20, and 30 min) at each of the amino acid concentrations (30, 50, 100, 200, 250, 300, 400, 500, 600, and 1,000 µM) for determination of gradients and key kinetic parameters from triplicate data sets, using the Michaelis-Menten analysis method in Prism software (GraphPad).
Deacylation assays.
Aminoacylation reactions were set up in four 200-µl reaction mixtures, each consisting of 30 mM HEPES (pH 7.6), 15 mM MgCl2, 10 mM diethiothreitol, 2 mM ATP, 2 µmol min−1 ml−1 inorganic pyrophosphatase (Roche), 250 µM [14C]Leu (with a specific activity of ~300 cpm/pmol), 10 µM S. pneumoniae tRNAIle transcript (prior to use, stock was resuspended in 2 mM MgCl2 and heated at 80°C for 10 min, followed by slow cooling to room temperature to allow refolding), 2 µM mol min-1 ml−1 inorganic pyrophosphatase, and 1 µM IleRS. The reaction mixtures were incubated at 37°C for 1 h, quenched with 20 µM of 3 M sodium acetate (pH 4.5), and processed as described elsewhere (27). Deacylation assays were carried out by incubation of 50 pM [14C]Leu-tRNAIle in buffer composed of 0.1 M Na-HEPES (pH 7.2), 30 mM KCl, and 10 mM MgCl2. In addition, 0.5 µM E. coli or S. pneumoniae IleRS, 0.5 µM MurM, or an equal volume of protein storage buffer was added to the reaction mixtures, which were monitored by TCA precipitation and scintillation counting. Attempts to utilize pneumococcal Val-tRNAIle for deacylation assays were not successful due to rapid spontaneous deacylation during isolation.
SUPPLEMENTAL MATERIAL
Predicted cloverleaf structures of S. pneumoniae tRNALys anticodons TTT (A) and CTT (B). Sequence variations between the two tRNALys isoacceptors are highlighted in red. All tRNA cloverleaf structures are shown without the CCA end. Download
Natural deacylation of mischarged pneumococcal Ala-tRNALys demonstrates its inherent instability. Deacylation time courses were carried out as described in the text with incubation of 10 pM [14C]Ala-tRNALys in buffer composed of 0.1 M Na-HEPES (pH 7.2), 30 mM KCl, and 10 mM MgCl2. Download
Predicted cloverleaf structures of E. coli (A) and S. pneumoniae (B) tRNAIle (anticodon GAU). Sequence variations from the S. pneumoniae tRNAIle sequence are highlighted in red. All tRNA cloverleaf structures are shown without the CCA end. Position 16 is boxed. Download
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (GM065183).
Footnotes
Citation Shepherd J, Ibba M. 2014. Relaxed substrate specificity leads to extensive tRNA mischarging by Streptococcus pneumoniae class I and class II aminoacyl-tRNA synthetases. mBio 5(5):e01656-14. doi:10.1128/mBio.01656-14.
REFERENCES
- 1. Pesakhov S, Benisty R, Sikron N, Cohen Z, Gomelsky P, Khozin-Goldberg I, Dagan R, Porat N. 2007. Effect of hydrogen peroxide production and the Fenton reaction on membrane composition of Streptococcus pneumoniae. Biochim. Biophys. Acta 1768:590–597. 10.1016/j.bbamem.2006.12.016 [DOI] [PubMed] [Google Scholar]
- 2. Regev-Yochay G, Trzcinski K, Thompson CM, Lipsitch M, Malley R. 2007. SpxB is a suicide gene of Streptococcus pneumoniae and confers a selective advantage in an in vivo competitive colonization model. J. Bacteriol. 189:6532–6539. 10.1128/JB.00813-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pericone CD, Overweg K, Hermans PW, Weiser JN. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 68:3990–3997. 10.1128/IAI.68.7.3990-3997.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Netzer N, Goodenbour JM, David A, Dittmar KA, Jones RB, Schneider JR, Boone D, Eves EM, Rosner MR, Gibbs JS, Embry A, Dolan B, Das S, Hickman HD, Berglund P, Bennink JR, Yewdell JW, Pan T. 2009. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature 462:522–526. 10.1038/nature08576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ling J, Söll D. 2010. Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proc. Natl. Acad. Sci. U. S. A. 107:4028–4033. 10.1073/pnas.1000315107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Storz G, Imlay JA. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188–194. 10.1016/S1369-5274(99)80033-2 [DOI] [PubMed] [Google Scholar]
- 7. Yesilkaya H, Andisi VF, Andrew PW, Bijlsma JJ. 2013. Streptococcus pneumoniae and reactive oxygen species: an unusual approach to living with radicals. Trends Microbiol. 21:187–195. 10.1016/j.tim.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 8. Carter CW., Jr. 1993. Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu. Rev. Biochem. 62:715–748. 10.1146/annurev.bi.62.070193.003435 [DOI] [PubMed] [Google Scholar]
- 9. Giegé R, Puglisi JD, Florentz C. 1993. tRNA structure and aminoacylation efficiency. Prog. Nucleic Acid Res. Mol. Biol. 45:129–206. 10.1016/S0079-6603(08)60869-7 [DOI] [PubMed] [Google Scholar]
- 10. Cusack S, Berthet-Colominas C, Härtlein M, Nassar N, Leberman R. 1990. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 Å. Nature 347:249–255. 10.1038/347249a0 [DOI] [PubMed] [Google Scholar]
- 11. Cusack S. 1993. Sequence, structure and evolutionary relationships between class 2 aminoacyl-tRNA synthetases: an update. Biochimie 75:1077–1081. 10.1016/0300-9084(93)90006-E [DOI] [PubMed] [Google Scholar]
- 12. Eriani G, Delarue M, Poch O, Gangloff J, Moras D. 1990. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature 347:203–206. 10.1038/347203a0 [DOI] [PubMed] [Google Scholar]
- 13. Burbaum JJ, Schimmel P. 1991. Structural relationships and the classification of aminoacyl-tRNA synthetases. J. Biol. Chem. 266:16965–16968 [PubMed] [Google Scholar]
- 14. Ibba M, Morgan S, Curnow AW, Pridmore DR, Vothknecht UC, Gardner W, Lin W, Woese CR, Söll D. 1997. A euryarchaeal lysyl-tRNA synthetase: resemblance to class I synthetases. Science 278:1119–1122. 10.1126/science.278.5340.1119 [DOI] [PubMed] [Google Scholar]
- 15. Ibba M, Soll D. 2000. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69:617–650. 10.1146/annurev.biochem.69.1.617 [DOI] [PubMed] [Google Scholar]
- 16. Carter P, Bedouelle H, Winter G. 1986. Construction of heterodimer tyrosyl-tRNA synthetase shows tRNATyr interacts with both subunits. Proc. Natl. Acad. Sci. U. S. A. 83:1189–1192. 10.1073/pnas.83.5.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yaremchuk A, Kriklivyi I, Tukalo M, Cusack S. 2002. Class I tyrosyl-tRNA synthetase has a class II mode of cognate tRNA recognition. EMBO J. 21:3829–3840. 10.1093/emboj/cdf373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McClain WH. 1993. Rules that govern tRNA identity in protein synthesis. J. Mol. Biol. 234:257–280. 10.1006/jmbi.1993.1582 [DOI] [PubMed] [Google Scholar]
- 19. Guth EC, Francklyn CS. 2007. Kinetic discrimination of tRNA identity by the conserved motif 2 loop of a class II aminoacyl-tRNA synthetase. Mol. Cell 25:531–542. 10.1016/j.molcel.2007.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ling J, Reynolds N, Ibba M. 2009. Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 63:61–78. 10.1146/annurev.micro.091208.073210 [DOI] [PubMed] [Google Scholar]
- 21. Fersht AR. 1977. Editing mechanisms in protein synthesis. Rejection of valine by the isoleucyl-tRNA synthetase. Biochemistry 16:1025–1030. 10.1021/bi00624a034 [DOI] [PubMed] [Google Scholar]
- 22. Silvian LF, Wang J, Steitz TA. 1999. Insights into editing from an Ile-tRNA synthetase structure with tRNAIle and mupirocin. Science 285:1074–1077. 10.1126/science.285.5430.1074 [DOI] [PubMed] [Google Scholar]
- 23. Eldred EW, Schimmel PR. 1972. Rapid deacylation by isoleucyl transfer ribonucleic acid synthetase of isoleucine-specific transfer ribonucleic acid aminoacylated with valine. J. Biol. Chem. 247:2961–2964 [PubMed] [Google Scholar]
- 24. Hale SP, Auld DS, Schmidt E, Schimmel P. 1997. Discrete determinants in transfer RNA for editing and aminoacylation. Science 276:1250–1252. 10.1126/science.276.5316.1250 [DOI] [PubMed] [Google Scholar]
- 25. Jakubowski H. 1999. Misacylation of tRNALys with noncognate amino acids by lysyl-tRNA synthetase. Biochemistry 38:8088–8093. 10.1021/bi990629i [DOI] [PubMed] [Google Scholar]
- 26. Shepherd J, Ibba M. 2013. Direction of aminoacylated transfer RNAs into antibiotic synthesis and peptidoglycan-mediated antibiotic resistance. FEBS Lett. 587:2895–2904. 10.1016/j.febslet.2013.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shepherd J, Ibba M. 2013. Lipid II-independent trans editing of mischarged tRNAs by the penicillin resistance factor MurM. J. Biol. Chem. 288:25915–25923. 10.1074/jbc.M113.479824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jakubowski H. 1997. Aminoacyl thioester chemistry of class II aminoacyl-tRNA synthetases. Biochemistry 36:11077–11085. 10.1021/bi970589n [DOI] [PubMed] [Google Scholar]
- 29. Shepherd J. 2011. Characterisation of pneumococcal peptidoglycan cross-linking enzymology. Ph.D. thesis. University of Warwick, Coventry, England [Google Scholar]
- 30. Baldwin AN, Berg P. 1966. Transfer ribonucleic acid-induced hydrolysis of valyladenylate bound to isoleucyl ribonucleic acid synthetase. J. Biol. Chem. 241:839–845 [PubMed] [Google Scholar]
- 31. Farrow MA, Nordin BE, Schimmel P. 1999. Nucleotide determinants for tRNA-dependent amino acid discrimination by a class I tRNA synthetase. Biochemistry 38:16898–16903. 10.1021/bi9920782 [DOI] [PubMed] [Google Scholar]
- 32. Ibba M, Francklyn C, Cusack S. 2005. The aminoacyl-tRNA synthetases. Landes Bioscience, Georgetown, TX [Google Scholar]
- 33. Reynolds NM, Lazazzera BA, Ibba M. 2010. Cellular mechanisms that control mistranslation. Nat. Rev. Microbiol. 8:849–856. 10.1038/nrmicro2472 [DOI] [PubMed] [Google Scholar]
- 34. Fersht AR, Kaethner MM. 1976. Enzyme hyperspecificity. Rejection of threonine by the valyl-tRNA synthetase by misacylation and hydrolytic editing. Biochemistry 15:3342–3346. 10.1021/bi00660a026 [DOI] [PubMed] [Google Scholar]
- 35. Hirst RA, Kadioglu A, O’Callaghan C, Andrew PW. 2004. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin. Exp. Immunol. 138:195–201. 10.1111/j.1365-2249.2004.02611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grange JM, Fox K, Morgan NL. 2009. Genetic manipulation: techniques and applications. John Wiley & Sons, Oxford, United Kingdom [Google Scholar]
- 37. Wannemacher RW, Jr, Powanda MC, Pekarek RS, Beisel WR. 1971. Tissue amino acid flux after exposure of rats to Diplococcus pneumoniae. Infect. Immun. 4:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wannemacher RW, Jr, Powanda MC, Dinterman RE. 1974. Amino acid flux and protein synthesis after exposure of rats to either Diplococcus pneumoniae or Salmonella typhimurium. Infect. Immun. 10:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wannemacher RW., Jr. 1977. Key role of various individual amino acids in host response to infection. Am. J. Clin. Nutr. 30:1269–1280 [DOI] [PubMed] [Google Scholar]
- 40. Istvan ES, Dharia NV, Bopp SE, Gluzman I, Winzeler EA, Goldberg DE. 2011. Validation of isoleucine utilization targets in Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 108:1627–1632. 10.1073/pnas.1011560108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Babbitt SE, Altenhofen L, Cobbold SA, Istvan ES, Fennell C, Doerig C, Llinás M, Goldberg DE. 2012. Plasmodium falciparum responds to amino acid starvation by entering into a hibernatory state. Proc. Natl. Acad. Sci. U. S. A. 109:3278–3287. 10.1073/pnas.1209823109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hou YM, Schimmel P. 1988. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature 333:140–145. 10.1038/333140a0 [DOI] [PubMed] [Google Scholar]
- 43. McClain WH, Chen YM, Foss K, Schneider J. 1988. Association of transfer RNA acceptor identity with a helical irregularity. Science 242:1681–1684. 10.1126/science.2462282 [DOI] [PubMed] [Google Scholar]
- 44. Gabriel K, Schneider J, McClain WH. 1996. Functional evidence for indirect recognition of G.U in tRNAAla by alanyl-tRNA synthetase. Science 271:195–197. 10.1126/science.271.5246.195 [DOI] [PubMed] [Google Scholar]
- 45. Beuning PJ, Yang F, Schimmel P, Musier-Forsyth K. 1997. Specific atomic groups and RNA helix geometry in acceptor stem recognition by a tRNA synthetase. Proc. Natl. Acad. Sci. U. S. A. 94:10150–10154. 10.1073/pnas.94.19.10150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ling B, Berger-Bächi B. 1998. Increased overall antibiotic susceptibility in Staphylococcus aureus femAB null mutants. Antimicrob. Agents Chemother. 42:936–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Giannouli S, Kyritsis A, Malissovas N, Becker HD, Stathopoulos C. 2009. On the role of an unusual tRNAGly isoacceptor in Staphylococcus aureus. Biochimie 91:344–351. 10.1016/j.biochi.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 48. Fiser A, Filipe SR, Tomasz A. 2003. Cell wall branches, penicillin resistance and the secrets of the MurM protein. Trends Microbiol. 11:547–553. 10.1016/j.tim.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 49. Filipe SR, Tomasz A. 2000. Inhibition of the expression of penicillin resistance in Streptococcus pneumoniae by inactivation of cell wall muropeptide branching genes. Proc. Natl. Acad. Sci. U. S. A. 97:4891–4896. 10.1073/pnas.080067697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Filipe SR, Severina E, Tomasz A. 2002. The murMN operon: a functional link between antibiotic resistance and antibiotic tolerance in Streptococcuspneumoniae. Proc. Natl. Acad. Sci. U. S. A. 99:1550–1555. 10.1073/pnas.032671699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pan T. 2013. Adaptive translation as a mechanism of stress response and adaptation. Annu. Rev. Genet. 47:121–137. 10.1146/annurev-genet-111212-133522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ke D, Boissinot M, Huletsky A, Picard FJ, Frenette J, Ouellette M, Roy PH, Bergeron MG. 2000. Evidence for horizontal gene transfer in evolution of elongation factor Tu in enterococci. J. Bacteriol. 182:6913–6920. 10.1128/JB.182.24.6913-6920.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roy H, Ling J, Irnov M, Ibba M. 2004. Post-transfer editing in vitro and in vivo by the beta subunit of phenylalanyl-tRNA synthetase. EMBO J. 23:4639–4648. 10.1038/sj.emboj.7600474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guillerez J, Lopez PJ, Proux F, Launay H, Dreyfus M. 2005. A mutation in T7 RNA polymerase that facilitates promoter clearance. Proc. Natl. Acad. Sci. U. S. A. 102:5958–5963. 10.1073/pnas.0407141102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilkinson AJ, Fersht AR, Blow DM, Winter G. 1983. Site-directed mutagenesis as a probe of enzyme structure and catalysis: tyrosyl-tRNA synthetase cysteine-35 to glycine-35 mutation. Biochemistry 22:3581–3586. 10.1021/bi00284a007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predicted cloverleaf structures of S. pneumoniae tRNALys anticodons TTT (A) and CTT (B). Sequence variations between the two tRNALys isoacceptors are highlighted in red. All tRNA cloverleaf structures are shown without the CCA end. Download
Natural deacylation of mischarged pneumococcal Ala-tRNALys demonstrates its inherent instability. Deacylation time courses were carried out as described in the text with incubation of 10 pM [14C]Ala-tRNALys in buffer composed of 0.1 M Na-HEPES (pH 7.2), 30 mM KCl, and 10 mM MgCl2. Download
Predicted cloverleaf structures of E. coli (A) and S. pneumoniae (B) tRNAIle (anticodon GAU). Sequence variations from the S. pneumoniae tRNAIle sequence are highlighted in red. All tRNA cloverleaf structures are shown without the CCA end. Position 16 is boxed. Download